You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Vicky Zhou and Version 1 by Tinne Dilles.
Pharmaceutical care necessitates significant efforts from patients, informal caregivers, the interprofessional team of health care professionals and health care system administrators. Collaboration, mutual respect and agreement amongst all stakeholders regarding responsibilities throughout the complex process of pharmaceutical care is needed before patients can take full advantage of modern medicine. Based on the literature and policy documents, in this position paper, we reflect on opportunities for integrated evidence-based pharmaceutical care to improve care quality and patient outcomes from a nursing perspective.
- nursing
- pharmaceutical care
- interprofessional collaboration
1. Introduction
Prescribed and purchased medicines are important aspects of patient management. Optimising and individualising each patient’s pharmacotherapy regimen, with maximum therapeutic gain and minimum adverse effects, can be challenging. Pharmaceutical care, with the focus on optimising medicine use and the improvement of health outcomes [1], necessitates significant efforts from patients, informal caregivers, the interprofessional team of health care professionals and health care system administrators. Collaboration, mutual respect and agreement amongst all stakeholders regarding responsibilities throughout the complex process of pharmaceutical care is needed before patients can take full advantage of modern medicine.
On 11 March 2020, the Council of Europe adopted a new resolution on pharmaceutical care [2]. Pharmaceutical care was defined as the responsible provision of pharmacotherapy for the purpose of achieving definite outcomes that improve a patient’s quality of life [2][3]. Examples of definite outcomes reported in core outcome sets are drug-related hospital admissions, drug overuse, drug underuse, potentially inappropriate medications/medication appropriateness, clinically significant drug–drug interactions, health-related quality of life, pain relief, adverse drug reactions, falls, medication regimen complexity, mortality, and medication side effects [4][5]. The resolution focuses on how pharmaceutical care can be implemented for the benefit of patients and health services. Patients and their families or friends are not only important partners in care, they also decide on care goals, informed by health care providers. They are key in the evaluation of care and the achievement of anticipated care goals.
The resolution identifies opportunities to optimise pharmaceutical care through interprofessional and patient-centred approaches, but also some challenges. Steps in pharmaceutical care, listed in the resolution, are (1) patient assessment of medication, health problems and health status; (2) identification and prioritisation of medication-related problems; (3) selection of interventions and formulation of pharmaceutical care plan; (4) patient agreement, implementation and monitoring; and (5) follow-up with the patient [2]. Other concepts are sometimes used to refer to (parts of) pharmaceutical care as defined above: one example is the concept of medicines optimisation, as defined by the UK National Health Service (NHS) [6]. This paper embraces these concepts to the extent that they accord with the definition of pharmaceutical care.
The resolution acknowledges the importance of an integrated interprofessional and multi-disciplinary approach to improving quality of care and patient outcomes. The World Health Organization (WHO) defines integrated health services as “health services that are managed and delivered so that people receive a continuum of health promotion, disease prevention, diagnosis, treatment, disease-management, rehabilitation and palliative care services, coordinated across the different levels and sites of care within and beyond the health sector, and according to their needs throughout the life course” [7]. Person- or people-centred care is a prerequisite for integrated care. In an editorial, J. Scerri et al. explain that person-centred care can impact the regulatory and decision-making context for the safe use of medicines at the clinical level [8]. Person- or people-centred care entails goal-oriented care, with a focus on the person instead of on the patient or the disease: it can be delivered in the absence of disease. It promotes equality in the relationship between health care providers and patients. This framework explores the needs and expectations of the person, considering the context of the patient, family and community. People-centred care aims to provide the education and support for individuals to make decisions and participate in their own care [7][9]. So, by definition, people-centred pharmaceutical care requires regular communication between patients and health care providers, patient education, monitoring and tailoring of care and interventions. Medication use needs to be adjusted in accordance with patients’ care goals and contextual factors such as patient competences, therapy expectations, financial means, informal care and beliefs about medication.
Integrated care requires intense interprofessional collaboration. To implement high-quality interprofessional relationships in pharmaceutical care, health care providers need to acknowledge shared, person-centred goals and respect each other’s competences and contributions.
2. Advices to Strengthen Integrated, Evidence-Based Pharmaceutical Care
Many factors impact the quality of integrated, evidence-based pharmaceutical care, as schematically presented in Figure 1. Measures to strengthen integrated, evidence-based pharmaceutical care can address these factors. To support implementation, nurse leaders could and probably should or even must: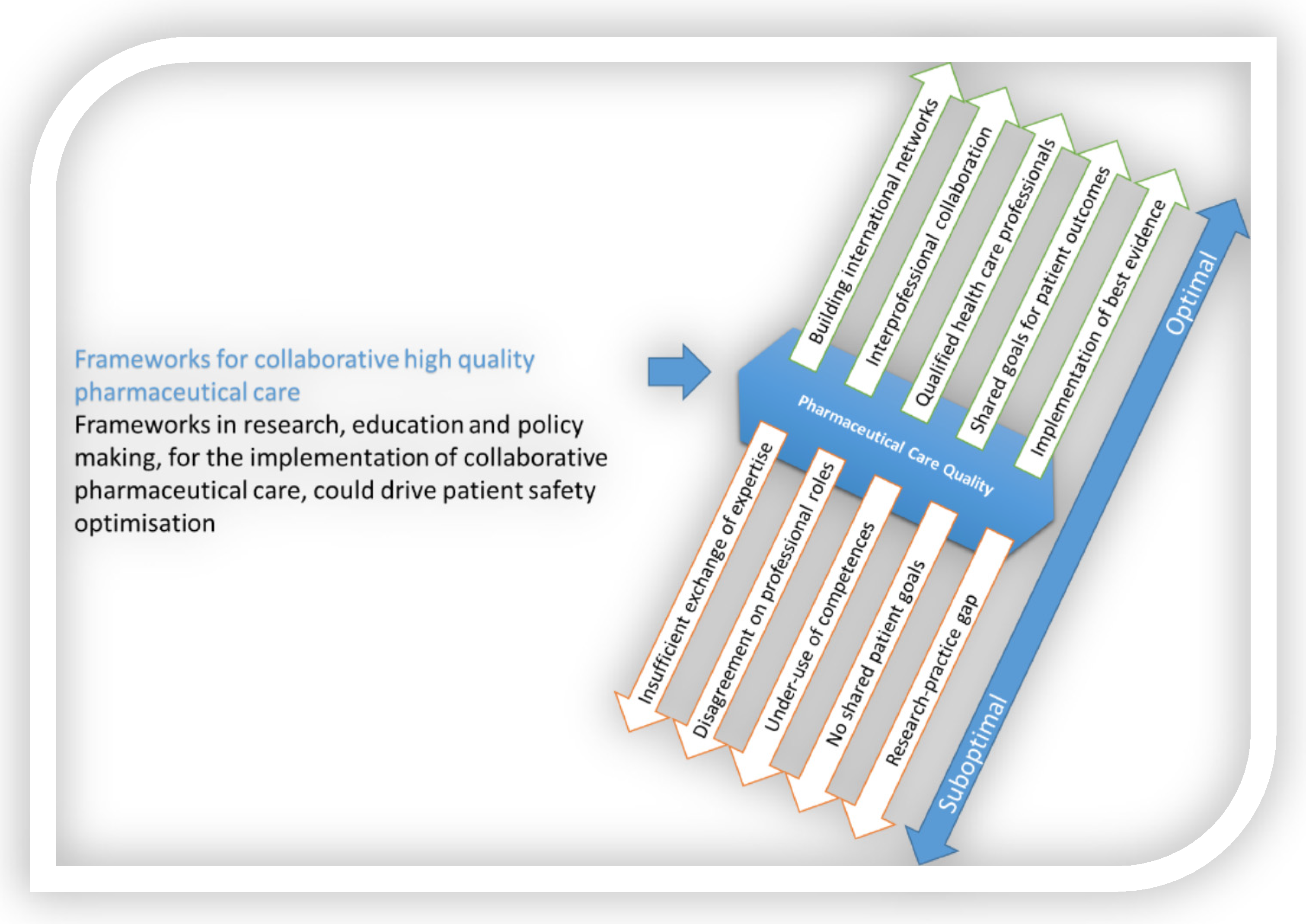 Figure 1. The need for frameworks to allow the implementation of interprofessional, integrated, evidence-based pharmaceutical care.
Figure 1. The need for frameworks to allow the implementation of interprofessional, integrated, evidence-based pharmaceutical care.-
Offer a framework for nurses’ contributions to integrated pharmaceutical care.Ensuring best use of medicinesNurses need to communicate clearly on how they can and do contribute to integrated pharmaceutical care.
-
Nurses, as the professionals working most closely with patients, should be fully integrated into the interprofessional team providing pharmaceutical care, including managing and monitoring patients’ medicines and the effects of these medicines on patientsExpand research on nursing interventions in pharmaceutical care.
- ]. There is little evidence that nurse substitutions [23] or nurse practitioners’ care [11][24] or costs [25] differ from those of doctors, particularly when prescribing practices are compared. The evidence suggests that non-medical prescribing is safe and can provide beneficial outcomes [26], even though nurses tend to prescribe less than doctors [27] and have reservations regarding working unsupported [28][11][27].Furthermore, nurses, together with pharmacists and physicians, need to explicitly take into account the impact of social diversity when researching and developing interventions or guidelines or policies or providing education in pharmaceutical care. Interprofessional collaboration with shared goals on the elimination of inequality in pharmaceutical care can help us move forward.Expand research on interprofessional collaboration in pharmaceutical care.As interprofessional collaboration in pharmaceutical is so fundamental to the quality of integrated, people-centred care and patient outcomes, research should continue to focus on facilitators of high-quality interprofessional pharmaceutical care [13] and the barriers to bringing this into practice. This research should also consider how gender, gender-based assumptions, stereotypes and preconceptions still influence participants [14].
-
Promoting patient safetyPatient safety related to pharmaceutical care is suboptimal [29]. The adoption of standardised international pharmacotherapy curricula and assessments for pre- and post-registration nurse education would provide the foundation for nurses to meet practice requirements and realise their full potential, whilst maintaining comparable standards of care, not only at national levels, but also at European and international levels [30][31]. Avoiding unsafe medication practices, minimising avoidable harm caused by medicines and meeting the WHO third patient safety challenge [29] requires a focus on patient outcomes.Ensure that available evidence is implemented in practice.Substantial parts of evidence resulting from research are not translated into practice and therefore fail to generate expected outcomes. Therefore, extra efforts from all professionals involved (clinicians, managers, regulators and policy makers) are needed to ensure research results are implemented in clinical practice [15].
-
Create and maintain an international network of nurses with expertise in pharmaceutical care.Such a network serves not only to enhance collaboration, exchange initiatives and disseminate information but also to provide a point of contact for other professional groups to identify nurse representatives to be engaged in research and policy making on pharmaceutical care. Engaging in debates at this level will develop interprofessional frameworks for the implementation of pharmaceutical care and strengthen nurses’ contribution to research, policy, education and clinical practice.
-
Collaborate with representatives of other disciplines to develop a framework for interprofessional pharmaceutical care.
-
Eliminating inequityThe framework should be developed and co-designed as a collaboration between all disciplines involved in pharmaceutical care. The goals are optimum care quality and patient outcomes, allowing for contextual factors, such as expertise, treatment availability and costs. Knowledge gained from implementation science models, such as the CICI framework, can be harnessed to improve the usability of such frameworks [16] and uncover and address any challenges in implementing the framework.
3. Conclusions
Initiatives have been taken to work on the implementation of integrated evidence-based pharmaceutical care. In 2015, the NuPhaC network was founded as a European collaboration to unite researchers, clinicians, educators and policy makers in promoting the quality of pharmaceutical care and patient outcomes by realising the potential of all professionals.
References
- Allemann, S.S.; van Mil, J.W.F.; Botermann, L.; Berger, K.; Griese, N.; Hersberger, K.E. Pharmaceutical care: The PCNE definition 2013. Int. J. Clin. Pharm. 2014, 36, 544–555.
- Committee of Ministers, Council of Europe. CM/Res(2020)3 Resolution on the Implementation of Pharmaceutical Care for the Benefit of Patients and Health Services. 2020. Available online: (accessed on 8 December 2020).
- Hepler, C.D.; Strand, L.M. Opportunities and Responsabilities in pharmaceutical care. Am. J. Hosp. Pharm. 1990, 47, 533–543.
- Beuscart, J.B.; Knol, W.; Cullinan, S.; Schneider, C.; Dalleur, O.; Boland, B.; Thevelin, S.; Jansen, P.A.F.; O’Mahony, D.; Rodondi, N.; et al. International core outcome set for clinical trials of medication review in multi-morbid older patients with polypharmacy. BMC Med. 2018, 16, 21.
- Rankin, A.; Cadogan, C.A.; In Ryan, C.; Clyne, B.; Smith, S.M.; Hughes, C.M. Core Outcome Set for Trials Aimed at Improving the Appropriateness of Polypharmacy in Older People in Primary Care. J. Am. Geriatr. Soc. 2018, 66, 1206–1212.
- NHS. Medicines Optimisation. Available online: (accessed on 27 May 2021).
- WHO. Framework on Integrated, People-Centered Health Services. 2016. Available online: (accessed on 27 May 2021).
- Scerri, J.; Churchill, J.; Banks, D.; Sultana, J. Advocating a person-centered care approach to drug safety. Exp. Opin. Drug Safety 2021, 20, 255–258.
- Maeseneer, J.D.; Weel, C.V.; Daeren, L.; Leyns, C.; Decat, P.; Boeckxstaens, P.; Avonts, D.; Willems, S. From “patient” to “person” to “people”: The need for integrated, people-centered healthcare. Int. J. Pers. Cent. Med. 2012, 2, 14.
- Smigorowsky, M.J.; Sebastianski, M.; Sean McMurtry, M.; Tsuyuki, R.T.; Norris, C.M. Outcomes of nurse practitioner-led care in patients with cardiovascular disease: A systematic review and meta-analysis. J. Adv. Nurs. 2020, 76, 81–95.
- Lovink, M.H.; Laurant, M.G.; van Vught, A.J.; Maassen, I.; Schoonhoven, L.; Persoon, A.; Koopmans, R.T. Substituting physicians with nurse practitioners, physician assistants or nurses in nursing homes: A realist evaluation case study. BMJ Open 2019, 9, e028169.
- McCleery, E.; Christensen, V.; Peterson, K.; Humphrey, L.; Helfand, M. Evidence Brief: The Quality of Care Provided by Advanced Practice Nurses; Department of Veterans Affairs (US): Washington, DC, USA, 2014.
- Kovačević, M.; Vezmar Kovačević, S.; Radovanović, S.; Steavanović, P.; Miljković, B. Abstracts 12th PCNE working conference ‘Partnering for better patient outcomes: Challenges and opportunities 3-6 February 2021, University of Basel, Switzerland (was held online). Int. J. Clin. Pharm. 2021.
- Leake, P. Nursing, Power, and Gender in Interprofessional Collaboration in Leslie Dan Faculty of Pharmacy. Ph.D. Thesis, University of Toronto, Ontario, TOR, Canada, 2018.
- Wensing, M.; Grol, R.; Grimshaw, J. (Eds.) Improving Patient Care. The Implementation of Change in Health Care, 3rd ed.; Willey Blackwell: Hoboken, NJ, USA, 2020.
- Pfadenhauer, L.M.; Gerhardus, A.; Mozygemba, K.; Lysdahl, K.B.; Booth, A.; Hofmann, B.; Wahlster, P.; Polus, S.; Burns, J.; Brereton, L.; et al. Making sense of complexity in context and implementation: The Context and Implementation of Complex Interventions (CICI) framework. Implement. Sci. 2017, 12, 21.
- Dijkstra, N.E. Potential Clinical Consequences of Medication Process Problems in Older Home Care Patients. J. Geriatr. Med. Gerontol. 2020, 6, 84.
- Dilles, T.; Vander Stichele, R.H.; van Bortel, L.M.; Elseviers, M.M. The development and test of an intervention to improve ADR screening in nursing homes. J. Am. Med. Dir. Assoc. 2013, 14, 379.e1–379.e6.
- Mardani, A.; Griffiths, P.; Vaismoradi, M. The Role of the Nurse in the Management of Medicines during Transitional Care: A Systematic Review. J. Multidiscip. Healthc. 2020, 13, 1347–1361.
- Jordan, S.; Gabe-Walters, M.E.; Watkins, A.; Humphreys, I.; Newson, L.; Snelgrove, S.; Dennis, M.S. Nurse-Led Medicines’ Monitoring for Patients with Dementia in Care Homes: A Pragmatic Cohort Stepped Wedge Cluster Randomised Trial. PLoS ONE 2015, 10, e0140203.
- Logan, V.; Keeley, S.; Akerman, K.; de Baetselier, E.; Dilles, T.; Griffin, N.; Matthews, L.; van Rompaey, B.; Jordan, S. Did we do everything we could have? Nurses’ contributions to medicines optimization: A mixed-methods study. Nurs. Open 2020, 8, 592–606.
- Wilson, M. A 5-year retrospective audit of prescribing by a critical care outreach team. Nurs. Crit. Care 2018, 23, 121–126.
- Laurant, M.; van der Biezen, M.; Wijers, N.; Watananirun, K.; Kontopantelis, E.; van Vught, A.J. Nurses as substitutes for doctors in primary care. Cochrane Database Syst. Rev. 2018, 7, CD001271.
- Kuethe, M.C.; Vaessen-Verberne, A.A.; Elbers, R.G.; Van Aalderen, W.M. Nurse versus physician-led care for the management of asthma. Cochrane Database Syst. Rev. 2013, 2, CD009296.
- Hollinghurst, S.; Horrocks, S.; Anderson, E.; Salisbury, C. Comparing the cost of nurse practitioners and GPs in primary care: Modelling economic data from randomised trials. Br. J. Gen. Pract. 2006, 56, 530–535.
- Noblet, T.; Marriott, J.; Graham-Clarke, E.; Shirley, D.; Rushton, A. Clinical and cost-effectiveness of non-medical prescribing: A systematic review of randomised controlled trials. PLoS ONE 2018, 13, e0193286.
- Karimi-Shahanjarini, A.; Shakibazadeh, E.; Rashidian, A.; Hajimiri, K.; Glenton, C.; Noyes, J.; Lewin, S.; Laurant, M.; Colvin, C.J. Barriers and facilitators to the implementation of doctor-nurse substitution strategies in primary care: A qualitative evidence synthesis. Cochrane Database Syst. Rev. 2019, 4, CD010412.
- De Baetselier, E.; Van Rompaey, B.; Batalha, L.M.; Bergqvist, M.; Czarkowska-Paczek, B.; de Santis, A.; Dijkstra, N.E.; Fernandes, M.I.; Filov, I.; Grondahl, V.A.; et al. EUPRON: Nurses’ practice in interprofessional pharmaceutical care in Europe. A cross-sectional survey in 17 countries. BMJ Open 2020, 10, e036269.
- WHO. WHO Global Patient Safety Challenge: Medication Without Harm. 2017. Available online: (accessed on 27 May 2021).
- Jordan, S.; Davies, S.; Green, B. The biosciences in the pre-registration nursing curriculum: Staff and students’ perceptions of difficulties and relevance. Nurse Educ. Today 1999, 19, 215–226.
- Newman, M.A. A position paper: The continuing dilemma in nursing education. Nurs. Outlook 2011, 59, e3–e5.
More
