The long-chain polyunsaturated fatty acids (LCPUFAs), docosahexaenoic acid,22:6n-3 (DHA), and arachidonic acid,20:4n-6 (ARA) are important nutrients required for fetal brain growth and development. The accumulation of DHA and ARA in the fetal brain predominantly occurs in the third trimester of a human pregnancy. The de novo synthesis of these LCPUFAs seems low in a growing fetus and placenta; the maternal intake of these fatty acids contributes a significant share for brain development.
- arachidonic acid
- 20:4n-6
- brain
- docosahexaenoic acid
- 22:6n-3
- fetus
- maternal diet
- cognitive
- infants
- neurodevelopment
- neurogenesis
1. Introduction
The neurodevelopmental process involves a complex interplay between nutrients, genes, and environmental factors that result in the optimal growth, development, and maturation of the brain. The development of the brain in utero critically depends on the maternal supply of several components for its well-regulated structural–developmental process, characterized by specifically defined developmental periods, growth, a cellular signaling system, and maturation. During the brain growth spurt, neurodevelopment is particularly vulnerable to nutritional deficiencies [1].
The long-chain polyunsaturated fatty acids (LCPUFAs), docosahexaenoic acid,22:6n-3 (DHA), and arachidonic acid,20:4n-6 (ARA) are important nutrients required for fetal brain growth and development. The de novo synthesis of these LCPUFAs seems low in a growing fetus and placenta [2]; the maternal intake of these fatty acids contributes a significant share for brain development. Several experimental studies suggested a crucial involvement of these two fatty acids in neural membrane formation and various roles of their metabolites, production of eicosanoids, and their influence on depression- and anxiety-related behaviors. Moreover, multiple trials have found that higher plasma or erythrocyte DHA levels positively correlate with infant neurocognitive outcomes [5,6,7,8,9,10][3][4][5][6][7][8].
High levels of DHA in the brain are achieved during early life and are maintained throughout life. DHA accretion to the brain continues into childhood, and the incorporation of DHA is still high despite its reduced accumulation rate. LCPUFA content in human milk also regulate the amount of DHA and ARA transferred to the infant during breastfeeding [13][9]. However, this phenomenon may depend on an optimal maternal dietary intake of DHA from the supplement or marine fish [14][10], whereas ARA levels are usually maintained due to high n-6/n-3 ratios in the diet.
In the central nervous system (CNS), the proportion of DHA with other membrane fatty acids increases as the brain size increases. DHA has significant neurobiochemical roles in ion channel and receptor functions, the release of neurotransmitters, synaptic plasticity, and gene expression in the neurons. Both DHA and ARA of synaptic membrane phospholipids are released as free fatty acids (FFAs) by activated phospholipase A2(PLA2) and are converted to different bioactive metabolites. These liberated fatty acids and the metabolites play critical roles during ischemia seizure activity, inflammation, and other types of brain disorders.
Maternal n-3 PUFA deficiency during pregnancy was associated with impaired brain development in offspring [15][11] and defective neuroblast migration [16][12]. The deficiency of n-3 LCPUFAs during development causes hypomyelination in the brain, resulting in mood and anxiety disorders [17,18][13][14]. Consuming a diet containing a high amount of n-3 fatty acids during pregnancy protected infants against the detrimental effects of maternal stress [19][15].
The essentiality of DHA is well recognized in childhood and adult life, as its deficiency causes cognitive decline and other psychiatric disorders [20][16]. Plasma DHA levels are inversely correlated with depressive symptoms in infants and adolescents with bipolar disorder [21][17]. Human breast milk containing higher n-3 and n-6 LCPUFAs was associated with decreased infant despair and distress [22][18]. The impact of n-3 PUFAs in human milk in influencing infant mood or anxiety is still not clearly established, since cortisol levels in milk are also associated with infant temperament [23][19].
Although n-3 LCPUFAs supplementation is beneficial in preventing and treating major depression, bipolar disorder, and anxiety disorders in adults [24[20][21],25], much less is known about how the imbalance of these LCPUFA levels impact the mood and behavior of infants. Studies in experimental models suggested that early exposure to n-3 fatty acids had a lasting effect on temperament and behavioral phenotypes of offspring [26][22]. Interventional studies in adults also showed an association between the n-3 PUFA status with improved mood and mental health [17,26][13][22].
High DHA levels in the phospholipids of synaptic membranes provide membrane flexibility and improve the efficiency of protein–protein interaction necessary for signal transduction [27][23]. Furthermore, several human brain diseases, such as Alzheimer’s and bipolar disorders, involve disturbed n-3 and n-6 LCPUFA uptake and metabolism. An additional neuroprotective mechanism may involve bioactive molecules derived from DHA and ARA, which are also involved in several cellular neuronal biochemical processes. These bioactive derivatives modify the functions of several genes in the brain by acting as ligands for transcription factors involved in critical brain functions, including signal transduction and synaptic plasticity.
A literature search was performed on the PubMed database by using search terms such as DHA, ARA, brain development, infant, fetal, breastfeeding nutrients deficiency, DHA and ARA supplementation. The articles retrieved in the first round of searches identified additional references by a manual search among the cited references. This review describes the latest development of the interplay of DHA and ARA transfer and their impacts on brain development, their complementarity, the structure–function relationship, and their mechanisms of action in the brain. Moreover, the evidence of the essentiality of ARA in brain development is summarized.
2. Roles of DHA and Its Metabolites in the Brain
DHA and its metabolites play vital roles in the functional brain development of the fetus in utero and infants and healthy brain function in adults. DHA and its metabolites play significant roles in cellular and biological functions. The oxidation of DHA by lipoxygenases produces several types of metabolites such as oxylipins that regulate various biochemical processes of the brain [126][24].
120 mediated gene activation to promote anti-inflammatory activities [127,128][25][26]. DHA also activates PPARs and upregulates the expression of genes responsible for increasing insulin sensitivity and reducing plasma triglyceride level and inflammation. DHA and its metabolites’ signaling pathways are involved in neurogenesis, anti-nociceptive effects, anti-apoptotic effects, the plasticity of the synapse, Ca2+homeostasis in the brain, and nigrostriatal activities [129][27]. DHA itself and its metabolites have a broad spectrum of actions at different levels and sites in the brain
DHA is metabolized by the P450 system, cyclooxygenase, and lipoxygenase enzymes under different metabolic conditions. 18-HEPE: 18-hydroxy-eicosapentaenoic acid; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; GPR32/37: G protein-coupled receptor 32/37; LC-PUFAs: long-chain polyunsaturated fatty acids; LOX: lipoxygenases.
DHA-derived specialized pro-resolving mediators (SPMs) such as DHA epoxides, oxo-derivatives (EFOX) of DHA, neuroprostanes, ethanolamines, acylglycerols, docosahexaenoyl amides of amino acids, and branched DHA esters of hydroxy fatty acids play important roles in brain functions [131,132][28][29]. As the most demanding by-products of DHA, resolvins are formed by either LOX15 or CYP or aspirin-treated COX-2 activity [28][30]. Both resolvin D1 and aspirin-triggered resolvin D1 improve brain functions and impede neuronal death by down-regulating several factors such as NFkB, TLR4, CD200, and IL6R [136,137][31][32]. However, the functions of other resolvins are still a mystery.
Another SPM, neuroprotectin D1(NPD1), derived from DHA, improves cell survival and cell repair in brain disorders [151][33]. In response to neuroinflammation, NPD1 is produced from endogenous DHA in the retina and brain [153,154][34][35]. Besides antiviral protection, NPD1 helps in neurocognitive functions [155,156,157][36][37][38]. NDP1 blocks the progression of Alzheimer’s disease by stimulating the expression of PPARγ, amyloid precursor protein-α, and reducing the β-amyloid precursor protein [156][37].Figure 3describes DHA metabolites and their global effects on gene expression and second messenger systems affecting multiple cellular functions in the brain.
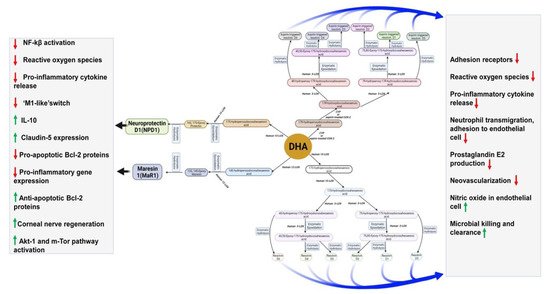
Figure 3. DHA metabolites formation and function in the brain.
Maresin 1(MaR1), neuroprotectin D1(NPD1), and resolvins are produced from DHA by human 12-LOX, 15-LOX, and CYP or aspirin-treated COX-2 enzymatically. These metabolites have multiple functions in the brain, which have been mentioned in the boxes. DHA is metabolized by the P450 system, cyclooxygenase, and lipoxygenase enzymes under different metabolic conditions.
Anti-inflammatory activities of EFOX and neuroprostanes protect neuroinflammation in various diseases, such as Parkinson’s disease and Alzheimer ’s disease [158,159,160][39][40][41]. Another DHA derivative, docosahexaenoyl ethanolamide, improves mood, pain, inflammation status, hunger, and glucose uptake by the brain endocannabinoid system [161,162,163,164,165,166][42][43][44][45][46][47]. The function of DHA metabolites is summarized inTable 1.
| Metabolites | Name | Biological Effects |
|---|---|---|
| DHA Metabolites | Maresins | Resolution of inflammation, wound healing, analgesic effects |
| Protectins | Resolution of inflammation, neuroprotection | |
| Resolvins | Resolution of inflammation and wound healing | |
| Electrophilic oxo-derivatives (EFOX) of DHA | Anti-inflammatory, anti-proliferative effects | |
| Epoxides | Anti-hypertensive, analgesic actions | |
| Neuroprostanes | Cardio-protection, wound healing | |
| DHA conjugates | Ethanolamines and glycerol esters | Neural development, immunomodulation, metabolic effects |
| Branched fatty acid esters of hydroxy fatty acids (FAHFA) | Immuno-modulation, resolution of inflammation | |
| N-acyl amides | Metabolic regulation, neuroprotection, neurotransmission | |
| ARA metabolites | Lipoxins A4 | Lowers neuroinflammation by inhibiting microglial activation |
| Lipoxins B4 | Promotes neuroprotection from acute and chronic injuries |
DHA glyceryl ester regulates the intake of food and neuroinflammation, similarly to the way docosahexaenoyl ethanolamide uses the endocannabinoid system [167,168][48][49]. The endocannabinoid system plays an integral part in memory, cognition, and pain perception [169,170][50][51]. DHA conjugates via cannabinoid receptors reduce neuroinflammation and improve neurogenesis [171,172][52][53].
DHA is involved in alleviating short-term stress, preventing anxiety and stress in later life [132,173][29][54]. DHA is reported to improve various psychiatric disorders such as schizophrenia, mood and anxiety disorders, obsessive-compulsive disorder, ADHD, autism, aggression, hostility and impulsivity, borderline personality disorder, substance abuse, and anorexia nervosa [174][55]. There is strong evidence that the consumption of marine fish reduces depression [175][56].
DHA was also shown to improve depressive symptoms of bipolar disorder by increasing N-acetyl-aspartate brain levels without affecting mania [174][55]. Even IQ outcomes in children and cognitive function in the aging brain are improved by DHA supplementation [2,24][2][20]. DHA may modulate the metabolism of cholesterol and apolipoprotein E, lipid raft assembly, and the cell signaling system in Alzheimer’s disease [178,179][57][58]. DHA protects neuronal brain function by reducing NO production, calcium influx, and apoptosis while activating antioxidant enzymes such as glutathione peroxidase and glutathione reductase [180][59].
3. DHA Deficiency in Utero and Human Brain Function
DHA deficiency is linked with different brain disorders such as major depressive and bipolar disorder [181,182][60][61]. DHA levels are positively correlated with improved learning and memory and reduced neuronal loss [24][20]. DHA deficiency affects epigenetic development in the feto-placental unit [183,184][62][63]. The improvement in attention scores, adaptability to new surroundings, mental development, memory performance, and hand–eye coordination are associated with higher maternal DHA delivery to the fetal brain [7,185][5][64].
DHA deficiency during pregnancy suggests the lower development of language learning skills in children [186][65]. DHA deficiency in the third trimester significantly causes preterm brain development due to the insufficient maternal consumption of n-3 fatty acids. Even following delivery, infants are entirely dependent on breast milk or formula milk for DHA and ARA. Reduced DHA consumption during this critical brain development period may influence brain functionalities in adult life [191][66].
Dementia has shown an inverse relationship with regular marine fish consumption in different continents [120,192][67][68] and higher blood DHA levels inversely related to dementia [193,194][69][70]. Various brain diseases/disorders, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, schizophrenia, and mood disorders, are related to disturbed fatty acid signaling [195,196][71][72]. The most prevalent dementia is Alzheimer’s disease, which is inversely related to brain DHA level. However, different randomized controlled trials (RCTs) found mixed results with DHA supplementation.
Multiple factors influence serotonin biosynthesis and function. The brain’s serotonin level correlates with various behavioral consequences, e.g., control of executive function, sensory gating, social behavior, and impulsivity [204][73]. DHA modulates the activity of serotonin in the brain. DHA increases serotonin receptor accessibility by increasing membrane fluidity in postsynaptic neurons [204][73].
Observational studies showed that ADHD also has a relationship with DHA levels. RCTs of DHA with EPA supplementation and the addition of medications have demonstrated significant improvement in ADHD symptoms [122,187,206,207,208,209,210,211,212,213,214,215,216][74][75][76][77][78][79][80][81][82][83][84][85][86]. However, DHA supplementation with methylphenidate did not improve ADHD symptoms [217][87].
There are mixed results in RCTs that have been observed in early psychosis symptoms improvement with DHA supplementation. When DHA is supplemented with EPA for at least 12 weeks, the functional improvement and reduction in psychiatric symptoms are visible in different studies [218,219,220][88][89][90]. DHA deficiency elicits the chances of schizophrenia by promoter hypermethylation of nuclear receptor genes RxR and PPAR, which results in the downregulation of the gamma-aminobutyric acid-ergic system and the prefrontal cortex involved in oligodendrocyte integrity [221][91]. However, recent RCTs found DHA has no role in improving the symptoms of bipolar disorder [20][16].
4. Roles of Arachidonic acid20:4n-6 (ARA) in Brain Development and Function
Using various knock-out models for enzymes involved in brain ARA metabolism, Bosetti showed that the ARA and its metabolites play a significant role in brain physiology via the PLA2/COX pathway [240][92]. Studies clearly show the requirement for both ARA and DHA in addition to the essential fatty acids (linoleic acid,18:2n-6 (LA), and ALA to support the optimal body, brain growth, and brain function. Although diverse roles of DHA are investigated, the roles of ARA in brain development and functions have not been investigated to a greater extent. Given that ARA and its precursor, LA, contribute significantly to the Western diet and its pleiotropic biological effects and its interactions with DHA, this n-6 LCPUFA is a crucial modifiable factor in brain development and preventive strategies of brain diseases.
Several studies have suggested that the structure–function and metabolism of the brain depend on levels of ARA and DHA and interactions of their metabolites [29][93]. The recycling (de-esterification–re-esterification) of these two fatty acids in the brain are independently carried out by ARA- and DHA-selective enzymes. Therefore, in studies using n-3 PUFA deficiency models, the homeostatic mechanisms show DHA loss in the brain while increasing ARA metabolism. sPLA2, and COX-2 and their effects on brain function and neuroinflammation.
Thus, the growing brain depends on a steady supply of ARA [243][94] from the maternal circulation or via breast milk. Upon its release, a portion of the unesterified ARA is converted to prostaglandins, leukotrienes, and lipoxins, a portion oxidized via β-oxidation, and the remainder (approximately 97% under basal conditions) is activated by ACSL and ultimately recycled and re-esterified into the sn-2 position of phospholipids [246][95]. N-methyl-d-aspartate receptors in conditions such as neuroinflammation and excitotoxicity. Although the signals that ARA and its derivatives relay are not entirely understood, they regulate blood flow, neuroinflammation, excitotoxicity, the sleep/wake cycle, and neurogenesis [247][96].
Like DHA, ARA is also directly involved in synaptic functions. The level of intracellular free ARA and the balance between the releasing and incorporating enzymes in membrane phospholipids may play critical roles in neuroinflammation and synaptic dysfunction. Both these events are observed in the murine model of Alzheimer’s disease before the amyloid plaques and the neurofibrillary tangles, respectively formed by the two agents known for Alzheimer’s disease agents, A β peptide and hyperphosphorylatedtau. Finally, western food, which contains excessive n-6/n-3 ratios, might favor more ARA levels and influence Alzheimer’s disease mechanisms.
A better understanding of the complex relationships between ARA and DHA and their brain mechanisms is required. ARA is directly involved in synaptic functions as a retrograde messenger and a regulator of neuro mediator exocytosis. Moreover, ARA has pleiotropic effects on brain disease, and it may be used in the fight against brain diseases. The dietary ARA and brain diseases about DHA should be investigated further to prevent the disease.
Aβ production in Alzheimer’s disease. Studies on the impact of dietary ARA on Alzheimer’s disease are required to identify the underlying mechanisms of action. ’s However, a DHA-rich diet did not show such effects [249][97].
ARA is involved in cell division and signaling during brain growth and development [251][98]. In addition, ARA mediates neuronal firing [252][99], signaling [253][100] and long-term potentiation [254][101]. The absolute levels of n-3 PUFAs and the ratio of n-6 and n-3 PUFA affect gene expression of controlling neurogenesis and neural function.
Released intracellular ARA activates protein kinases and ion channels, inhibits the uptake of neurotransmitters, and enhances synaptic transmission, and modulates neuronal excitability [251][98]. Neurite growth closely correlates with the ARA-mediated activation of STX-3 in membrane expansion at growth cones [257][102]. The SNARE proteins are involved in producing a fusion of vesicular and plasma membranes in the brain. The formation of this SNARE complex mediated by ARA drives membrane fusion, leading to the release of vesicular cargo into the extracellular spaces [258][103].
The most abundant prostaglandins in the brain are PGD2and PGE2. They are synthesized from ARA by PGD2and PGE2synthases. COX-2 is overexpressed in the cortex and hippocampus of Alzheimer’s disease patients [259][104]. PGE2increases neuroinflammation and amplifies Alzheimer’s disease pathology through various mechanisms.
Figure 4describes different metabolites of ARA involved in brain function. ARA-derived lipoxins are anti-inflammatory eicosanoids distinct from pro-inflammatory leukotriene and prostaglandin. Lipoxin biosynthesis occurs via two different pathways. LipoxinA4 lowers neuroinflammation by reducing microglial activation.
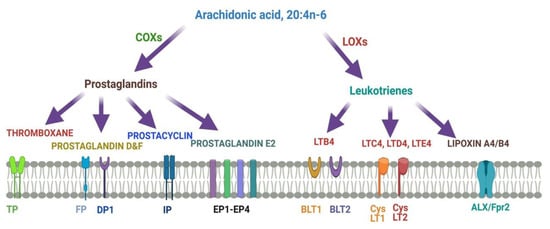
Figure 4. Metabolites of ARA and their receptors.
Lipoxins mediate their action on endothelial cells to offer an inflammation resolution process. LipoxinA4 lowers neuroinflammation by reducing microglial activation. LTA4, LTB4, LTC4, LTD4, and LTE4 are leukotrienes A4, B4, C4, D4, and E4, respectively; DP1, EP1-EP4, FP, and IP, prostaglandin receptors; TP, thromboxane A2 receptor; BLT1 and BLT2, leukotriene B4 receptor; CysLT1 and CysLT2: ALXR: lipoxins receptor.
12(S)-HETE and 15(S)-HETE were reported in frontal and temporal brain regions’ cerebrospinal fluid, respectively, in Alzheimer’s disease patients.
The endocannabinoids containing ARA are involved in Alzheimer’s disease via the CB2 receptor. Although neuroinflammation is related to Alzheimer’s disease, there is no evidence that higher brain contents of ARA would produce inflammation.
Several animal studies indicate that ARA has beneficial effects on cognition and synaptic plasticity [260][105]. ARA induces presynaptic long-term depression associated with a Ca2+influx and the activation of metabotropic glutamate receptors [261][106]. In addition, ARA can induce neurotransmitter exocytosis in the presynaptic neuron via activation of the soluble N-ethylmaleimide-attachment receptors (SNARE). Age and Aβ concentration or other physiological factors could modify ARA’s effects on synapse and neuronal cells.
References
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front. Hum. Neurosci. 2013, 7, 97.
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harslof, L.B.; Ciappolino, V.; Agostoni, C. DHA Effects in brain development and function. Nutrients 2016, 8, 6.
- Agostoni, C.; Trojan, S.; Bellu, R.; Riva, E.; Giovannini, M. Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice: The role of long-chain polyunsaturated fatty acids. Pediatr. Res. 1995, 38, 262–266.
- Agostoni, C.; Trojan, S.; Bellu, R.; Riva, E.; Bruzzese, M.G.; Giovannini, M. Developmental quotient at 24 months and fatty acid composition of diet in early infancy: A follow up study. Arch. Dis. Child 1997, 76, 421–424.
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44.
- Drover, J.R.; Hoffman, D.R.; Castañeda, Y.S.; Morale, S.E.; Garfield, S.; Wheaton, D.H.; Birch, E.E. Cognitive function in 18-month-old term infants of the DIAMOND study: A randomized, controlled clinical trial with multiple dietary levels of docosahexaenoic acid. Early Hum. Dev. 2011, 87, 223–230.
- Innis, S.M. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J. Pediatr. 2003, 143, S1–S8.
- Jensen, C.L.; Voigt, R.G.; Prager, T.C.; Zou, Y.L.; Fraley, J.K.; Rozelle, J.C.; Turcich, M.R.; Llorente, A.M.; Anderson, R.E.; Heird, W.C. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am. J. Clin. Nutr. 2005, 82, 125–132.
- Lauritzen, L.; Carlson, S.E. Maternal fatty acid status during pregnancy and lactation and relation to newborn and infant status. Matern. Child Nutr. 2011, 7, 41–58.
- Browning, L.M.; Walker, C.G.; Mander, A.P.; West, A.L.; Madden, J.; Gambell, J.M.; Young, S.; Wang, L.; Jebb, S.A.; Calder, P.C. Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 2012, 96, 748–758.
- Innis, S.M.; Friesen, R.W. Essential n-3 fatty acids in pregnant women and early visual acuity maturation in term infants. Am. J. Clin. Nutr. 2008, 87, 548–557.
- Yavin, E.; Himovichi, E.; Eilam, R. Delayed cell migration in the developing rat brain following maternal omega 3 alpha linolenic acid dietary deficiency. Neuroscience 2009, 162, 1011–1022.
- Su, K.P. Biological mechanism of antidepressant effect of omega-3 fatty acids: How does fish oil act as a ‘mind-body interface’? Neurosignals 2009, 17, 144–152.
- Su, K.P.; Matsuoka, Y.; Pae, C.U. Omega-3 polyunsaturated fatty acids in prevention of mood and anxiety disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 129–137.
- Brunst, K.J.; Enlow, M.B.; Kannan, S.; Carroll, K.N.; Coull, B.A.; Wright, R.J. Effects of prenatal social stress and maternal dietary fatty acid ratio on infant temperament: Does race matter? Epidemiology 2014, 4.
- Ciappolino, V.; DelVecchio, G.; Prunas, C.; Andreella, A.; Finos, L.; Caletti, E.; Siri, F.; Mazzocchi, A.; Botturi, A.; Turolo, S.; et al. The Effect of DHA supplementation on cognition in patients with bipolar disorder: An exploratory randomized control trial. Nutrients 2020, 12, 708.
- Clayton, E.H.; Hanstock, T.L.; Hirneth, S.J.; Kable, C.J.; Garg, M.L.; Hazell, P.L. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids 2008, 43, 1031–1038.
- Hahn-Holbrook, J.; Fish, A.; Glynn, L.M. Human milk omega-3 fatty acid composition is associated with infant temperament. Nutrients 2019, 11, 2964.
- Grey, K.R.; Davis, E.P.; Sandman, C.A.; Glynn, L.M. Human milk cortisol is associated with infant temperament. Psychoneuroendocrinology 2013, 38, 1178–1185.
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients 2016, 8, 99.
- Lin, P.Y.; Su, K.P. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J. Clin. Psychiatry 2007, 68, 1056–1061.
- Bhatia, H.S.; Agrawal, R.; Sharma, S.; Huo, Y.X.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PLoS ONE 2011, 6, e28451.
- Mallick, R.; Basak, S.; Duttaroy, A.K. Docosahexaenoic acid,22:6n-3: Its roles in the structure and function of the brain. Int. J. Develop. Neurosci. Off. J. Int. Soc. Develop. Neurosci. 2019, 79, 21–31.
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421.
- Oh, D.Y.; Walenta, E.; Akiyama, T.E.; Lagakos, W.S.; Lackey, D.; Pessentheiner, A.R.; Sasik, R.; Hah, N.; Chi, T.J.; Cox, J.M.; et al. A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat. Med. 2014, 20, 942–947.
- Diep, Q.N.; Touyz, R.M.; Schiffrin, E.L. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-alpha ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension 2000, 36, 851–855.
- Chitre, N.M.; Wood, B.J.; Ray, A.; Moniri, N.H.; Murnane, K.S. Docosahexaenoic acid protects motor function and increases dopamine synthesis in a rat model of Parkinson’s disease via mechanisms associated with increased protein kinase activity in the striatum. Neuropharmacology 2020, 167, 107976.
- Anderson, E.; Taylor, D. Stressing the heart of the matter: Re-thinking the mechanisms underlying therapeutic effects of n-3 polyunsaturated fatty acids. F1000 Med. Rep. 2012, 4, 13.
- Robertson, R.; Guihéneuf, F.; Schmid, M.; Stengel, D.; Fitzgerald, G.; Ross, P.; Stanton, C. Algae-Derived Polyunsaturated Fatty Acids: Implications for Human Health; Nova Sciences Publishers, Inc.: New York, NY, USA, 2013; p. 43.
- Duvall, M.G.; Levy, B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016, 785, 144–155.
- Bisicchia, E.; Sasso, V.; Catanzaro, G.; Leuti, A.; Besharat, Z.M.; Chiacchiarini, M.; Molinari, M.; Ferretti, E.; Viscomi, M.T.; Chiurchiù, V. Resolvin D1 halts remote neuroinflammation and improves functional recovery after focal brain damage via ALX/FPR2 receptor-regulated microRNAs. Mol. Neurobiol. 2018, 55, 6894–6905.
- Recchiuti, A.; Krishnamoorthy, S.; Fredman, G.; Chiang, N.; Serhan, C.N. MicroRNAs in resolution of acute inflammation: Identification of novel resolvin D1-miRNA circuits. FASEB J. 2010, 25, 544–560.
- Bazan, N.G.; Molina, M.F.; Gordon, W.C. Docosahexaenoic acid signalolipidomics in nutrition: Significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Ann. Rev. Nutr. 2011, 31, 321–351.
- Bazan, N.G. Neuroprotectin D1 (NPD1): A DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005, 15, 15267–15278.
- Calandria, J.M.; Marcheselli, V.L.; Mukherjee, P.K.; Uddin, J.; Winkler, J.W.; Petasis, N.A.; Bazan, N.G. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 2009, 284, 17877–17882.
- Morita, M.; Kuba, K.; Ichikawa, A.; Nakayama, M.; Katahira, J.; Iwamoto, R.; Watanebe, T.; Sakabe, S.; Daidoji, T.; Nakamura, S.; et al. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell 2013, 153, 112–125.
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS ONE 2011, 6, e15816.
- Garcia-Sastre, A. Lessons from lipids in the fight against influenza. Cell 2013, 154, 22–23.
- Gladine, C.; Laurie, J.-C.; Giulia, C.; Dominique, B.; Corinne, C.; Nathalie, H.; Bart, S.; Giuseppe, Z.; Alessio, P.; Jean-Marie, G.; et al. Neuroprostanes, produced by free-radical mediated peroxidation of DHA, inhibit the inflammatory response of human macrophages. Free Radic. Biol. Med. 2014, 75, S15–S25.
- Groeger, A.L.; Cipollina, C.; Cole, M.P.; Woodcock, S.R.; Bonacci, G.; Rudolph, T.K.; Rudolph, V.; Freeman, B.A.; Schopfer, F.J. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat. Chem. Biol. 2010, 6, 433–441.
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015, 7, 52.
- De Petrocellis, L.; Melck, D.; Bisogno, T.; Milone, A.; Di Marzo, V. Finding of the endocannabinoid signalling system in Hydra, a very primitive organism: Possible role in the feeding response. Neuroscience 1999, 92, 377–387.
- Kim, J.; Carlson, M.E.; Watkins, B.A. Docosahexaenoyl ethanolamide improves glucose uptake and alters endocannabinoid system gene expression in proliferating and differentiating C2C12 myoblasts. Front. Physiol. 2014, 5.
- Soderstrom, K. Endocannabinoids link feeding state and auditory perception-related gene expression. J. Neurosci. 2004, 24, 10013–10024.
- Valenti, M.; Cottone, E.; Martinez, R.; De Pedro, N.; Rubio, M.; Viveros, M.P.; Franzoni, M.F.; Delgado, M.J.; Di Marzo, V. The endocannabinoid system in the brain of Carassius auratus and its possible role in the control of food intake. J. Neurochem. 2005, 95, 662–672.
- Chiang, N.; Takano, T.; Arita, M.; Watanabe, S.; Serhan, C.N. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br. J. Pharmacol. 2003, 139, 89–98.
- Piazza, P.V.; Lafontan, M.; Girard, J. Integrated physiology and pathophysiology of CB1-mediated effects of the endocannabinoid system. Diabetes Metab. 2007, 33, 97–107.
- Masoodi, M.; Kuda, O.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Lipid signaling in adipose tissue: Connecting inflammation & metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 503–518.
- D’Addario, C.; Micioni Di Bonaventura, M.V.; Pucci, M.; Romano, A.; Gaetani, S.; Ciccocioppo, R.; Cifani, C.; Maccarrone, M. Endocannabinoid signaling and food addiction. Neurosci. Biobehav. Rev. 2014, 47, 203–224.
- Wagner, J.J.; Alger, B.E. Increased neuronal excitability during depolarization-induced suppression of inhibition in rat hippocampus. J. Physiol. 1996, 495, 107–112.
- Wilson, R.I.; Nicoll, R.A. Neuroscience: Endocannabinoid signaling in the brain. Science 2002, 296, 678–682.
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662.
- Lu, H.C.; MacKie, K. An introduction to the endogenous cannabinoid system. Biol. Psychiatry 2016.
- Song, C.; Manku, M.S.; Horrobin, D.F. Long-chain polyunsaturated fatty acids modulate interleukin-1β–induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J. Nutr. 2018.
- Bozzatello, P.; Brignolo, E.; De Grandi, E.; Bellino, S. Supplementation with omega-3 fatty acids in psychiatric disorders: A review of literature data. J. Clin. Med. 2016, 5, 67.
- Hibbeln, J.R. Fish consumption and major depression. Lancet 1998, 351, 1213.
- Boudrault, C.; Bazinet, R.P.; Ma, D.W.L. Experimental models and mechanisms underlying the protective effects of n-3 polyunsaturated fatty acids in Alzheimer’s disease. J. Nutr. Biochem. 2009, 20, 1–10.
- Hashimoto, M.; Hossain, S.; Agdul, H.; Shido, O. Docosahexaenoic acid-induced amelioration on impairment of memory learning in amyloid β-infused rats relates to the decreases of amyloid β and cholesterol levels in detergent-insoluble membrane fractions. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1738, 91–98.
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6.
- McNamara, R.K. DHA Deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J. Nutr. 2010, 140, 864–868.
- McNamara, R.K.; Hahn, C.G.; Jandacek, R.; Rider, T.; Tso, P.; Stanford, K.E.; Richtand, N.M. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol. Psychiatry 2007, 62, 17–24.
- Basak, S.; Vilasagaram, S.; Duttaroy, A.K. Maternal dietary deficiency of n-3 fatty acids affects metabolic and epigenetic phenotypes of the developing fetus. Prostaglandins Leukot. Essent. Fatty Acids 2020, 158, 102109.
- Srinivas, V.; Molangiri, A.; Mallepogu, A.; Kona, S.R.; Ibrahim, A.; Duttaroy, A.K.; Basak, S. Maternal n-3 PUFA deficiency alters uterine artery remodeling and placental epigenome in the mice. J. Nutr. Biochem. 2021, in press.
- Boucher, O.; Burden, M.J.; Muckle, G.; Saint-Amour, D.; Ayotte, P.; Dewailly, E.; Nelson, C.A.; Jacobson, S.W.; Jacobson, J.L. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am. J. Clin. Nutr. 2011, 93, 1025–1037.
- Mulder, K.A.; King, D.J.; Innis, S.M. Omega-3 fatty acid deficiency in infants before birth identified using a randomized trial of maternal DHA supplementation in pregnancy. PLoS ONE 2014, 9, e83764.
- Smith, S.L.; Rouse, C.A. Docosahexaenoic acid and the preterm infant. Mater. Health Neonatol. Perinatol. 2017, 3, 22.
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary patterns and risk of dementia: A systematic review and meta-analysis of cohort studies. Mol. Neurobiol. 2016, 53, 6144–6154.
- Albanese, E.; Dangour, A.D.; Uauy, R.; Acosta, D.; Guerra, M.; Guerra, S.S.; Huang, Y.; Jacob, K.S.; de Rodriguez, J.L.; Noriega, L.H.; et al. Dietary fish and meat intake and dementia in Latin America, China, and India: A 10/66 Dementia Research Group population-based study. Am. J. Clin. Nutr. 2009, 90, 392–400.
- Milte, C.M.; Sinn, N.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Howe, P.R. Erythrocyte polyunsaturated fatty acid status, memory, cognition and mood in older adults with mild cognitive impairment and healthy controls. Prostaglandins Leukot. Essent. Fatty Acids 2011, 84, 153–161.
- Yin, Y.; Fan, Y.; Lin, F.; Xu, Y.; Zhang, J. Nutrient biomarkers and vascular risk factors in subtypes of mild cognitive impairment: A cross-sectional study. J. Nutr. Health Aging. 2015, 19, 39–47.
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785.
- Shamim, A.; Mahmood, T.; Ahsan, F.; Kumar, A.; Bagga, P. Lipids: An insight into the neurodegenerative disorders. Clin. Nutr. Exp. 2018, 20, 1–19.
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222.
- Sinn, N.; Milte, C.M.; Street, S.J.; Buckley, J.D.; Coates, A.M.; Petkov, J.; Howe, P.R. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Nutr. 2012, 107, 1682–1693.
- Bos, D.J.; Oranje, B.; Veerhoek, E.S.; Van Diepen, R.M.; Weusten, J.M.H.; Demmelmair, H.; Koletzko, B.; De Sain-Van Der Velden, M.G.M.; Eilander, A.; Hoeksma, M.; et al. Reduced symptoms of inattention after dietary omega-3 fatty acid supplementation in boys with and without attention deficit/hyperactivity disorder. Neuropsychopharmacology 2015, 40, 2298–2306.
- Sinn, N.; Bryan, J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J. Dev. Behav. Pediatr. 2007, 28, 82–91.
- Sinn, N.; Bryan, J.; Wilson, C. Cognitive effects of polyunsaturated fatty acids in children with attention deficit hyperactivity disorder symptoms: A randomised controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 311–326.
- Belanger, S.A.; Vanasse, M.; Spahis, S.; Sylvestre, M.P.; Lippe, S.; L'Heureux, F.; Ghadirian, P.; Vanasse, C.M.; Levy, E. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: A randomized, double-blind, placebo-controlled study. Paediatr. Child Health 2009, 14, 89–98.
- Johnson, M.; Ostlund, S.; Fransson, G.; Kadesjo, B.; Gillberg, C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: A randomized placebo-controlled trial in children and adolescents. J. Atten. Disord. 2009, 12, 394–401.
- Gustafsson, P.A.; Birberg-Thornberg, U.; Duchen, K.; Landgren, M.; Malmberg, K.; Pelling, H.; Strandvik, B.; Karlsson, T. EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010, 99, 1540–1549.
- Hariri, M.; Djazayery, A.; Djalali, M.; Saedisomeolia, A.; Rahimi, A.; Abdolahian, E. Effect of n-3 supplementation on hyperactivity, oxidative stress and inflammatory mediators in children with attention-deficit-hyperactivity disorder. Malays J. Nutr. 2012, 18, 329–335.
- Behdani, F.; Hebrani, P.; Naseraee, A.; Haghighi, M.B.; Akhavanrezayat, A. Does omega-3 supplement enhance the therapeutic results of methylphenidate in attention deficit hyperactivity disorder patients? J. Res. Med. Sci. 2013, 18, 653–658.
- Dashti, N.; Hekmat, H.; Soltani, H.R.; Rahimdel, A.; Javaherchian, M. Comparison of therapeutic effects of omega-3 and methylphenidate (ritalin((R))) in treating children with attention deficit hyperactivity disorder. Iran J. Psychiatry Behav. Sci. 2014, 8, 7–11.
- Anand, P.; Sachdeva, A. Effect of poly unsaturated fatty acids administration on children with attention deficit hyperactivity disorder: A randomized controlled trial. J. Clin. Diagn. Res. 2016, 10, OC01–OC05.
- Assareh, M.; Davari Ashtiani, R.; Khademi, M.; Jazayeri, S.; Rai, A.; Nikoo, M. Efficacy of Polyunsaturated Fatty Acids (PUFA) in the treatment of attention deficit hyperactivity disorder. J. Atten. Disord. 2017, 21, 78–85.
- Kean, J.D.; Sarris, J.; Scholey, A.; Silberstein, R.; Downey, L.A.; Stough, C. Reduced inattention and hyperactivity and improved cognition after marine oil extract (PCSO-524(R)) supplementation in children and adolescents with clinical and subclinical symptoms of attention-deficit hyperactivity disorder (ADHD): A randomised, double-blind, placebo-controlled trial. Psychopharmacology 2017, 234, 403–420.
- Voigt, R.G.; Llorente, A.M.; Jensen, C.L.; Fraley, J.K.; Berretta, M.C.; Heird, W.C. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J. Pediatr. 2001, 139, 189–196.
- Amminger, G.P.; Schafer, M.R.; Papageorgiou, K.; Klier, C.M.; Cotton, S.M.; Harrigan, S.M.; Mackinnon, A.; McGorry, P.D.; Berger, G.E. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: A randomized, placebo-controlled trial. Arch. Gen. Psychiatry 2010, 67, 146–154.
- Pawelczyk, T.; Grancow-Grabka, M.; Kotlicka-Antczak, M.; Trafalska, E.; Pawelczyk, A. A randomized controlled study of the efficacy of six-month supplementation with concentrated fish oil rich in omega-3 polyunsaturated fatty acids in first episode schizophrenia. J. Psychiatr Res. 2016, 73, 34–44.
- Ammann, E.M.; Pottala, J.V.; Harris, W.S.; Espeland, M.A.; Wallace, R.; Denburg, N.L.; Carnahan, R.M.; Robinson, J.G. omega-3 fatty acids and domain-specific cognitive aging: Secondary analyses of data from WHISCA. Neurology 2013, 81, 1484–1491.
- Maekawa, M.; Watanabe, A.; Iwayama, Y.; Kimura, T.; Hamazaki, K.; Balan, S.; Ohba, H.; Hisano, Y.; Nozaki, Y.; Ohnishi, T.; et al. Polyunsaturated fatty acid deficiency during neurodevelopment in mice models the prodromal state of schizophrenia through epigenetic changes in nuclear receptor genes. Transl. Psychiatry 2017, 7, e1229.
- Bosetti, F. Arachidonic acid metabolism in brain physiology and pathology: Lessons from genetically altered mouse models. J. Neurochem. 2007, 102, 577–586.
- Contreras, M.A.; Rapoport, S.I. Recent studies on interactions between n-3 and n-6 polyunsaturated fatty acids in brain and other tissues. Curr. Opin. Lipidol. 2002, 13, 267–272.
- Coronary heart disease in seven countries. Summary. Circulation 1970, 41, I186–I195.
- Rapoport, S.I. Arachidonic acid and the brain. J. Nutr. 2008, 138, 2515–2520.
- Duncan, R.E.; Bazinet, R.P. Brain arachidonic acid uptake and turnover: Implications for signaling and bipolar disorder. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 130–138.
- Hosono, T.; Mouri, A.; Nishitsuji, K.; Jung, C.G.; Kontani, M.; Tokuda, H.; Kawashima, H.; Shibata, H.; Suzuki, T.; Nabehsima, T.; et al. Arachidonic or docosahexaenoic acid diet prevents memory impairment in Tg2576 mice. J. Alzheimers Dis. 2015, 48, 149–162.
- Katsuki, H.; Okuda, S. Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol. 1995, 46, 607–636.
- Sanchez-Mejia, R.O.; Newman, J.W.; Toh, S.; Yu, G.Q.; Zhou, Y.; Halabisky, B.; Cisse, M.; Scearce-Levie, K.; Cheng, I.H.; Gan, L.; et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2008, 11, 1311–1318.
- Vijayaraghavan, S.; Huang, B.; Blumenthal, E.M.; Berg, D.K. Arachidonic acid as a possible negative feedback inhibitor of nicotinic acetylcholine receptors on neurons. J. Neurosci. 1995, 15, 3679–3687.
- Williams, J.H.; Errington, M.L.; Lynch, M.A.; Bliss, T.V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature 1989, 341, 739–742.
- Darios, F.; Davletov, B. Omega-3 and omega-6 fatty acids stimulate cell membrane expansion by acting on syntaxin 3. Nature 2006, 440, 813–817.
- Darios, F.; Ruiperez, V.; Lopez, I.; Villanueva, J.; Gutierrez, L.M.; Davletov, B. Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010, 11, 528–533.
- Yasojima, K.; Schwab, C.; McGeer, E.G.; McGeer, P.L. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999, 830, 226–236.
- McGahon, B.; Clements, M.P.; Lynch, M.A. The ability of aged rats to sustain long-term potentiation is restored when the age-related decrease in membrane arachidonic acid concentration is reversed. Neuroscience 1997, 81, 9–16.
- Angelova, P.R.; Muller, W.S. Arachidonic acid potently inhibits both postsynaptic-type Kv4.2 and presynaptic-type Kv1.4 IA potassium channels. Eur. J. Neurosci. 2009, 29, 1943–1950.
