Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Rossella Svigelj and Version 2 by Rita Xu.
Deep Eutectic Solvents (DESs) are a new class of solvents characterized by a remarkable decrease in melting point compared to those of the starting components. The eutectic mixtures can be simply prepared by mixing a Hydrogen Bond Acceptor (HBA) with a Hydrogen Bond Donor (HBD) at a temperature of about 80 °C.
- deep eutectic solvents
- biosensors
- DNA
- enzymes
- MIPs
- aptamers
- nanomaterials
1. Deep Eutectic Solvents (DESs)
Lately, a new class of solvents, called Deep Eutectic Solvents (DESs), has been successfully employed in different research fields. In 2004, Abbott et al. described the generation of eutectic mixtures as complexes formed between halide salts and a range of compounds such as amides and carboxylic acids [1]. Indeed, DESs can be easily prepared by mixing at least two components able to form a eutectic mixture with a melting point lower than that of the starting components [2][3][2,3]. This group of solvents share some characteristics with ionic liquids (ILs), a class of organic salts with a low melting point, generally obtained by combining an organic cation (commonly imidazolium-based cations) with a large variety of anions (i.e., Cl−, BF4−, PF6−, [NTf2]−) [4]. However, DESs have demonstrated to have some interesting advantages over ILs. As a matter of fact, the definition of ILs as “green” species is largely contested in the current literature [5]. Therefore, to overcome the high cost of their production and their toxicity, DESs have arisen as a cheaper and greener alternative [6].
Recently, another class of solvents, called Natural Deep Eutectic Solvents (NADESs), has been introduced [7]. This new category was described after observing the occurrence of certain natural mixtures. In fact, the presence of eutectic liquids in living organisms could explain a great number of biological processes where poorly water-soluble metabolites are involved [8]. In different types of microbial, animal, and plant cells, the presence of substances, including sugars, some amino acids, choline, and some organic acids such as malic acid, citric acid, and lactic acid, could lead to the formation of NADESs as a third type of solvent besides water and lipids. Furthermore, the existence of these natural solvents could explain the survival of some organisms through extreme conditions such as cold or water shortage [8].
DESs are usually prepared by mixing a Hydrogen Bond Acceptor (HBA) with a Hydrogen Bond Donor (HBD) at a temperature of about 80 °C. Among the HBAs, different quaternary ammonium salts can be found, while amines, carboxylic acids, alcohols, or carbohydrates belong to the group of HBDs (Figure 1). DESs have found applications in various research areas, including organic synthesis, electrochemistry, and bio-catalysis [9]. Furthermore, they display improved biodegradability and lower toxicity compared to other solvents, characteristics that make them particularly interesting in green chemistry [10].
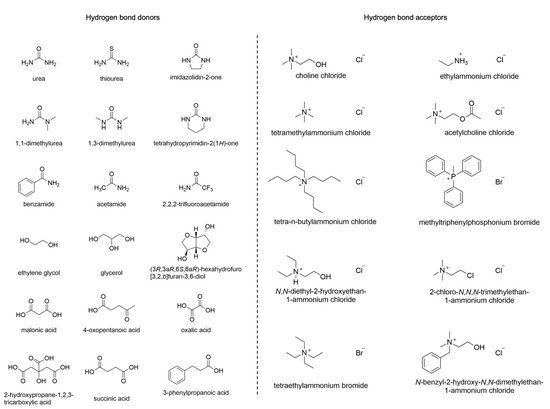
Figure 1. Typical HBDs and HBAs employed in DESs.
Interestingly, both DESs and NADESs provide a network of hydrogen bonds that make possible the solubilization of a wide range of molecules [11]. Their characteristics make them potential universal solvents capable of extracting a wide variety of non-polar and polar compounds [7]. Thus, they could become an appealing alternative to several standard and toxic organic solvents. The structures of their components strongly influence all DESs’ physicochemical properties, such as melting point, density, conductivity, and viscosity [12]. Moreover, studies on the three-dimensional structure of DESs revealed that the interaction between HBDs and HBAs depends mainly on hydrogen bonds, but they also interact with the anion by arranging themselves around it [13]. In general, the presence of a broad hydrogen-bonding network among the components is responsible for the high viscosity of DESs and the restrained mobility of free species inside the solvent. Additional phenomena, such as van der Waals and electrostatic interactions, may also be responsible for the high viscosity of DESs. The freezing point of DESs is characterized by a consistent drop, generally wider than 150 °C. The difference in the freezing point of a binary mixture in the eutectic composition compared to that of a theoretical ideal mixture is related to the magnitude of the interaction of the components [10]. DESs exhibit, in general, higher densities than water, with values ranging from 1.041 g cm−3 to 1.63 g cm−3 [10]. Among the different quaternary ammonium salts used in eutectic mixtures, choline chloride (ChCl) is the most employed thanks to its low cost and biodegradability. ChCl can be combined with HBDs to provide DESs with different physical and chemical properties such as freezing point, viscosity, conductivity, and pH [14]. Table 1 shows some physical parameters of ChCl-based DESs.
Table 1. Physical parameters of some ChCl-based DESs.
| DESs | Salt/HBD Molar Ratio | Viscosity | a | Pa·s | Conductivity | a | μS·cm | −1 | Density | a | g/cm | 3 | T | f | /°C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ChCl:urea | 1:2 | - | - | 1.25 | 12 | ||||||||||
| ChCl:acetamide | 1:2 | 0.127 | 2710 | 1.09 | 51 | ||||||||||
| ChCl:glycerol | 1:2 | 0.177 | 1647 | 1.19 | −40 | ||||||||||
| ChCl:1,4-butanediol | 1:4 | 0.047 | 2430 | 1.04 | - | ||||||||||
| ChCl:triethylene glycol | 1:4 | 0.044 | 1858 | 1.12 | −66 | ||||||||||
| ChCl:xylitol | 1:1 | 3.867 | 172.6 | 1.24 | - | ||||||||||
| ChCl:D-sorbitol | 1:1 | 13.736 | 63.3 | 1.28 | - | ||||||||||
| ChCl:oxalic acid | 1:1 | 0.089 | 2350 | 1.24 | 34 | ||||||||||
| ChCl:levulinic acid | 1:2 | 0.119 | 1422 | 1.13 | - | ||||||||||
| ChCl:malonic acid | 1:1 | 0.616 | 732 | 1.21 | 10 | ||||||||||
| ChCl:malic acid | 1:1 | 11.475 | 41.4 | 1.28 | - | ||||||||||
| ChCl:citric acid | 1:1 | 45.008 | 18.4 | 1.33 | 69 | ||||||||||
| ChCl:tartaric acid | 2:1 | 66.441 | 14.3 | 1.27 | 47 |
To date, DESs have found many applications in analytical chemistry, such as in the extraction of analytes from complex liquid and solid matrices [15], as modification media for nanomaterials [16][17][16,17], for elution in dispersive solid-phase extractions, and as a mobile-phase modifier in chromatography [18][19][18,19]. They have also been employed in the extraction of bioactive compounds [20], including flavonoids, phenolic acids, polyphenols, saponins, and anthraquinones, from various types of natural sources [21][22][23][21,22,23]. In addition, DESs display a good capability of solubilizing many other compounds, such as drugs, metal oxides, and carbon dioxide [24][25][24,25].
Taking into account all their properties and their biocompatibility, this class of solvents could replace the classic aqueous buffers that are normally used in the development of biosensors. DESs could also improve biosensor performance, for example, in the analysis of molecules that are not soluble in water.
2. Biosensors
Biosensors exploit the sensitivity of transducers combined with the high specificity of biological recognition elements, which are able to interact selectively with analytes [26]. Generally, biosensors can be categorized on the basis of their transduction mechanism, which can be optical (including optical fiber and surface plasmon resonance biosensors), electrochemical (including voltammetric, amperometric, and impedance biosensors), or piezoelectric (including quartz crystal microbalance biosensors) [27]. Alternatively, their classification is based on the sensing element, in which case they are named immunosensors, aptasensors, genosensors, enzymatic biosensors, and molecularly imprinted sensors when the biological sensing elements are antibodies, aptamers, nucleic acids, enzymes, and MIPs (molecularly imprinted polymers), respectively (Figure 2) [28][29][28,29]. The impact of biosensing is gaining importance in all sectors, such as clinical, environmental, and food-related fields [30][31][30,31]. Biosensors can be considered very versatile and powerful tools because of their low cost and compatibility with portable and compact instrumentation, as well as their easy and rapid use.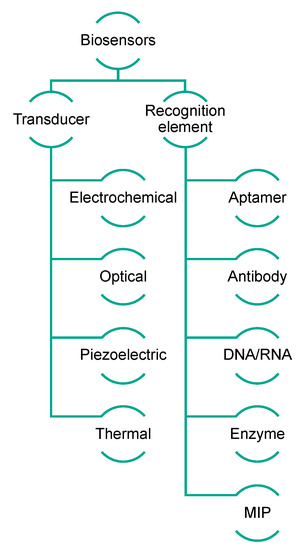
Figure 2. Schematic representation of biosensor classification.
3. Graphene, Carbon Paste, and Carbon Nanotubes in DESs
Several materials have been modified with DESs, including graphene, carbon paste, and nanotubes. The modification of these materials with the eutectic mixtures has shown several advantages, including increased charge transfer and conductivity, increased surface area, and the generation of new functional groups.
Graphene and DESs have separately demonstrated encouraging properties in the development of electroanalytical platforms [35]. Recently, Fuchs et al. combined the use of graphene and DESs, studying their coadjuvant effects in electrochemistry [36]. They considered the electrochemical performance of a centimeter-scale graphene monolayer generated by chemical vapor deposition and afterward moved onto insulating SiO2/Si supports in the DES ethaline formed by 1:2 ChCl and ethylene glycol. They provided a first approach for the use of graphene combined with a DES for electroanalytical applications, covering also the characterization of the graphene/DES electrochemical behavior. The authors also investigated the modification of graphene with metal (Zn) and metalloid (Ge) in the DES, evaluating their electrochemical performances. What emerged was that the behavior of graphene in ethaline is close to the characteristics of glassy carbon or highly ordered pyrolytic graphite; however, other features of the graphene/ethaline systems are uncommon, thanks to the two-dimensional nature of graphene. Hence, this work gave the first framework for graphene–DES systems.
A recent work by Cariati and Buoro reported the employment of natural deep eutectic solvents (NADESs) for the modification of carbon paste electrodes [37]. Enhanced conductivity and charge transfer rate were noticed when the cyclic voltammograms of potassium ferrocyanide were recorded with the NADES-modified carbon paste electrode compared to the bare carbon paste, revealing smaller peak potential separation. An improvement in the diffusion of the probe to the graphite surface was achieved with the integration of KCl in the NADES solution, decreasing in this way the binder layer viscosity. Better performance when using a reline (ChCl and urea, 1:2) modified carbon paste electrode was also noted for the oxidation of dopamine and ascorbic acid.
Further, Ibrahim et al. prepared two DESs using ethylene glycol combined with N,N-diethylethanolammonium chloride, or ChCl. In this study, the capability of DESs for carbon nanotube functionalization was investigated by studying the changes occurring after the functionalization process, using KMnO4 as the oxidizing agent. After the modifications, the functionalized carbon nanotubes were tested for the absorption of methyl orange from water. The effect of DESs was an increased surface area of carbon nanotubes and the generation of new functional groups on the carbon nanotubes’ surfaces without causing any damage to their structure, demonstrating its usefulness in the development of modified carbo-nanotubes for water treatment [38]. Furthermore, Rozas et al. studied the properties of carbon, boron nitride, silicon, germanium, and molybdenum disulfide nanotubes in reline by applying classical molecular dynamics simulations [39]. In this study, the interactions between reline and nanotubes revealed the development of a strongly adsorbed layer, demonstrating a great affinity of reline for the considered nanotubes and, among them, especially for the boron nitride one. The authors described a sustainable DES-based nanofluid system applicable to many different research fields. The nanotube size effect was also studied and, in every case, reline was able to solvate the nanotubes, demonstrating that nanofluids based on reline can be synthesized for a wide range of nanotube sizes and chemical compositions for different nanotechnology applications.
4. Nano and Magnetic Particles in DESs
Nanoparticles and magnetic particles have proved their importance in the development of different analytical platforms. Although they present several advantages, among the disadvantages are their aggregation and lack of stability over time; regarding these aspects, the use of eutectic liquids could help expand their use.
In 2008, Liao et al. reported the synthesis of gold nanoparticles (AuNPs) with diverse and unique shapes such as stars, snowflakes, and thorns using reline [40]. The authors obtained star-shaped AuNPs using HAuCl4 and l-ascorbic acid in DES at room temperature. They outlined that the water content present in the DES can modulate different shapes. SEM images illustrated pentagonal star-shaped AuNPs of about 300 nm; the images also revealed the presence of three- and four-branch star-shaped nanoparticles. The fact that DESs can influence the shape of nanoparticles could lead to progress in the synthetic approaches for nanomaterials.
In 2014, another synthesis and growth mechanism of AuNPs in DES was described [41]. In this study, a low-energy, soft-sputtering deposition technique was employed for gold nanoparticle formation in DES. The authors reported that DES can act as a structure-directing agent in the self-assembly (SA) of gold nanoparticles, and this feature can be modulated by anions, cations, and neutral components, such as urea. In addition, high concentrations of AuNPs in the eutectic mixture resulted in SA in the first and second shell short-range ordering of the gold nanoparticles. The growth and the ordering of AuNPs occurred on the surface as well as inside the bulk DES. The SA process depended on the nature of the components forming the eutectic mixture, and the authors employed reline, which is based on ChCl and urea. They obtained AuNPs of 5 nm size and SA in short-range order when they employed reline, while when using a non-templating agent such as castor oil they obtained AuNPs but without the SA. Moreover, the use of DES ensured that the particles remained stable, showing no aging effect, demonstrating the stabilizing effect of DES.
Svigelj et al. monitored the behavior of magnetic particles (MPs) modified with the peptide 33-mer in buffer and in ethaline with backscattering experiments. The MPs dispersed in ethaline remained in suspension longer than those in buffer, where they precipitated (speed of 0.06 mm/h) after undergoing agglomeration, which was detected as an increase in the backscattering of light in the middle zone of the measurement cell. In the DES, the particles displayed a homogeneous distribution throughout the entire cell and remained stable for 24 h, with no precipitation [42]. Among the advantages of the use of DES combined with MPs, the authors highlighted the stabilization and the ease of manipulation.
More recently, Baby et al. employed DESs in the solid-state synthesis of phase-pure magnesium ferrite nanoparticles that were subsequently exploited for the concomitant detection of nitrofurantoin (NFT) and 4-nitrophenol (4-NP) [43]. Five different DESs, based on the combination of ChCl with malonic acid, oxalic acid, urea, ethylene glycol, and fructose, were employed in the synthesis of the magnesium ferrite nanoparticles (Figure 3).
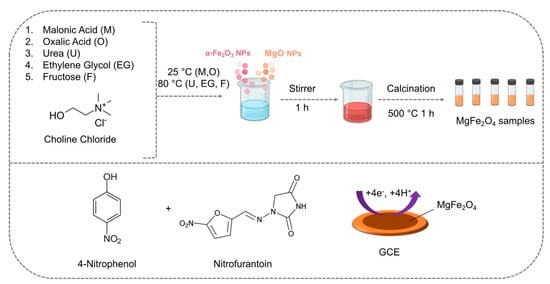

Figure 3. Synthetic path of various DES-assisted MgFe2O4 and their use in the electrochemical detection of 4-NP and NFT. Reprinted (adapted) with permission from Baby, J.N.; Sriram, B.; Wang, S.-F.; George, M. Effect of Various Deep Eutectic Solvents on the Sustainable Synthesis of MgFe2 O4 Nanoparticles for Simultaneous Electrochemical Determination of Nitrofurantoin and 4-Nitrophenol. ACS Sustain. Chem. Eng. 2020, 8, 1479–1486. Copyright 2020, American Chemical Society [43].
The authors noted that the type of DES employed in the synthesis was decisive for the shape of the NPs. The use of DESs based on ChCl and malonic acid or oxalic acid provided NPs with a variety of cubical and spherical shapes, whilst NPs synthesized in the DES based on ChCl and urea consisted of cubical nanoparticles. When ChCl in combination with ethylene glycol was used, nanospheres and spherical agglomerations were obtained, while the use of a DES based on ChCl and fructose led to the formation of nanograins. The electrode modified with NPs synthesized in the ChCl and fructose DES showed the highest sensitivity and lowest detection limits and a linear range between 0 and 342.6 μM, showing also an excellent selectivity and a good reproducibility.
