You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Jorge Caldeira.
Matrix metalloproteinases are enzymes that degrade the extracellular matrix. They have different substrates but similar structural organization. Matrix metalloproteinases are involved in many physiological and pathological processes and there is a need to develop inhibitors for these enzymes in order to modulate the degradation of the extracellular matrix (ECM). There exist two classes of inhibitors: endogenous and synthetics. The development of synthetic inhibitors remains a great challenge due to the low selectivity and specificity, side effects in clinical trials, and instability.
- matrix metalloproteinases
- TIMP
- synthetic inhibitors
1. Introduction
Matrix metalloproteinases (MMPs) are a protein family within the metzincin superfamily, comprising zinc-dependent endopeptidases with similar structural characteristics but with different substrate preferences. MMPs are produced and secreted from cells as inactive proenzymes depending, herein, on a structural alteration for activation [1,2,3,4,5,6][1][2][3][4][5][6]. In human tissues, there are 23 different types of MMPs expressed and they can be subdivided according to their substrate specificity, sequential similarity, and domain organization [1,2,4,7,8,9,10,11,12,13,14,15,16,17][1][2][4][7][8][9][10][11][12][13][14][15][16][17] (Table 1).
Table 1. Matrix metalloproteinases (MMPs) classes.
| Class | MMP | |
|---|---|---|
| Collagenases | MMP-1, Collagenase-1, Interstitial or Fibroblast collagenases | |
| MMP-8, Collagenase-2, or Neutrophil collagenases | ||
| MMP-13 or Collagenase 3 | ||
| Gelatinases | MMP-2 or Gelatinase A | |
| MMP-9 or Gelatinase B | ||
| Stromelysin | MMP-3 or Stromelysin-1 | |
| MMP-10 or Stromelysin-2 | ||
| MMP-11 | ||
| Matrilysin | MMP-7 | |
| MMP-26, Matrilysin-2, or Endometase | ||
| Membrane-type | Type I transmembrane protein | MMP-14 or MT1-MMP |
| MMP-15 or MT2-MMP | ||
| MMP-16 or MT3-MMP | ||
| MMP-24 or MT5-MMP | ||
| Glycosylphosphatidylinositol (GPI)-anchored | MMP17 or MT4-MMP | |
| MMP-25 or MT6-MMP | ||
| Other MMPs | MMP-12 | |
| MMP-19 | ||
| MMP-20 | ||
| MMP-21 | ||
| MMP-23 | ||
| MMP-27 | ||
| MMP-28 | ||
The most common structural features shared by MMPs are [1,2,4,5,7,8,10,11,12,13,14,16,18][1][2][4][5][7][8][10][11][12][13][14][16][18] (Figure 1) a pro-domain, a catalytic domain, a hemopexin-like domain, and a transmembrane domain for membrane-type MMPs (MT-MMPs) although some MMPS do not have all the structural features represented in the figure. The pro-domain keeps MMP inactive by a cysteine switch, which interacts with the catalytic zinc making it impossible to connect the substrate. The catalytic domain has two zinc ions, three calcium ions, and three histidine residues, which are highly conserved [1,2,3,4,5,6,7,8,9,11,12,13,14,15,16,17,18,19,20][1][2][3][4][5][6][7][8][9][11][12][13][14][15][16][17][18][19][20]. In the terminal zone of the catalytic domain, there is a region that forms the outer wall of the S1’ pocket [1,14,17][1][14][17]. This pocket is the most variable region in MMPs and it is a determining factor for substrate specificity [1,2,6,7,11,17,18][1][2][6][7][11][17][18]. However, there are six pockets (P1, P2, P3, P1’, P2’, and P3’) and the fragments of the substrates or inhibitors are named depending on the interaction with these pockets (R1, R2, R3, R1’ or Ra, R2’, and R3’). The linker is proline-rich, of variable length, allowing inter-domain flexibility and enzyme stability [4,8,12,13][4][8][12][13]. The hemopexin-like domain is necessary for collagen triple helix degradation and is important for substrate specificity [3,4,7,9,19][3][4][7][9][19].
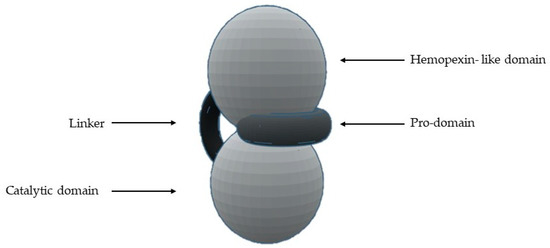
Figure 1. Schematic representation of the general structure of MMP.
The MMPs can process ECM proteins and glycoproteins, membrane receptors, cytokines, hormones, chemokines, adhesion molecules, and growth factors [1,3,4,6,7,9,10,11,13,14,20,21,22,23,24,25,26][1][3][4][6][7][9][10][11][13][14][20][21][22][23][24][25][26]. However, the presence and the activity of MMPs have been demonstrated to be intracellular [25,26][25][26]. For example, some studies show intracellular localization of MMP-2 in cardiac myocytes and colocalization of MMP-2 with troponin I in cardiac myofilaments [23]. The MMP-2 activity has also been detected in nuclear extracts from the human heart and rat liver [23]. The MMPs are involved in many biologic processes, such as tissue repair and remodulation, cellular differentiation, embryogenesis, angiogenesis, cell mobility, morphogenesis, wound healing, inflammatory response, apoptosis, ovulation, and endometrial proliferation [1,2,4,6,8,10,11,13,16,17,18,20,27][1][2][4][6][8][10][11][13][16][17][18][20][27]. The deregulation of MMPs activity leads to the progression of various pathologies depending on which enzyme is involved [1,6,10,13,14,15,16,17,20,27][1][6][10][13][14][15][16][17][20][27]: cancer and metastasis, inflammatory processes, arthritis, ulcers, periodontal diseases, brain degenerative diseases, liver cirrhosis, fibrotic lung diseases, otosclerosis, atherosclerosis, multiple sclerosis, dilated cardiomyopathy, aortic aneurysm, or varicose veins.
Although therapeutic strategies for specific inhibition of MMPs have been long researched, they are difficult to develop because these enzymes are involved in a myriad of pathways [2,5][2][5]. However, this inhibition can be done at the biomolecular expression and active enzyme terms [2,5,18][2][5][18]. The MMPs inhibitors can be divided into endogenous inhibitors, which can be specific or non-specific, and synthetic inhibitors [1,2,4,7,10,12,13,14,16,20,28,29][1][2][4][7][10][12][13][14][16][20][28][29] (Table 2).
Table 2. MMPs inhibitors classification.
| Specific Inhibitor | Tissue Inhibitor of Metalloproteinases (TIMP) | |
|---|---|---|
| Endogenous inhibitor | Non-specifics inhibitors | α2-macroglobulin |
| Tissue factor pathway inhibitor (TFPI) | ||
| The membrane-bound β-amyloid precursor protein | ||
| C-terminal proteinases enhancer protein | ||
| Reversion-inducing cysteine-rich protein with Kasal domain motifs (RECK) | ||
| GPI-anchored glycoprotein | ||
| Synthetic inhibitor | Hydroxamate-based inhibitors | |
| Non-hydroxamate-based inhibitors | ||
| Catalytic domain (non-zinc binding) inhibitors | ||
| Allosteric and exosite inhibitors | ||
| Antibody-based inhibitors | ||
2. Specific Endogenous Inhibitor-Tissue Inhibitors of Metalloproteinases (TIMPs)
Tissue inhibitors of metalloproteinases (TIMPs) are endogenous proteins responsible for the regulation of MMPs activity, but also of families such as the disintegrin metalloproteinases (ADAM and with thrombospondin motifs ADAMTS) and therefore for maintaining the physiological balance between ECM degradation and MMPs activity [1,2,8,9,18,30][1][2][8][9][18][30]. There are four TIMPs (TIMP-1, -2, -3, and -4) (Table 3), with 22–29 KDa and 41%–52% sequential similarity [2,4,12,13,16,20,31][2][4][12][13][16][20][31].
Table 3. Tissue inhibitors of metalloproteinases (TIMPs) classification.
| TIMP | Expression | Inhibition | Inhibition Mode |
|---|---|---|---|
| 1 | Several tissues with transcription inducible by cytokines and hormones | Strong interaction with MMP-1, -2, -3, and -9 Weak interaction with MT1-MMP, MT3-MMP, MT5-MMP, and MMP-19 |
TIMP-1 forms a complex with pro-MMP-9 by binding to the hemopexin domain |
| 2 | Constitutive expression | Strong interaction with MMP-2 | TIMP-2 has four residues in the N-terminal domain and an adjacent CD-loop region, which allows interaction between TIMP and the active center of MMP-2 |
| 3 | In response to mitogenic stimulation and during cell cycle progression | MMP-1, -2, -3, -9, and -13 | The inhibition mode is different from the other TIMPs for its unusual localization, as it is largely sequestered into the extracellular matrix or at the cell surface via heparan sulphate proteoglycans |
| 4 | Especially abundant in the heart, but is also expressed in injured tissue | MMP-2 and -14 | - |
TIMPs consist of a N- and C-terminal domain with 125 and 65 amino acids, respectively, each containing six conserved cysteine residues, which form three conserved disulphide bonds [2,4,7,8,9,12,31,32][2][4][7][8][9][12][31][32] (Figure 2a). The N-terminal domain is an independent unit, which can be inhibited by MMPs, in a 1:1 ratio [2,4,8,9,10,12,13,16,20][2][4][8][9][10][12][13][16][20]. This domain has two groups of four residues: Cys-Thr-Cys-Val and Glu-Ser-Val-Cys (Figure 2b), which are connected by disulphide bonds which are important for TIMP activity [7,12][7][12]. This is the main domain responsible for MMP inhibition through its binding to the catalytic site in a substrate-like manner [31]. The several domains allow the TIMP and pro-gelatinases interactions [4].
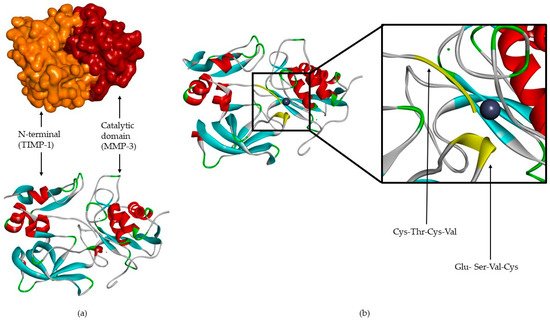
Figure 2. (a) TIMP-1-catalytic domain of the MMP-3 complex. (b) TIMP-1-catalytic domain of the MMP-3 complex, where two conserved groups, Cys-Thr-Cys-Val and Glu-Ser-Val-Cys, are represented in yellow.
3. Non-Specific Endogenous Inhibitors
Non-specific endogenous inhibitors have been reported to inhibit MMPs (Table 4), however, the inhibition mechanism details have only been partially discovered [7,12][7][12].
| Non-Specific Inhibitor | Inhibition |
|---|---|
| α2-macroglobulin | MMP-2 and -9 |
| Tissue factor pathway inhibitor | MMP-1 and -2 |
| Membrane-bound β-amyloid precursor protein | MMP-2 |
| C-terminal proteinase enhancer protein | MMP-2 |
| Reversion-inducing-cysteine-rich protein with Kasal motifs (RECK) | MMP-2, -9, and -14 |
| GPI-anchored glycoprotein | - |
Human α2-macroglobulin is a glycoprotein with four identical subunits that act by entrapping MMP and the complex is cleared by endocytosis [2]. The α2-macroglobulin has been found in blood and tissue fluid [2,31][2][31]. The tissue factor pathway inhibitor (TFPI) is a serine proteinase inhibitor, which targets MMP-1 and -2, but this inhibition mode is still unknown [7,12][7][12]. The C-terminal proteinase enhancer protein and tissue factor pathway inhibitor have sequences with certain similarities to the N-terminal domain of TIMPs [31].
References
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73.
- Liu, J.; Khalil, R.A. Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders. Prog. Mol. Biol. Transl. Sci. 2017, 148, 355–420.
- Klein, T.; Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids 2011, 41, 271–290.
- Maskos, K. Crystal structures of MMPs in complex with physiological and pharmacological inhibitors. Biochimie 2005, 87, 249–263.
- Cerofolini, L.; Fragai, M.; Luchinat, C. Mechanism and Inhibition of Matrix Metalloproteinases. Curr. Med. Chem. 2019, 26, 2609–2633.
- Fischer, T.; Senn, N.; Riedl, R. Design and Structural Evolution of Matrix Metalloproteinase Inhibitors. Chemistry 2019, 25, 7960–7980.
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573.
- Amălinei, C.; Căruntu, I.D.; Bălan, R.A. Biology of metalloproteinases. Rom. J. Morphol. Embryol. 2007, 48, 323–334.
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839.
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta. 2010, 1803, 20–28.
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268.
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Asp. Med. 2008, 29, 290–308.
- Mannello, F.; Medda, V. Nuclear localization of matrix metalloproteinases. Prog. Histochem. Cytochem. 2012, 47, 27–58.
- Rangasamy, L.; Geronimo, B.D.; Ortín, I.; Coderch, C.; Zapico, J.M.; Ramos, A.; de Pascual-Teresa, B. Molecular Imaging Probes Based on Matrix Metalloproteinase Inhibitors (MMPIs). Molecules 2019, 24, 2982.
- Vandenbroucke, R.E.; Dejonckheere, E.; Libert, C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur. Respir. J. 2011, 38, 1200–1214.
- Nuti, E.; Tuccinardi, T.; Rossello, A. Matrix metalloproteinase inhibitors: New challenges in the era of post broad-spectrum inhibitors. Curr. Pharm. Des. 2007, 13, 2087–2100.
- Georgiadis, D.; Yiotakis, A. Specific targeting of metzincin family members with small-molecules inhibitors: Progress toward a multifarious challenge. Bioorg. Med. Chem. 2008, 8781–8794.
- Jacobsen, J.A.; Major Jourden, J.L.; Miller, M.T.; Cohen, S.M. To bind zinc or not to bind zinc: An examination of innovative approaches to improved metalloproteinase inhibition. Biochim. Biophys. Acta 2010, 1803, 72–94.
- Whittaker, M.; Floyd, C.D.; Brown, P.; Gearing, A.J. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999, 99, 2735–2776.
- Tokuhara, C.K.; Santesso, M.R.; Oliveira, G.S.N.; Ventura, T.M.D.S.; Doyama, J.T.; Zambuzzi, W.F.; Oliveira, R.C. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J. Appl. Oral Sci. 2019, 27, e20180596.
- Liu, Y.; Tjäderhane, L.; Bresch, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in Bonding to Dentin and Experimental Strategies to Prevent Bond Degradation. J. Dent. Res. 2011, 90, 953–968.
- Young, D.; Das, N.; Anowai, A.; Dufour, A. Matrix Metalloproteases as Influencers of the Cells’ Social Media. Int. J. Mol. Sci. 2019, 20, 3847.
- Kwan, J.A.; Schulze, C.J.; Wang, W.; Leon, H.; Sariahmetoglu, M.; Sung, M.; Sawicka, J.; Sims, D.E.; Sawicki, G.; Schulz, R. Matrix metalloproteinase-2 (MMP-2) is present in the nucleus of cardiac myocytes and is capable of cleaving poly (ADP-ribose) polymerase (PARP) in vitro. Faseb J. 2004, 18, 690–692.
- Cauwe, B.; Van den Steen, P.E.; Opdenakker, G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 113–185.
- Cauwe, B.; Opdenakker, G. Intracellular substrate cleavage: A novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev. Biochem. Mol. Biol. 2010, 45, 351–423.
- Jobin, P.G.; Butler, G.S.; Overall, C.M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim Biophys Acta Mol. Cell Res. 2017, 1864, 2043–2055.
- Fields, G.B. The Rebirth of Matrix Metalloproteinase Inhibitors: Moving Beyond the Dogma. Cells 2019, 8, 984.
- Li, K.; Tay, F.R.; Yiu, C.K.Y. The past, present and future perspectives of matrix metalloproteinase inhibitors. Pharmacol. Ther. 2020, 207, 107465.
- Hu, J.; Van den Steen, P.E.; Sang, Q.X.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498.
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011, 12, 233.
- Folgueras, A.R.; Pendás, A.M.; Sánchez, L.M.; López-Otín, C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int. J. Dev. Biol 2004, 48, 411–424.
- Maskos, K.; Bode, W. Structural basis of matrix metalloproteinases and tissue inhibitors of metalloproteinases. Mol. Biotechnol. 2003, 25, 241–266.
- Arbeláez, L.F.; Bergmann, U.; Tuuttila, A.; Shanbhag, V.P.; Stigbrand, T. Interaction of matrix metalloproteinases-2 and -9 with pregnancy zone protein and alpha2-macroglobulin. Arch. Biochem. Biophys. 1997, 347, 62–68.
- Serifova, X.; Ugarte-Berzal, E.; Opdenakker, G.; Vandooren, J. Homotrimeric MMP-9 is an active hitchhiker on alpha-2-macroglobulin partially escaping protease inhibition and internalization through LRP-1. Cell Mol. Life Sci. 2019.
More
