You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by VIRGINIA MARTINEZ and Version 2 by Rita Xu.
BODIPY dyes have recently attracted attention as potential photosensitizers. Commercial and novel photosensitizers (PSs) based on BODIPY chromophores (haloBODIPYs and orthogonal dimers strategically designed with intense bands in the blue, green or red region of the visible spectra and high singlet oxygen production) were covalently linked to mesoporous silica nanoparticles (MSNs) further functionalized with PEG and folic acid (FA).
- BODIPY-based photosensitizers
- functionalized silica nanoparticles
- folic acid
- PEG
- photodynamic therapy
1. Introduction
Currently, several alternatives are used to treat cancer, including surgery and chemo, radio- or immune-therapy, although depending on the type of cancer an effective method has not been found yet. In this regard, Photodynamic Therapy (PDT) is a complementary treatment that can be applied as a combined therapy to enhance anticancer efficiency by a synergic or additive effect with conventional methods. PDT involves a light source, a photosensitizer (PS), and oxygen. During PDT, PS is activated under light at a specific wavelength to generate reactive oxygen species (ROS), mainly singlet oxygen (1O2), a cytotoxic species able to promote apoptosis or necrosis of cancer cells [1]. Nowadays, preclinical and clinical trials have proven PDT to be effective in early-stage tumors or the palliation of advanced cancers, such as skin, head, neck, esophageal, or lung cancer, improving patient survival [2][3][4][5][2,3,4,5]. PDT is considered a less invasive and more precise treatment (locally controlled by the light irradiation of malignant tissue), without inducing long-term side effects, and it has a lower cost with respect to other treatments. Nevertheless, the limitations of PDT are mainly related to the availability of the PSs. Despite there being several PSs approved by the FDA, most of them are hydrophobic and/or tend to have poor selectivity to malignant tissues [6][7][8][9][6,7,8,9].
The ideal PS to be used as a photoactive drug for PDT should be non-cytotoxic in dark conditions, selective to cancer tissues, and display limited stability in vivo to minimize side effects; it should have intense absorption bands (ε ≥ 50,000 M−1 cm−1), preferentially in the phototherapeutic window to ensure deeper penetration of light into tissues [10] (630–850 nm), and high singlet oxygen production to reduce doses and irradiation time; it should be photoresistant to avoid the photodecomposition of the PS during treatment; and finally, it should present an amphiphilic nature, being soluble in water as well as permeable through the cell membrane. At the moment, few PSs fulfill these requirements, and new molecular designs are required [6][7][11][12][13][14][6,7,11,12,13,14]. One approach for obtaining new molecules is focused on the synthesis of improved PS to overcome their limitations, but this usually requires multistep chemistry, increasing the costs and production time, hampering the implementation for clinical uses. In this context, BODIPY dyes have recently attracted attention as potential photosensitizers [15][16][17][18][15,16,17,18]. They are characterized by intense absorption and emission bands in the green region, and resistance to photobleaching [19][20][19,20]. Despite being highly fluorescent chromophores (antagonistic property to ROS generation) and poorly soluble in water, their synthesis allows easy, versatile, and selective modification of their molecular structure to increase the population of the triplet state, and consequently their singlet oxygen generation, while also shifting their spectroscopic bands into the clinical window. These modifications include the addition of iodine heavy atoms, π-conjugated systems in the BODIPY skeleton, or the design of orthogonal BODIPY dimers [15][17][18][21][22][23][24][25][26][27][28][29][30][31][32][33][15,17,18,21,22,23,24,25,26,27,28,29,30,31,32,33]. Further functionalization of the BODIPY chromophore is related with the incorporations of different targets to increase their solubility in aqueous media and enhance their selectivity to cancer cells [14][34][35][36][37][38][39][14,34,35,36,37,38,39].
Another alternative is the use of nanomaterials as (photo)drug carriers. They have a large surface-to-volume ratio, which allows the administration of a large amount of active components, preventing their degradation or inactivation by plasma components, delivering soluble and stable formulations in aqueous media, and enhancing their accumulation inside tumor tissues by so-called passive targeting due to the enhanced permeability and retention (EPR) effect [11][40][41][42][43][44][45][46][47][48][49][11,40,41,42,43,44,45,46,47,48,49]. Additionally, the selectivity to cancer cells can be improved by active targeting through surface modifications with target ligands, such as proteins, polysaccharides, nucleic acids, peptides and small molecules that bind to specific receptors overexpressed on the surface of malignant cells but not on healthy cells [9][50][51][52][9,50,51,52].
Currently, there are many different types of nanoparticles based on liposomes, polymeric, micellar, metallic, or protein for medical use [40][41][42][43][44][45][46][53][54][55][40,41,42,43,44,45,46,53,54,55]. In this regard, silica nanoparticles (SN) have attracted attention as carriers for drug delivery due to their properties, which include reduced toxicity, good biocompatibility, high surface area, easy functionalization, optical transparency, and low cost [56][57][56,57]. PS-loaded silica nanoparticles have been reported as promising singlet oxygen generator platforms, improving the photoactive drug delivery by enhancing PS poor solubility and selectivity for cancer cells [58][59][60][61][62][58,59,60,61,62]. The PS can be physically encapsulated or covalently attached to the internal or external surface of the silica nanoparticles [63][64][65][66][67][63,64,65,66,67]. Briefly, loading PS within the nanostructure ensures a high photostability but restrains the diffusion of oxygen species (molecular oxygen towards inside and singlet oxygen towards outside). It has been demonstrated that nanoparticles with draped-PS outside lead to better 1O2 productivity than PS located inside [68][69][70][68,69,70].
In the last few years, diverse nanoplatform designs have been used as vehicles to carry BODIPY-PS [71][72][73][74][75][76][77][78][79][80][81][71,72,73,74,75,76,77,78,79,80,81], or even BODIPY-based nanoparticles, through the self-assembly process [79][80][82][83][84][85][86][87][88][79,80,82,83,84,85,86,87,88]. However, despite the advantageous properties of SN, mentioned above, few examples can be found in the literature of their use as carriers for BODIPY-PSs [89][90][89,90].
In this work, different PSs (Figure 1) were tethered to the external surface of 50 nm MSNs. First, three commercially available PSs, Rose Bengal (RB), Chlorin e6 (C6), and Thionine (Th), recognized as suitable singlet oxygen generators and extensively employed in PDT, were used [6][9][10][11][59][91][92][93][6,9,10,11,59,91,92,93]. These dyes already have functional groups in their molecular structure (carboxylic in Rose Bengal and Chlorin e6 and amine in Thionine) and can be easily grafted to the external surface of MSNs. Afterward, seven custom-made BODIPY-based PSs were used, which were rationally designed to effectively generate high singlet oxygen production under illumination at different wavelengths of the visible spectra (blue, green or red light) [12][18][24][31][94][95][12,18,24,31,94,95], and to endow suitable graftable groups to be anchored at MSNs.
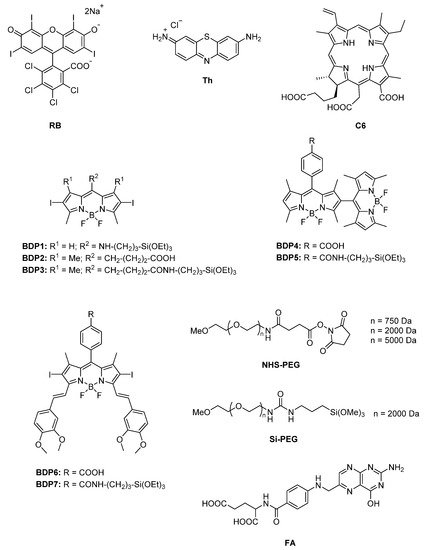
Figure 1. Molecular structure of the different compounds anchored to MSN: commercial (RB, Th, C6) and custom-made BODIPY photosensitizers (BDP1-BDP7), PEG derivatives with different functional groups (Si-PEG and NHS-PEG) and molecular weight (750 Da, 2000 Da and 5000 Da), and FA.
Additionally, MSNs were externally coated with polyethylene glycol (PEG), as it is usually required to stabilize nanoparticle systems, enhance their life-time in the blood system and avoid the induction of immune responses [44][47][49][94][95][96][97][98][44,47,49,94,95,96,97,98]. For that, several PEG derivatives (Figure 1) with different graftable groups at one end of the chain (succinimide group or with silyl group Si-PEG) were tethered at the MSN shell. The length of the polymer chain (750 Da, 2000 Da, and 5000 Da) was also adjusted to improve nanoparticle stabilization in water.
2. Silica Nanoparticles Characterization
Mesoporous silica nanoparticles with a suitable size for medical applications and particularly for PDT [98][99][98,106] were synthesized by the modified Stöber method [100][107] as described elsewhere [101][105]. The external surface of mesoporous nanoparticles surface was functionalized with amino group (NH-MSN) or carboxylic group (COOH-MSN). The latter type was obtained after conversion of CN-MSN in acidic conditions, according to the synthesis route described in Materials and methods section and Supporting Material. Bare MSNs analyzed by SEM and TEM showed spherical morphology and mesoporous structure (Figure 2 and Figure S1), with a size distribution of 50 ± 10 nm. The external functionalization of MSNs was studied by XPS (Table S1). The presence of 5% of nitrogen atoms in both NH-MSN and CN-MSN confirmed the existence of amine/cyano functional groups located outside of the nanoparticles whereas the absence of nitrogen atoms in the COOH-MSN indicates an effective conversion of CN into COOH groups. In the case of FTIR spectra (Figure S2), an intense peak located at υ = 1110–1000 cm−1 as well as a wide band placed at υ = 3650–3200 cm−1 were recorded in every sample spectrum, and are assigned to Si-O-C and O-H groups, respectively. A characteristic band of cyane group (C≡N) at υ = 2260–2240 cm−1 was recorded in CN-MSN, which disappeared in the COOH-MSN system (Figure S2 blue), indicating again the total conversion from -CN to -COOH. Furthermore, the typical band of COOH group (COO-H υ = 3550–2550 cm−1, C=O υ = 1775–1650 cm−1) was also registered in COOH-MSN.
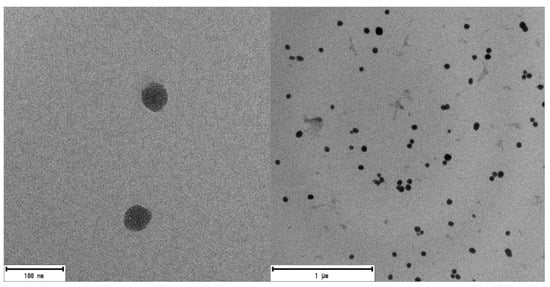

Figure 2. TEM images of MSNs. There are no noticeable differences between any of the synthesized MSNs (NH-MSN, CN-MSN and COOH-MSN).
The sizes of the three types of nanosystems were also characterized in water suspension by DLS. Both NH-MSN and COOH-MSN showed similar hydrodynamic diameter, of around 60–70 nm, to the size of the nanoparticles by TEM (Table 1), whereas the larger diameter, derived for CN-MSN, of 280 nm, indicates a tendency to form aggregates. Zeta potential values obtained for CN-MSN but also for NH-MSN (≤±25 mv) [102][108] confirm a poorer stability in water with respect to COOH-MSN system. The higher stability of this later functionalized COOH-MSN is attributed to the presence of carboxylic groups at the external surface, partially deprotonated (COO−) in aqueous media and the superficial negative charge makes the nanosystem more stable by electrostatic repulsion, Table 1.
Table 1.
DLS and Zeta potential of mesoporous silica nanoparticles in water.
| Name | Shell | DLS (nm) |
Z Pot (mV) |
||
|---|---|---|---|---|---|
| NH-MSN | NH | 2 | /OH | 71 | −3.96 |
| CN-MSN | CN/OH | 280 | −7.06 | ||
| COOH-MSN | COOH/OH | 66 | −39.7 |
Actually, the MSNs’ stability in aqueous media is certainly controlled by the type and the number of molecules lodged at the external surface. It has been demonstrated that the presence of organic PSs makes the external surface of MNS more hydrophobic, promoting nanoparticles agglomeration, hindering their stability in aqueous media [103][109]. Note here that the particle–particle aggregation is detrimental to singlet oxygen production and should be avoided or minimized. To optimize the PS loading and to ensure the stability of the nanoparticles in suspension, several syntheses were carried out for the amine-functionalized MSN (NH-MSN), employing commercial RB as PS and different PEG derivatives (RB-PEGn-NH-MSN). The combinations were focused on the variation of (i) the functional group of the MSN (OH-, or NH2-) at which PS and PEG molecules were attached, and (ii) the length of PEG chain (750 Da, 2000 Da, and 5000 Da).
3. Optimization of the Functionalization of Silica Nanoparticles with Rose Bengal as PS
Rose Bengal is a commercial PS with an intense absorption band (λmax = 556 nm; ε = 9.8·104 M−1cm−1) and high singlet oxygen production (Φ∆ = 0.86 in CH3OD). The carboxylic function in the RB molecular structure allows the covalent grafting to be inserted to amine groups or to the intrinsic hydroxyl groups of the external surface of MSN [103][109]. Nevertheless, both RB-MSN nanosystems (RB grafted at the external NH2 or OH) showed instantaneous flocculation in water media and the suspension was only viable in less polar solvents. Since stable nanoparticle suspension in water is crucial to obtain competitive hybrid nanocarriers for PDT [103][109], pegylation of the outside of MSNs is required to avoid the precipitation of the nanoparticles. Firstly, to optimize the stabilization of the system, NHS-PEG of different chain length (750 Da, 2000 Da and 5000 Da) were linked to the amine groups of the silica, while RB was anchored in OH groups (samples RB-PEG750-NP-a, RB-PEG2000-NP-a, and RB-PEG5000-NP-a in Table 2).
Table 2. RB amount, nanoparticle size and their Zeta potential by DLS in water of the different RB-PEG-MSNs.
| System | Characteristic | PEG Length (Da) |
DLS Size (nm) |
ZPOT (mV) |
[RB] (μmol/g) |
||||
|---|---|---|---|---|---|---|---|---|---|
| RB-PEG | 750 | -NP-a | RB-OH-MSN PEG-NH | 2 | -MSN | 750 | 130 | −4.3 | 20 |
| RB-PEG | 2000 | -NP-a | RB-OH-MSN PEG-NH | 2 | -MSN | 2000 | 99 | −25.0 | 20 |
| RB-PEG | 5000 | -NP-a | RB-OH-MSN PEG-NH | 2 | -MSN | 5000 | 114 | −25.0 | 20 |
| RB-PEG-NP-b | RB-NH | 2 | -MSN PEG-OH-MSN |
2000 | 95 | −29.0 | 10 | ||
| RB-PEG-NP-c | RB-OH-MSN PEG-OH-MSN |
2000 | 88 | −31.0 | 20 |
According to zeta potential (Table 2), the least favored value (−4.3 mV) was registered for sample RB-PEG750-NP-a with the shortest PEG chain in this series, indicating its inefficiency at improving the stability of RB-MSN in water. Indeed, a similar value of around -4 mV was obtained for NH-MSN without RB (Table 1). In contrast, PEG of higher molecular weight, 2000 Da and 5000 Da (RB-PEG2000-NP-a and RB-PEG5000-NP-a in Table 2) rendered Zpot values of −25 mV, indicating good stability of these nanosystems in water. The longer PEG-5000 did not lead to an improvement of the stability with respect to PEG-2000, which could likely be assigned to a different conformation adopted at the external surface [104][110]. Additionally, long PEG chains can also impede the internalization of nanoparticles into the cells [105][106][111,112]. Thus, a PEG of 2000 Da was selected as the most suitable, and was employed in the rest of the samples.
Next, different anchorages between PEG and MSN (at a fixed PEG length of 2000 Da) were also tested. The anchoring of Si-PEG (silylated PEG of 2000 Da, Figure 1) to the external OH-groups of MSN, samples RB-NP-b and RB-NP-c, led to even higher Zpot values with respect to sample RB-PEG2000-NP-a (with PEG at the amine groups), Table 2. This fact is possibly due to a higher presence of PEG at the surface because there are more accessible OH-groups than NH2-groups at the silica external surface [97]. This assumption was confirmed for RB, showing a double dye loading when was tethered to OH with respect to NH2 groups of the MSN external surface (RB-PEG-NP-b vs. RB-PEG-NP-c in Table 2) [103][109].
The stability of the nanoparticles can also be studied by the absorption spectra of the RB-PEG-MSNs samples in water suspension (Figure 3). The registered bands for RB-PEG2000-a and RB-PEG5000-a, practically identical, showed more prominent shoulders at both sides of the main absorption band, indicative of a higher dye aggregation tendency. Indeed, according to the absorption spectra, the dye aggregation follows the tendency RB-PEG5000-NP-a ≈ RB-PEG2000-NP-a > RB-PEG-NP-c > RB-PEG-NP-b. For the samples RB-PEG2000-NP-a, RB-PEG5000-NP-a, and RB-PEG-NP-c (RB grafted at the hydroxyl groups of MSNs, Table 2) the estimated RB loading was equal, and consequently, the observed dye aggregation in these samples should be assigned to interparticle processes, as supported by Zpot values and previously attributed to a lower presence of PEG molecules at the external surface. Sample RB-PEG-NP-b, with RB loading at the amine groups half of that obtained for the RB at the hydroxyl groups (sample RB-PEG-NP-b vs. sample RB-PEG-NP-a in Table 2), showed a narrower absorption band, not much different from that recorded for RB in diluted solution [101][105]. However, reducing the cargo of PS per nanoparticle would compel a higher concentration of nanoparticles per volume to reach effective PS doses for PDT in the cells, which would also promote particle-particle agglomeration. For this reason, the optimization of the samples is not a trivial task, and the quantification of their singlet oxygen production would be a good indicator of their applicability in cells. Significantly, all the samples, except for RB-PEG750-NP-a, showed a similar singlet oxygen quantum yield, with values around ΦΔ ≈ 0.80–0.85 in deuterated methanol (CH3OD), similar to that registered for RB in the same solvent (ΦΔ = 0.86). The fact that RB grafted to MSN can generate singlet oxygen as efficiently as the RB in solution is indicative of the potential use of these nanosystems in PDT [65][67][102][65,67,108].
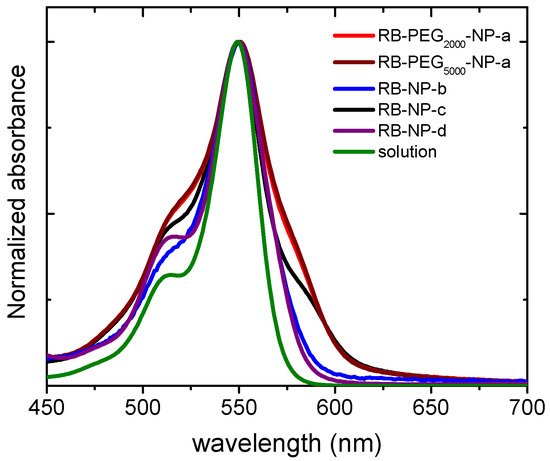

Figure 3. Normalized absorption spectra of RB-PEG2000-NP-a (red), RB-PEG5000-NP-a (brown), RB-PEG-NP-b (blue), RB-PEG-NP-c (black), RB-PEG-NP-d (purple) in water suspension (0.5 mg/mL) and RB in diluted aqueous solution (green). The absorption spectra were recorded after stirring the nanosystems for at least 24 h.
