Apoptosis is the physiological mechanism of cell death and can be modulated by endogenous and exogenous factors, including stress and metabolic alterations. Reactive oxygen species (ROS), as well as ROS-dependent lipid peroxidation products (including isoprostanes and reactive aldehydes including 4-hydroxynonenal) are proapoptotic factors. These mediators can activate apoptosis via mitochondrial-, receptor-, or ER stress-dependent pathways. Phospholipid metabolism is also an essential regulator of apoptosis, producing the proapoptotic prostaglandins of the PGD and PGJ series, as well as the antiapoptotic prostaglandins of the PGE series, but also 12-HETE and 20-HETE. The effect of endocannabinoids and phytocannabinoids on apoptosis depends on cell type-specific differences. Cells where cannabinoid receptor type 1 (CB1) is the dominant cannabinoid receptor, as well as cells with high cyclooxygenase (COX) activity, undergo apoptosis after the administration of cannabinoids. In contrast, in cells where CB2 receptors dominate, and cells with low COX activity, cannabinoids act in a cytoprotective manner. Therefore, cell type-specific differences in the pro- and antiapoptotic effects of lipids and their (oxidative) products might reveal new options for differential bioanalysis between normal, functional, and degenerating or malignant cells, and better integrative biomedical treatments of major stress-associated diseases.
- apoptosis
- lipid mediators
- phospholipids
- ROS
- oxidative stress
- endocannabinoids
1. Introduction
2. Participation of Phospholipid Metabolism Products in Apoptosis
Lipid mediators, produced from phospholipids, are critical influencers of apoptosis. Their generation is enhanced by the action of ROS [47][12]. Phospholipids are structural elements of biological membranes, and the lipid bilayer represents an important platform for proteins involved in cell signaling that affect intercellular communication, gene expression, and immune response [48][13]. However, under the influence of biological, chemical, and physical pathological factors, membrane phospholipids are metabolized to lipid mediators through ROS- and enzyme-dependent mechanisms Figure 51. These mediators—through various metabolic pathways—can modulate the process of apoptosis [47][12].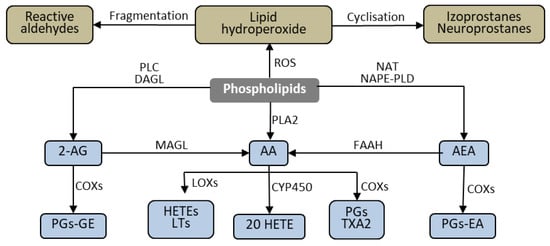
2.1. ROS-Dependent Lipid Peroxidation Products
2.2. Enzymes-Dependent Lipid Metabolism Products
2.2.1. Eicosanoids
2.2.2. Endocannabinoids
2.2.3. Exogenous Cannabinoids
2.3. Cross Talk between Lipids, Glucose, and Glutamine
References
- Suen, A.Y.W.; Baldwin, T.A. Proapoptotic protein Bim is differentially required during thymic clonal deletion to ubiquitous versus tissue-restricted antigens. Proc. Natl. Acad. Sci. USA 2012, 109, 893–898.
- Koyani, C.N.; Windischhofer, W.; Rossmann, C.; Jin, G.; Kickmaier, S.; Heinzel, F.R.; Groschner, K.; Alavian-Ghavanini, A.; Sattler, W.; Malle, E. 15-deoxy-Δ12,14-PGJ2 promotes inflammation and apoptosis in cardiomyocytes via the DP2/MAPK/TNFα axis. Int. J. Cardiol. 2014, 173, 472–480.
- Lee, C.-H.; Wu, S.-B.; Hong, C.-H.; Yu, H.-S.; Wei, Y.-H. Molecular Mechanisms of UV-Induced Apoptosis and Its Effects on Skin Residential Cells: The Implication in UV-Based Phototherapy. Int. J. Mol. Sci. 2013, 14, 6414–6435.
- Mahajan, A.; Herrmann, M.; Muñoz, L.E. Clearance Deficiency and Cell Death Pathways: A Model for the Pathogenesis of SLE. Front. Immunol. 2016, 7, 35.
- Gęgotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396.
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Dobrzyńska, I.; Skrzydlewska, E. Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox. Biol. 2017, 12, 733–744.
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827.
- Sznarkowska, A.; Olszewski, R.; Zawacka-Pankau, J. Pharmacological activation of tumor suppressor, wild-type p53 as a promising strategy to fight cancer. Postepy Hig. Med. Dosw. 2010, 64, 396–407.
- Lee, S.J.; Kim, M.S.; Park, J.Y.; Woo, J.S.; Kim, Y.K. 15-Deoxy-Δ12,14-prostaglandin J2 induces apoptosis via JNK-mediated mitochondrial pathway in osteoblastic cells. Toxicology 2008, 248, 121–129.
- Lamkanfi, M.; Kanneganti, T.-D. Caspase-7: A protease involved in apoptosis and inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 21–24.
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159.
- Soliman, E.; Henderson, K.L.; Danell, A.S.; Van Dross, R. Arachidonoyl-ethanolamide activates endoplasmic reticulum stress-apoptosis in tumorigenic keratinocytes: Role of cyclooxygenase-2 and novel J-series prostamides. Mol. Carcinog. 2015, 55, 117–130.
- Hu, J.; Lipowsky, R.; Weikl, T.R. Binding constants of membrane-anchored receptors and ligands depend strongly on the nanoscale roughness of membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 15283–15288.
- Pamplona, R. Membrane phospholipids, lipoxidative damage and molecular integrity: A causal role in aging and longevity. BBA-BIOENERGETICS 2008, 1777, 1249–1262.
- Gęgotek, A.; Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids 2019, 221, 46–52.
- Gęgotek, A.; Biernacki, M.; Ambrożewicz, E.; Surażyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117.
- Csala, M.; Kardon, T.; Legeza, B.; Lizák, B.; Mandl, J.; Margittai, É.; Puskás, F.; Száraz, P.; Szelényi, P.; Bánhegyi, G. On the role of 4-hydroxynonenal in health and disease. BBA-MOL. BASIS DIS. 2015, 1852, 826–838.
- Łuczaj, W.; Gęgotek, A.; Skrzydlewska, E. Antioxidants and HNE in redox homeostasis. Free Radic. Biol. Med. 2017, 111, 87–101.
- Łuczaj, W.; Skrzydlewska, E. DNA damage caused by lipid peroxidation products. Cell. Mol. Biol. Lett. 2003, 8, 391–413.
- Sharma, A.; Sharma, R.; Chaudhary, P.; Vatsyayan, R.; Pearce, V.; Jeyabal, P.V.S.; Zimniak, P.; Awasthi, S.; Awasthi, Y.C. 4-Hydroxynonenal induces p53-mediated apoptosis in retinal pigment epithelial cells. Arch. Biochem. Biophys. 2008, 480, 85–94.
- Shearn, C.T.; Fritz, K.S.; Reigan, P.; Petersen, D.R. Modification of Akt2 by 4-Hydroxynonenal Inhibits Insulin-Dependent Akt Signaling in HepG2 Cells. Biochemistry 2011, 50, 3984–3996.
- Ji, G.; Yu, N.; Xue, X.; Li, Z. 4-Hydroxy-2-nonenal Induces Apoptosis by Inhibiting AKT Signaling in Human Osteosarcoma Cells. Sci. World J. 2014, 2014, 873525.
- Abarikwu, S.O.; Pant, A.B.; Farombi, E.O. 4-Hydroxynonenal induces mitochondrial-mediated apoptosis and oxidative stress in SH-SY5Y human neuronal cells. Basic Clin. Pharmacol. Toxicol. 2012, 110, 441–448.
- Ji, Y.; Dai, Z.; Wu, G.; Wu, Z. 4-Hydroxy-2-nonenal induces apoptosis by activating ERK1/2 signaling and depleting intracellular glutathione in intestinal epithelial cells. Sci. Rep. 2016, 6, 32929.
- Lee, J.Y.; Je, J.H.; Kim, D.H.; Chung, S.W.; Zou, Y.; Kim, N.D.; Yoo, M.A.; Baik, H.S.; Yu, B.P.; Chung, H.Y. Induction of endothelial apoptosis by 4-hydroxyhexenal. Eur. J. Biochem. 2004, 271, 1339–1347.
- Kreuzer, T.; Grube, R.; Wutte, A.; Zarkovic, N.; Schaur, R.J. 4-Hydroxynonenal modifies the effects of serum growth factors on the expression of the c-fos proto-oncogene and the proliferation of HeLa carcinoma cells. Free Radic. Biol. Med. 1998, 25, 42–49.
- Zarkovic, N.; Ilic, Z.; Jurin, M.; Schaur, R.J.; Puhl, H.; Esterbauer, H. Stimulation of HeLa cell growth by physiological concentrations of 4-hydroxynonenal. Cell Biochem. Funct. 1993, 11, 279–286.
- Bauer, G.; Zarkovic, N. Revealing mechanisms of selective, concentration-dependent potentials of 4-hydroxy-2-nonenal to induce apoptosis in cancer cells through inactivation of membrane-associated catalase. Free Radic. Biol. Med. 2015, 81, 128–144.
- Gasparovic, A.C.; Milkovic, L.; Sunjic, S.B.; Zarkovic, N. Cancer growth regulation by 4-hydroxynonenal. Free Radic. Biol. Med. 2017, 111, 226–234.
- Zarkovic, K.; Jakovcevic, A.; Zarkovic, N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic. Biol. Med. 2017, 111, 110–126.
- Živković, N.P.; Petrovečki, M.; Lončarić, Č.T.; Nikolić, I.; Waeg, G.; Jaganjac, M.; Žarković, K.; Žarković, N. Positron emission tomography-computed tomography and 4-hydroxynonenal-histidine immunohistochemistry reveal differential onset of lipid peroxidation in primary lung cancer and in pulmonary metastasis of remote malignancies. Redox. Biol. 2017, 11, 600–605.
- Zhong, H.; Xiao, M.; Zarkovic, K.; Zhu, M.; Sa, R.; Lu, J.; Tao, Y.; Chen, Q.; Xia, L.; Cheng, S.; et al. Mitochondrial control of apoptosis through modulation of cardiolipin oxidation in hepatocellular carcinoma: A novel link between oxidative stress and cancer. Free Radic. Biol. Med. 2017, 102, 67–76.
- Milkovic, L.; Zarkovic, N.; Saso, L. Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox. Biol. 2017, 12, 727–732.
- Sovic, A.; Borovic, S.; Loncaric, I.; Kreuzer, T.; Zarkovic, K.; Vukovic, T.; Wäg, G.; Hrascan, R.; Wintersteiger, R.; Klinger, R.; et al. The carcinostatic and proapoptotic potential of 4-hydroxynonenal in HeLa cells is associated with its conjugation to cellular proteins. Anticancer. Res. 2001, 21, 1997–2004.
- Borovic, S.; Cipak, A.; Meinitzer, A.; Kejla, Z.; Perovic, D.; Waeg, G.; Zarkovic, N. Differential sensitivity to 4-hydroxynonenal for normal and malignant mesenchymal cells. Redox. Rep. 2007, 12, 50–54.
- Zarkovic, K.; Larroque-Cardoso, P.; Pucelle, M.; Salvayre, R.; Waeg, G.; Nègre-Salvayre, A.; Zarkovic, N. Elastin aging and lipid oxidation products in human aorta. Redox. Biol. 2014, 4, 109–117.
- Jaganjac, M.; Milkovic, L.; Gegotek, A.; Cindric, M.; Zarkovic, K.; Skrzydlewska, E.; Zarkovic, N. The relevance of pathophysiological alterations in redox signaling of 4-hydroxynonenal for pharmacological therapies of major stress-associated diseases. Free Radic. Biol. Med. 2019.
- Milkovic, L.; Cipak Gasparovic, A.; Zarkovic, N. Overview on major lipid peroxidation bioactive factor 4-hydroxynonenal as pluripotent growth-regulating factor. Free Radic. Res. 2015, 49, 850–860.
- Milkovic, L.; Siems, W.; Siems, R.; Zarkovic, N. Oxidative stress and antioxidants in carcinogenesis and integrative therapy of cancer. Curr. Pharm. Des. 2014, 20, 6529–6542.
- Cesar, V.; Jozić, I.; Begović, L.; Vuković, T.; Mlinarić, S.; Lepeduš, H.; Borović Šunjić, S.; Žarković, N. Cell-Type-Specific Modulation of Hydrogen Peroxide Cytotoxicity and 4-Hydroxynonenal Binding to Human Cellular Proteins In Vitro by Antioxidant Aloe vera Extract. Antioxidants 2018, 7, 125.
- Luo, H.; Zhai, L.; Yang, H.; Xu, L.; Liu, J.; Liang, H.; Tang, H. Dichloroacetonitrile induces cytotoxicity through oxidative stress-mediated and p53-dependent apoptosis pathway in LO2 cells. Toxicol. Mech. Methods. 2017, 27, 575–581.
- Yan, P.; Tang, S.; Zhang, H.; Guo, Y.; Zeng, Z.; Wen, Q. Palmitic acid triggers cell apoptosis in RGC-5 retinal ganglion cells through the Akt/FoxO1 signaling pathway. Metab. Brain. Dis. 2017, 32, 453–460.
- Sun, J.; Wei, X.; Lu, Y.; Cui, M.; Li, F.; Lu, J.; Liu, Y.; Zhang, X. Glutaredoxin 1 (GRX1) inhibits oxidative stress and apoptosis of chondrocytes by regulating CREB/HO-1 in osteoarthritis. Mol. Immunol. 2017, 90, 211–218.
- Syta-Krzyżanowska, A.; Jarocka-Karpowicz, I.; Kochanowicz, J.; Turek, G.; Rutkowski, R.; Gorbacz, K.; Mariak, Z.; Skrzydlewska, E. F2-isoprostanes and F4-neuroprostanes as markers of intracranial aneurysm development. Adv. Clin. Exp. Med. 2018, 27, 673–680.
- Musiek, E.S.; Breeding, R.S.; Milne, G.L.; Zanoni, G.; Morrow, J.D.; McLaughlin, B. Cyclopentenone isoprostanes are novel bioactive products of lipid oxidation which enhance neurodegeneration. J. Neurochem. 2006, 97, 1301–1313.
- El-Osta, H.; Circu, M.L. Mitochondrial ROS and Apoptosis. In Mitochondrial Mechanisms of Degeneration and Repair in Parkinson’s Disease; Buhlman, L.M., Ed.; Springer International Publishing: Cham, Switzerland, 2016.
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox. Biol. 2018.
- Bauer, J.; Ripperger, A.; Frantz, S.; Ergün, S.; Schwedhelm, E.; Benndorf, R.A. Pathophysiology of isoprostanes in the cardiovascular system: Implications of isoprostane-mediated thromboxane A2 receptor activation. Br. J. Pharmacol. 2014, 171, 3115–3131.
- Xue, L.; Barrow, A.; Pettipher, R. Novel Function of CRTH2 in Preventing Apoptosis of Human Th2 Cells through Activation of the Phosphatidylinositol 3-Kinase Pathway. J. Immunol. 2009, 182, 7580–7586.
- Czapski, G.A.; Czubowicz, K.; Strosznajder, J.B.; Strosznajder, R.P. The Lipoxygenases: Their Regulation and Implication in Alzheimer’s Disease. Neurochem. Res. 2016, 41, 243–257.
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523.
- Morimoto, K.; Shirata, N.; Taketomi, Y.; Tsuchiya, S.; Segi-Nishida, E.; Inazumi, T.; Kabashima, K.; Tanaka, S.; Murakami, M.; Narumiya, S.; et al. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J. Immunol. 2014, 192, 1130–1137.
- Schratl, P.; Royer, J.F.; Kostenis, E.; Ulven, T.; Sturm, E.M.; Waldhoer, M.; Hoefler, G.; Schuligoi, R.; Lippe, I.T.; Peskar, B.A.; et al. The Role of the Prostaglandin D2 Receptor, DP, in Eosinophil Trafficking. J. Immunol. 2007, 179, 4792–4799.
- Bittleman, D.B.; Casale, T.B. 5-Hydroxyeicosatetraenoic acid (HETE)-induced neutrophil transcellular migration is dependent upon enantiomeric structure. Am. J. Respir. Cell Mol. Biol. 1995, 12, 260–267.
- Caramia, G. The essential fatty acids omega-6 and omega-3: From their discovery to their use in therapy. Minerva Pediatr. 2008, 60, 219–233.
- Zhu, F.; Wang, P.; Kontrogianni-Konstantopoulos, A.; Konstantopoulos, K. Prostaglandin (PG)D2 and 15-deoxy-Δ12,14-PGJ2, but not PGE2, Mediate Shear-Induced Chondrocyte Apoptosis via Protein Kinase A-dependent Regulation of Polo-like Kinases. Cell Death Differ. 2010, 17, 1325–1334.
- Luo, G.; Li, F.; Li, X.; Wang, Z.-G.; Zhang, B. TNF-α and RANKL promote osteoclastogenesis by upregulating RANK via the NF-κB pathway. Mol. Med. Rep. 2018, 17, 6605–6611.
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286.
- Sperandio, M.; Demasi, A.P.D.; Martinez, E.F.; Saad, S.O.; Pericole, F.V.; Vieira, K.P.; Freitas, N.S.; Araújo, V.C.; Brown, A.L.; Clemente-Napimoga, J.T.; et al. 15d-PGJ2 as an endoplasmic reticulum stress manipulator in multiple myeloma in vitro and in vivo. Exp. Mol. Pathol. 2017, 102, 434–445.
- Zuo, S.; Kong, D.; Wang, C.; Liu, J.; Wang, Y.; Wan, Q.; Yan, S.; Zhang, J.; Tang, J.; Zhang, Q.; et al. CRTH2 promotes endoplasmic reticulum stress-induced cardiomyocyte apoptosis through m-calpain. EMBO Mol. Med. 2018, 10, e8237.
- Rasheed, Z.; Haqqi, T.M. Endoplasmic reticulum stress induces the expression of COX-2 through activation of eIF2α, p38-MAPK and NF-κB in advanced glycation end products stimulated human chondrocytes. Biochim. Biophys. Acta. 2012, 1823, 2179–2189.
- Yue, L.; Haroun, S.; Parent, J.-L.; de Brum-Fernandes, A.J. Prostaglandin D(2) induces apoptosis of human osteoclasts through ERK1/2 and Akt signaling pathways. Bone 2014, 60, 112–121.
- Shen, Z.-N.; Nishida, K.; Doi, H.; Oohashi, T.; Hirohata, S.; Ozaki, T.; Yoshida, A.; Ninomiya, Y.; Inoue, H. Suppression of chondrosarcoma cells by 15-deoxy-Δ12,14-prostaglandin J2 is associated with altered expression of Bax/Bcl-xL and p21. Biochem. Biophys. Res. Commun. 2005, 328, 375–382.
- Jung, W.-K.; Park, I.-S.; Park, S.-J.; Yea, S.S.; Choi, Y.H.; Oh, S.; Park, S.-G.; Choi, I.-W. The 15-Deoxy-Δ12,14-prostaglandin J2 inhibits LPS-stimulated AKT and NF-κB activation and suppresses interleukin-6 in osteoblast-like cells MC3T3E-1. Life Sci. 2009, 85, 46–53.
- Chen, Y.; Medhora, M.; Falck, J.R.; Pritchard, K.A.; Jacobs, E.R. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L378–L385.
- Munkarah, A.R.; Morris, R.; Baumann, P.; Deppe, G.; Malone, J.; Diamond, M.P.; Saed, G.M. Effects of prostaglandin E(2) on proliferation and apoptosis of epithelial ovarian cancer cells. J. Soc. Gynecol. Investig. 2002, 9, 168–173.
- Kalouche, G.; Boucher, C.; Coste, A.; Debussche, L.; Orsini, C.; Baudouin, C.; Debeir, T.; Vigé, X.; Rostène, W. Prostaglandin EP2 receptor signaling protects human trabecular meshwork cells from apoptosis induced by ER stress through down-regulation of p53. BBA-MOL. CELL. RES. 2016, 1863, 2322–2332.
- Li, F.; You, Y.; Zhu, H. 15-HETE protects pulmonary artery smooth muscle cells against apoptosis via SIRT1 regulation during hypoxia. Biomed. Pharmacother. 2018, 108, 325–330.
- Liu, Q.; Tan, W.; Che, J.; Yuan, D.; Zhang, L.; Sun, Y.; Yue, X.; Xiao, L.; Jin, Y. 12-HETE facilitates cell survival by activating the integrin-linked kinase/NF-κB pathway in ovarian cancer. Cancer. Manag. Res. 2018, 10, 5825–5838.
- Dhanasekaran, A.; Bodiga, S.; Gruenloh, S.; Gao, Y.; Dunn, L.; Falck, J.R.; Buonaccorsi, J.N.; Medhora, M.; Jacobs, E.R. 20-HETE increases survival and decreases apoptosis in pulmonary arteries and pulmonary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H777–H786.
- Omori, K.; Morikawa, T.; Kunita, A.; Nakamura, T.; Aritake, K.; Urade, Y.; Fukayama, M.; Murata, T. Lipocalin-type prostaglandin D synthase-derived PGD2 attenuates malignant properties of tumor endothelial cells. J. Pathol. Bacteriol. 2018, 244, 84–96.
- Ibsen, M.S.; Connor, M.; Glass, M. Cannabinoid CB1 and CB2 Receptor Signaling and Bias. Cannabis Cannabinoid Res. 2017, 2, 48–60.
- Costa, M.A.; Fonseca, B.M.; Teixeira, N.A.; Correia-da-Silva, G. The endocannabinoid anandamide induces apoptosis in cytotrophoblast cells: Involvement of both mitochondrial and death receptor pathways. Placenta 2015, 36, 69–76.
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. The endocannabinoid anandamide induces apoptosis of rat decidual cells through a mechanism involving ceramide synthesis and p38 MAPK activation. Apoptosis 2013, 18, 1526–1535.
- Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Mackie, K.; Pacher, P. Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells. Br. J. Pharmacol. 2010, 160, 688–700.
- Orellana-SerradelL, O.; Pooblete, C.E.; Sanchez, C.; Castellón, E.A.; Gallegos, I.; Huidobro, C.; Llanos, M.N.; Conteras, H.R. Proapoptotic effect of endocannabinoids in prostate cancer cells. Oncol. Rep. 2015, 33, 1599–1608.
- Signorello, M.G.; Leoncini, G. Effect of 2-arachidonoylglycerol on myosin light chain phosphorylation and platelet activation: The role of phosphatidylinositol 3 kinase/AKT pathway. Biochimie 2014, 105, 182–191.
- Blázquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; García-Rincón, D.; Sendtner, M.; Timmusk, T.; Lutz, B.; Galve-Roperh, I.; et al. The CB1 cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015, 22, 1618–1629.
- Pokrywka, M.; Góralska, J.; Solnica, B. Cannabinoids—a new weapon against cancer? Postepy. Hig. Med. Dosw. 2016, 70, 1309–1320.
- Salazar, M.; Carracedo, A.; Salanueva, Í.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 2009, 119, 1359–1372.
- Greijer, A.E.; van der Wall, E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 2004, 57, 1009–1014.
- Abán, C.; Martinez, N.; Carou, C.; Albamonte, I.; Toro, A.; Seyahian, A.; Franchi, A.; Leguizamón, G.; Trigubo, D.; Damiano, A.; et al. Endocannabinoids participate in placental apoptosis induced by hypoxia inducible factor-1. Apoptosis 2016, 21, 1094–1105.
- Sun, H.-J.; Lu, Y.; Wang, H.-W.; Zhang, H.; Wang, S.-R.; Xu, W.-Y.; Fu, H.-L.; Yao, X.-Y.; Yang, F.; Yuan, H.-B. Activation of Endocannabinoid Receptor 2 as a Mechanism of Propofol Pretreatment-Induced Cardioprotection against Ischemia-Reperfusion Injury in Rats. Oxid. Med. Cell Longev. 2017, 2017, 2186383.
- Zhou, H.; Yang, W.; Li, Y.; Ren, T.; Peng, L.; Guo, H.; Liu, J.; Zhou, Y.; Zhao, Y.; Yang, L.; et al. Oleoylethanolamide attenuates apoptosis by inhibiting the TLR4/NF-κB and ERK1/2 signaling pathways in mice with acute ischemic stroke. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 77–84.
- Cui, H.-J.; Liu, S.; Yang, R.; Fu, G.-H.; Lu, Y. N-stearoyltyrosine protects primary cortical neurons against oxygen-glucose deprivation-induced apoptosis through inhibiting anandamide inactivation system. Neurosci. Res. 2017, 123, 8–18.
- Biernacki, M.; Łuczaj, W.; Jarocka-Karpowicz, I.; Ambrożewicz, E.; Toczek, M.; Skrzydlewska, E. The Effect of Long-Term Administration of Fatty Acid Amide Hydrolase Inhibitor URB597 on Oxidative Metabolism in the Heart of Rats with Primary and Secondary Hypertension. Molecules 2018, 23, 2350.
- Biernacki, M.; Ambrożewicz, E.; Gęgotek, A.; Toczek, M.; Bielawska, K.; Skrzydlewska, E. Redox system and phospholipid metabolism in the kidney of hypertensive rats after FAAH inhibitor URB597 administration. Redox. Biol. 2018, 15, 41–50.
- Biernacki, M.; Skrzydlewska, E. Metabolism of endocannabinoids. Postepy. Hig. Med. Dosw. 2016, 70, 830–843.
- Siegmund, S.V.; Wojtalla, A.; Schlosser, M.; Schildberg, F.A.; Knolle, P.A.; Nüsing, R.M.; Zimmer, A.; Strassburg, C.P.; Singer, M.V. Cyclooxygenase-2 contributes to the selective induction of cell death by the endocannabinoid 2-arachidonoyl glycerol in hepatic stellate cells. Biochem. Biophys. Res. Commun. 2016, 470, 678–684.
- Kuc, C.; Jenkins, A.; Van Dross, R.T. Arachidonoyl ethanolamide (AEA)-induced Apoptosis is Mediated by J-series Prostaglandins and is Enhanced by Fatty Acid Amide Hydrolase (FAAH) Blockade. Mol. Carcinog. 2012, 51, 139–149.
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Patel, V.; Saito, K.; Matsumoto, S.; Kashiwaya, Y.; Horváth, B.; Mukhopadhyay, B.; Becker, L.; et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J. Am. Coll. Cardiol. 2010, 56, 2115–2125.
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141.
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol. Pharmacol. 2006, 70, 897–908.
- Wu, H.-Y.; Chang, A.-C.; Wang, C.-C.; Kuo, F.-H.; Lee, C.-Y.; Liu, D.-Z.; Jan, T.-R. Cannabidiol induced a contrasting pro-apoptotic effect between freshly isolated and precultured human monocytes. Toxicol. Appl. Pharmacol. 2010, 246, 141–147.
- Sultan, A.S.; Marie, M.A.; Sheweita, S.A. Novel mechanism of cannabidiol-induced apoptosis in breast cancer cell lines. Breast 2018, 41, 34–41.
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; McAllister, S.; Soroceanu, L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015, 6, e1601.
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-talk between Apoptosis and Autophagy. Mol. Cancer. Ther. 2011, 10, 1161–1172.
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066.
- Kim, J.L.; Kim, B.R.; Kim, D.Y.; Jeong, Y.A.; Jeong, S.; Na, Y.J.; Park, S.H.; Yun, H.K.; Jo, M.J.; Kim, B.G.; et al. Cannabidiol Enhances the Therapeutic Effects of TRAIL by Upregulating DR5 in Colorectal Cancer. Cancers 2019, 11, 642.
- Kisková, T.; Mungenast, F.; Suváková, M.; Jäger, W.; Thalhammer, T. Future Aspects for Cannabinoids in Breast Cancer Therapy. Int. J. Mol. Sci 2019, 20, 1673.
- Mecha, M.; Torrao, A.S.; Mestre, L.; Carrillo-Salinas, F.J.; Mechoulam, R.; Guaza, C. Cannabidiol protects oligodendrocyte progenitor cells from inflammation-induced apoptosis by attenuating endoplasmic reticulum stress. Cell Death Dis. 2012, 3, e331.
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-γ Confer Cannabidiol-Induced Apoptosis of Human Lung Cancer Cells. Mol. Cancer Ther. 2013, 12, 69–82.
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q.; et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules 2019, 9, 302.
- Do, Y.; McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Activation through Cannabinoid Receptors 1 and 2 on Dendritic Cells Triggers NF-κB-Dependent Apoptosis: Novel Role for Endogenous and Exogenous Cannabinoids in Immunoregulation. J. Immunol. 2004, 173, 2373–2382.
- Herrera, B.; Carracedo, A.; Diez-Zaera, M.; Gómez del Pulgar, T.; Guzmán, M.; Velasco, G. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp. Cell Res. 2006, 312, 2121–2131.
- Coe, G.L.; Redd, P.S.; Paschall, A.V.; Lu, C.; Gu, L.; Cai, H.; Albers, T.; Lebedyeva, I.O.; Liu, K. Ceramide mediates FasL-induced caspase 8 activation in colon carcinoma cells to enhance FasL-induced cytotoxicity by tumor-specific cytotoxic T lymphocytes. Sci. Rep. 2016, 6, 30816.
- Pedrycz, A.; Siermontowski, P.; Lonc, G.; Tomasiak, M. Zewnętrzna droga indukcji sygnału do apoptozy: Receptory śmierci. Pol. Hyperb. Res. 2012, 4, 147–157.
- Chipuk, J.E.; McStay, G.P.; Bharti, A.; Kuwana, T.; Clarke, C.J.; Siskind, L.J.; Obeid, L.M.; Green, D.R. Sphingolipid Metabolism Cooperates with BAK and BAX to Promote the Mitochondrial Pathway of Apoptosis. Cell 2012, 148, 988–1000.
- Blázquez, C.; Galve-Roperh, I.; Guzmán, M. De novo-synthesized ceramide signals apoptosis in astrocytes via extracellular signal-regulated kinase. FASEB J. 2000, 14, 2315–2322.
- Lorente, M.; Torres, S.; Salazar, M.; Carracedo, A.; Hernández-Tiedra, S.; Rodríguez-Fornés, F.; García-Taboada, E.; Meléndez, B.; Mollejo, M.; Campos-Martín, Y.; et al. Stimulation of the midkine/ALK axis renders glioma cells resistant to cannabinoid antitumoral action. Cell Death Differ. 2011, 18, 959–973.
- Niaudet, C.; Bonnaud, S.; Guillonneau, M.; Gouard, S.; Gaugler, M.-H.; Dutoit, S.; Ripoche, N.; Dubois, N.; Trichet, V.; Corre, I.; et al. Plasma membrane reorganization links acid sphingomyelinase/ceramide to p38 MAPK pathways in endothelial cells apoptosis. Cell. Signal. 2017, 33, 10–21.
- Alnahdi, A.; John, A.; Raza, H. Augmentation of Glucotoxicity, Oxidative Stress, Apoptosis and Mitochondrial Dysfunction in HepG2 Cells by Palmitic Acid. Nutrients 2019, 11, 1979.
- El-Assaad, W.; Buteau, J.; Peyot, M.-L.; Nolan, C.; Roduit, R.; Hardy, S.; Joly, E.; Dbaibo, G.; Rosenberg, L.; Prentki, M. Saturated Fatty Acids Synergize with Elevated Glucose to Cause Pancreatic β-Cell Death. Endocrinology 2003, 144, 4154–4163.
- Kim, J.-W.; Yoon, K.-H. Glucolipotoxicity in Pancreatic β-Cells. Diabetes Metab. J. 2011, 35, 444–450.
- Solinas, G.; Naugler, W.; Galimi, F.; Lee, M.-S.; Karin, M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proc. Natl. Acad. Sci. USA 2006, 103, 16454–16459.
- Kang, S.; Song, J.H.; Kang, H.; Kim, S.; Lee, Y.; Park, D.-B. Insulin can block apoptosis by decreasing oxidative stress via phosphatidylinositol 3-kinase- and extracellular signal-regulated protein kinase-dependent signaling pathways in HepG2 cells. Eur. J. Endocrinol. 2003, 148, 147–155.
- Kaifu, K.; Kiyomoto, H.; Hitomi, H.; Matsubara, K.; Hara, T.; Moriwaki, K.; Ihara, G.; Fujita, Y.; Sugasawa, N.; Nagata, D.; et al. Insulin attenuates apoptosis induced by high glucose via the PI3-kinase/Akt pathway in rat peritoneal mesothelial cells. Nephrol. Dial. Transplant. 2009, 24, 809–815.
- Wang, Y.; Liu, N.; Zhu, W.; Zhang, K.; Si, J.; Bi, M.; Lv, X.; Wang, J. Protective effect of β-hydroxybutyrate on glutamate induced cell death in HT22 cells. Int. J. Clin. Exp. Med. 2016, 9, 23433–23439.
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47.
- Halama, A.; Kulinski, M.; Dib, S.S.; Zaghlool, S.B.; Siveen, K.S.; Iskandarani, A.; Satheesh, N.J.; Bhagwat, A.M.; Uddin, S.; Kastenmüeller, G.; et al. Accelerated lipid catabolism and autophagy are cancer survival mechanisms under inhibited glutaminolysis. bioRxiv 2018.
