Soybeans are rich in proteins and lipids and have become a staple part of the human diet. Besides their nutritional excellence, they have also been shown to contain various functional components, including isoflavones, and have consequently received increasing attention as a functional food item. Isoflavones are structurally similar to 17-β-estradiol and bind to estrogen receptors (ERα and ERβ). The estrogenic activity of isoflavones ranges from a hundredth to a thousandth of that of estrogen itself. Isoflavones play a role in regulating the effects of estrogen in the human body, depending on the situation. Thus, when estrogen is insufficient, isoflavones perform the functions of estrogen, and when estrogen is excessive, isoflavones block the estrogen receptors to which estrogen binds, thus acting as an estrogen antagonist. In particular, estrogen antagonistic activity is important in the breast, endometrium, and prostate, and such antagonistic activity suppresses cancer occurrence.
1. Introduction
In general, substances that interfere with the endocrine system are known as endocrine disruptors. Most are artificial chemicals. These estrogen disruptors (including pesticides such as dichlorodiphenyltrichloroethane (DDT), polychlorinated biphenyls (PCBs), and bisphenol A) not only cause reproductive problems, but also induce cancer and obesity
[1]. In contrast, phytoestrogens are estrogen-like non-steroid substances found in plants (hence the prefix “phyto”) and are effective in preventing cancer, arteriosclerosis, osteoporosis, menopausal symptoms, and obesity
[2]. Twenty different types of phytoestrogens are known so far, including lignans (secoisolariciresinol, matairesinol, pinoresinol, and lariciresinol), isoflavones (genistein, daidzein, glycitein, and formononetin), coumestans (coumestrol), and prenylflavonoids
[3]. Such phytoestrogens are abundant in grains, vegetables, and fruits. Soybeans contain particularly high amounts of isoflavones, making it advantageous that soybeans are easy to consume. Barley, sunflower seeds, lentils, arrowroot, broccoli, and cauliflower also contain isoflavones
[4]. The most well-known isoflavones are genistein, daidzein, formononetin, biochanin A, and coumestrol. Genistein is the most abundant isoflavone in legumes. Among legumes, soybeans contain the highest amount of genistein (26.8–102.5 mg/100 g dry weight), followed by arrowroot (12.6 mg/100 g dry weight)
[5]. Compared to soybeans, other food sources contain very small amounts of genistein. In addition to genistein (40–60%), daidzein (30–50%) and glycitein (12–13%) are other well-known isoflavones
[5].
Isoflavones protect plants exposed to the external environment. Although the recommended daily intake of isoflavones has not yet been established, the FDA recommends an intake of 50 mg per day, which is considered to be safe
[6]. Currently, there is a lack of information on the side effects of ingesting high concentrations of isoflavones. Isoflavone intake is exceptionally high in Asian countries; China, Japan, and South Korea have a daily soy isoflavone intake in the range of 25–50 mg, in contrast to the United States and European countries, where the average daily intake is less than 2 mg
[6].
Asian countries consume isoflavones in tofu, tempeh, miso, natto, and cheonggukjang; in Western countries, it is mainly consumed in the form of dairy substitutes, such as soy milk, soy cheese, and soy yogurt
[7][8][7,8]. Isoflavones occur in food as either sugar-bound glycosides or as aglycones, without a sugar molecule; aglycones are known to be more bioavailable than glycosides
[9]. In non-fermented foods like tofu and bean sprouts, isoflavones mostly exist as glycosides; however, in fermented foods like doenjang (soybean paste) and cheonggukjang, large amounts of isoflavones are broken down, and these foods thus provide highly bioavailable isoflavones
[10]. Chemically, isoflavones are compounds of the flavonoid family with a 3-phenylchromone skeleton that are abundant in soybeans, particularly in its hypocotyl
[11].
Isoflavones show structural similarity to the female hormone estrogen as well as similar biological activities, and consequently they are called phytoestrogens
[12]. Phytoestrogens have been found to serve as a potential alternative therapy for hormone-dependent diseases, including cancer, menopausal syndrome, cardiovascular diseases, and osteoporosis. Isoflavones also play a role in strengthening the bones by increasing bone density
[13]. There are 12 types of soy isoflavones, classified into glycosides and aglycones according to their chemical structure. Of them, genistein and daidzein in particular been attracting attention for their anti-cancer and antioxidative effects, and are thus hypothesized to have the potential to prevent and treat not only various types of cancers, but also various lifestyle diseases
[9][13][9,13]. They have been found to inhibit the action of enzymes involved in the proliferation of cancer cells; several studies have reported their ability to inhibit cancers such as prostate cancer. In addition, studies have shown that they bind weakly to estrogen receptors and inhibit the development of breast cancer cells that require estrogen activity
[6][14][6,14]. Isoflavones exhibit weak estrogen activity and can also prevent bone loss by promoting bone cell proliferation. Various studies have reported their role in the prevention of osteoporosis caused by menopause and aging
[6][15][6,15]. Due to the recent increasing interest in soybean extracts, isoflavones are now being used as a material in food medicines for a variety of commercialized products such as functional health supplements, cosmetics, and in beverages
[5][12][5,12].
2. Content, Structure, and Metabolism of Isoflavones
2.1. Isoflavone Content in Soybean
According to the USDA Database for the Isoflavone Content of Selected Foods (Release 2.1) published by the US Department of Agriculture (
http://doi.org/10.15482/USDA.ADC/1324538 or
http://www.ars.usda.gov/nutrientdata/isoflv; accessed on 1 January 2021), soybean is the only type of beans, in comparison to other beans, to contain high levels of isoflavones
[16]. The isoflavone content for soybeans varies slightly between countries, as in 100 g soybean it was found to be 118.28 mg in China, 130.65 mg in Japan 159.98 mg in the US, and it was highest in Korea at 178.81 mg. The global average was 154.53 mg
[16]. This indicates that the isoflavone content of soybeans varies according to the cultivar and growth conditions; but nevertheless, it generally make up 0.2–0.3% of the dry weight. The daily recommended intake of isoflavones is around 40–50 mg/day, and to achieve this 25 g of boiled soybean or 100 g of common tofu should be consumed daily
[5].
2.2. Isoflavone Biosynthesis and Regulation
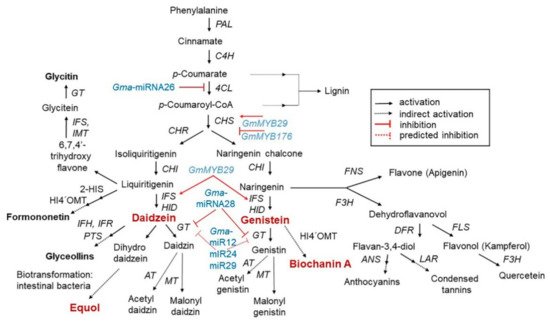
The isoflavone biosynthetic pathway is divided into several branches that generally share common substrates resulting in strong and regulated flux channeling. Isoflavones have a distinct group of plant secondary metabolites that are produced from the phenylpropanoid pathway
[17]. The precursor in the multistep pathway of isoflavone biosynthesis is the amino acid L–phenylalanine, which in the initiating step is stripped of its amine group to generate cinnamic acid after non-oxidative deamination via the enzyme phenylalanine ammonia lyase (PAL). Then, cinnamic acid is translated into
p-coumaryol CoA by 4–hydroxylase (C4H) and 4–coumarate CoA ligase (4CL). The first critical enzymes involved in isoflavone biosynthesis are from the multigenic chalcone synthase (CHS) family, although not all are expressed in the seeds at detectable levels. Of all the CHS homologous copies, CHS7 and CHS8, which are seed specific in soybean, have the highest expression levels in seeds and they catalyze the conversion of
p–coumaryol CoA into naringenin chalcone. Chalcone isomerase (CHI) and chalcone reductase (CHR) are the other important enzymes that are required for isoflavone synthesis. CHI converts chalcones to flavanones and CHR is required for daidzein and glycitein formation (
Figure 1)
[18][19][18,19].
Figure 1. Schematic representation of the phenylpropanoid pathway, showing the key intermediates and enzymes associated with isoflavone biosynthesis, and partial regulation pathway. Isoflavones are biosynthesized via a phenylalanine-dependent pathway in the presence of a wide range of enzymes. Regarding regulation, GmMYB29 activates chalcone synthase (CHS) expression, whereas GmMYB176 inhibits CHS expression. Furthermore, Gma-miRNA26, Gma-miRNA269, and Gma-miRNA28 suppress the gene expression or enzymatic activity of 4-coumarate-CoA ligase (4CL), isoflavone synthase (IFS), and glucosyltransferase (GT), respectively. Dotted lines represent the unclear role of miRNA in the pathway
[18][19][18,19]. This isoflavone biosynthetic pathway is adapted from “USDA Database for the Isoflavone Content of Selected Foods Release 2.0 (
http://www.ars.usda.gov/nutrientdata; accessed on 1 March 2021).
Isoflavone synthase (IFS) is a cytochrome P450 monooxygenase that is important for distinguishing between isoflavone-producing and isoflavone-lacking plants. The soybean genome harbors two IFS genes (IFS1 and IFS2) with 14 different amino acids. The two IFS isoforms catalyze the first reaction of isoflavone biosynthesis to form 2-hydroxyisoflavanone, which in turn is dehydrated to form daidzein and genistein naturally or by intervention of 2-hydroxyisoflavanone dehydratase (HID)
[20]. Soybean synthesizes daidzein and genistein in the cytoplasm, which are additionally glycosylated into daidzin and genistin by UDP-glucose:isoflavone 7-
O-glucosyltransferase, or malonylated into malonyldaidzin/genistin by malonyl-CoA:isoflavone 7-
O-glucoside 6″-
O-malonyltransferase
[21]. Malonylated products accumulate in vacuoles in which daidzein and genistein are directly secreted to the root or rhizosphere through membrane transport by ATP-binding cassette-type transporters or are indirectly secreted into apoplasts as isoflavone glucosides. This process is mediated by isoflavone conjugates-hydrolyzing beta-glucosidase (ICHG) present in the apoplasts
[22]. In addition to isoflavone biosynthesis, the phenylpropanoid pathway is involved in the synthesis of lignins, stilbene, phlobaphenes, proanthocyanidins, and anthocyanins through specific branches
[23][24][23,24]. The differences in isoflavone accumulation among several soybean varieties result from genetic and environmental interactions, which regulatory mechanisms remain unclear.
All steps of the isoflavone biosynthesis pathway are regulated by other genes that determine the accumulation patterns of other compounds in the pathway
[18]. Currently, five microRNAs (Gma–miRNA12, Gma–miRNA24, Gma–miRNA26, Gma–miRNA28, and Gma–miRNA29) are known to regulate isoflavone biosynthesis. The differential expression of Gma-miRNA26 and Gma-miRNA28, and their corresponding target genes (Glyma.10g197900 and Glyma.09g127200) were directly related to the total isoflavone content
[18]. In contrast, some MYB transcription factors involved in the regulation of the isoflavone biosynthesis pathway have been identified in soybean
[25]. For example, the R1-type MYB transcription factor GmMYB176 affects isoflavone synthesis by regulating chalcone synthase 8 (CHS8) expression. R2R3-type MYB transcription factors (GmMYB39 and GmMYB100), are believed to negatively regulate isoflavone biosynthesis by inhibiting the expression of structural biosynthesis genes
[25][26][25,26]. Recently, the R2R3–type MYB transcription factor GmMYB29 (Glyma.20g209700) was found to activate a promoter related to IFS2 and CHS8
[25][26][25,26]. Overexpression of GmMYB29 in hairy roots increased, whereas isoflavone levels were reduced upon its suppression
[26]. Thus, a better understanding of the isoflavone biosynthesis mechanism may aid in the development of novel plant varieties based on molecular breeding.
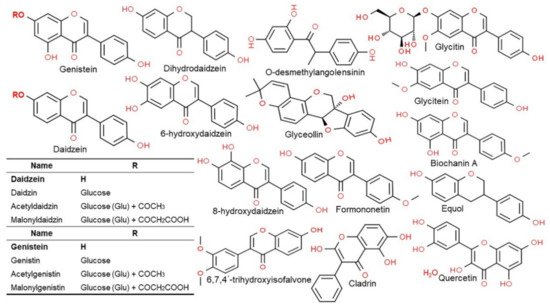
2.3. Structure and Type of Isoflavones
Isoflavones are compounds with a 3-phenylchrome structure, and the best known are genistein, daidzein, formononetin, biochanin A, and coumestrol. Soy isoflavones are divided into glycosides and aglycones depending on their chemical structures. Twelve isoflavones have been identified in soybean, including three aglycones—genistein, daidzein, and glycitein—and their corresponding glycosides daidzin, genistein, and glycitin, as well as malonyl glycoside and acetyl glycoside. The glycosides in soybean are often found as malonyl glycoside. The other forms include the glycosides of daidzin and genistin, which are daidzein and genistein, respectively, with a carbohydrate attached to C7 (7-
O-glucosides) and 6′-
O-acetyl daidzin and 6′-
O-acetylgenistin, which have a carbohydrate attached to C6 (6′-
O-acetylglucosides). There are also 6′-
O-malonyl daidzin and 6′-
O-malonyl genistin (6′-
O-malonylglucosides) (
Figure 2)
[5]. For absorption in the intestine the glycosides with a polysaccharide attached (7′-
O-glucosides, 6′-
O-acetylglucosides, and 6′-
O-malonylglucosides) are converted to aglycones, which are free isoflavones without the sugar, by an enzyme called β-glucosidase secreted by the intestinal microflora
[9][27][9,27]. The chemical structure of soy isoflavones resembles estrogen and they have an affinity for the estrogen receptor. Such isoflavone compounds are mainly distributed in the epicotyl and hypocotyl of the plant as mentioned above, while their contents vary greatly among the different cultivars and even within the same cultivar, variation in content has also been reported to be depended on growth conditions
[13][28][13,28].
Figure 2. Chemical structures of the major classes of isoflavones and their metabolites. Structures were drawn using the ChemSpider. See the genistein and daidzein backbone in bold before reviewing respective molecules.
2.4. Functionality of Isoflavones in Plants
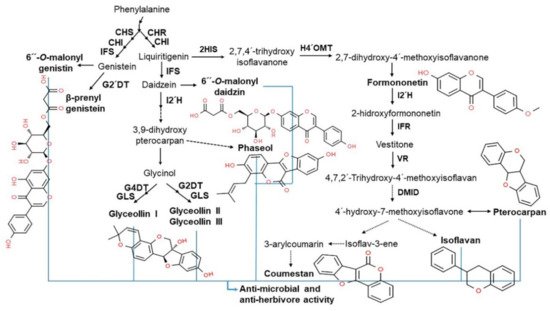
In plants, isoflavones play an important role in plant-microbial interactions, including defense and symbiosis. In plant defense systems, isoflavones serve as stress-resistant mediators with antioxidant activity that help neutralize reactive oxygen species (ROS) induced by stressful conditions. They are precursors of phytoalexins, a classic plant metabolite with antibacterial and antiviral effects and/or antiherbivore activities that protect plants from pathogen infections (
Figure 3)
[26][29][30][31][32][26,29,30,31,32]. One of the most well-known soybean phytoalexins is prenylated pterocarpans derived from glyceollins and daidzein, which are released in response to pathogens, such as
Phytophthora sojae and
Macrophomina phaseolina [33]. In addition to their role in the plant defense system, isoflavones are known to have health benefits for humans, including the prevention and amelioration of various diseases including cancer, cardiovascular disease, neurologic disorders, climacteric syndrome, obesity, inflammation, and aging, due to its phytoestrogen and antioxidant properties
[13][34][13,34]. Recently, isoflavones were reported as desirable ingredients in bean-based infant formulas
[35]. Therefore, the various benefits of soybean-derived isoflavones to plants and humans highlight their importance and the need for more relevant research (especially in plants).
Figure 3. Relationship between isoflavone products and antimicrobial activity. Phenylalanine-derived biosynthetic isoflavone products, including 6″-
O-malonylgenistein, glyceollins (I, II, and III), coumestan, isoflavan, and pterocarpan, are capable of antimicrobial and antiherbivore activity for defense against abiotic stress, such as pathogens
[29][30][31][32][29,30,31,32].
As aforementioned, isoflavone biosynthesis is believed to be regulated by environmental conditions
[5]. For example, high concentrations of heavy metals can suppress plant growth and development. Environmental factors can directly or indirectly influence various organism structures and processes through the production of ROS
[36]. In particular, vanadium compounds have a negative impact on plant growth and development, as they can bind to phosphates of enzymes and inhibit their activation, such as protein kinases, ribonucleases, or ATPases
[37][38][39][37,38,39]. Despite these known negative effects and toxicity, vanadium compounds have also been observed in vivo as promising plant-induced substances that affect the production and exudation of secondary metabolites, and have been reported as potential therapeutic agents for several diseases
[37]. Moreover, some vanadium compounds can activate genes and enzymes involved in the phenylpropanoid pathway, thereby facilitating flavonoid production and release
[38].
The complex surrounding environment of plants is continuously evolving. Thus, to protect themselves from harmful substances, plants are equipped with a natural immune system, divided into acquired and induced systemic resistance
[40][41][40,41]. In plants, phytoestrogens do not function as hormones, instead they have an important role as phytoalexins, small molecule compounds with antifungal, antibacterial, antiviral, and antioxidant properties that are produced in response to environmental stress and pathogenic attack
[5]. Among these compounds, isoflavones play a crucial role in plant-microbe interactions, such as rhizobia-legume symbiosis and defense responses in legumes, including soybeans
[42][43][42,43]. In plant defense, isoflavones act as a precursor of antibacterial and/or antiherbivore activities of phytoalexins that protect the plant from pathogen infection
[44][45][44,45]. One of the most well-known phytoalexins in soybean is prenylated pterocarpans derived from glyceollins and daidzein, which are induced in response to pathogens such as
P. sojae and
M. phaseolina [33][46][33,46]. For example, daidzein is metabolized to produce glyceollins, which are important elements in the defense mechanism against fungal pathogens. Concerning symbiosis, isoflavones are signaling molecules that contribute to the formation of nitrogen-fixing root nodules in legumes
[47]. In addition to these functions in plants, isoflavones (such as daidzein and genistein) are released into rhizospheres—a small area around legume roots—where they will serve as signaling molecules for rhizobia to form nodules in the roots
[22][48][22,48]. Therefore, isoflavones play an important role in biological communication with soil microbes. Research on the functions of isoflavones in plants is lacking compared with that available in humans; therefore, this topic warrants more attention and investigation.