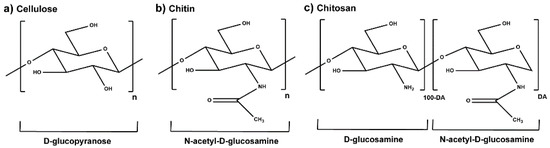
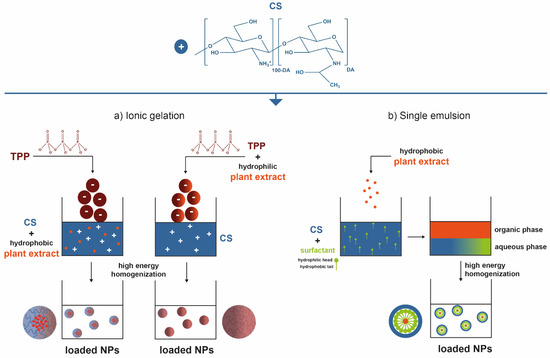
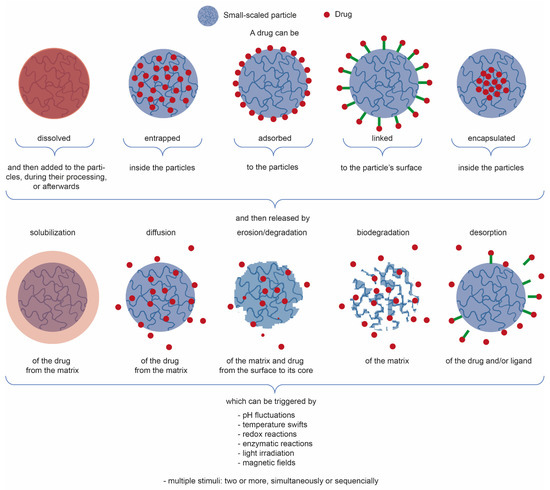
Plant extracts are widely used as natural drugs in conventional medicine, given their high availability from nature (e.g., seeds, bark, wood, roots, leaves, flowers, and fruits), bioactivity, operating facility, reduced capital costs, and scalability
[31][32][65,66]. Plants synthetize a large panoply of structurally different compounds such as simple phenols and phenolic acids, quinones, flavonoids, tannins, coumarins, terpenes and terpenoids, alkaloids, lectins and polypeptides, among other phytochemicals, each having a specific and distinct role in the plant’s bioactivity
[32][33][34][35][36][37][66,67,68,69,70,71]. Some, such as terpenoids, also give plants their odors; others (quinones and tannins) offer to plants their pigmentation
[35][69]. These biomolecules can exert strong antioxidant, anticancer, anti-inflammatory, and antimicrobial properties at their site of action
[14][38][39][40][41][14,72,73,74,75]. Their ability to inactivate free radicals is mostly mediated by phenolic biomolecules within its composition, namely the hydrogen atoms of the adjacent hydroxyl groups (o-diphenol), the double bonds of the benzene ring, and the double bond of the oxo functional group of some flavonoids. They reduce tissue lipid oxidation, this way delaying aging, decreasing inflammation, oxidative stress, as well as the chances of developing some diseases, namely cardiovascular pathologies (e.g., myocardial infarction and atherosclerosis), cancer, metabolic (e.g., diabetes) and neurological disorders (e.g., depression)
[14][36][42][14,70,76]. Plant-based metabolites act as defense mechanisms against invasive microorganisms, insects, and herbivores. They wield antibacterial activity via multiple mechanisms, acting in consonance for increased host protection. Their chemical versatility has additionally enabled the synthesis of a large variety of functionalized skeletons. Modes of action are variable, yet potent
[43][44][45][46][77,78,79,80]. Inhibition of cell wall synthesis, permeabilization and disintegration of bacterial peripheral layers, restriction of bacterial physiology, oxygen uptake and oxidative phosphorylation, efflux pump inhibition, modulation of antibiotic susceptibility, biofilm inhibition, hindrance of the microbial protein adhesion to the host’s polysaccharide receptors, and attenuation of bacterial virulence, are known and acclaimed mechanisms of action of such elements
[33][35][36][47][67,69,70,81]. Compounds such as lectins even allow specific recognition and reversible interaction to either free carbohydrates or glycoconjugates, without modifying their structure. They may form ion channels in the microbial membrane or inhibit adhesion of microbial proteins to host polysaccharide receptors. Hence, they are capable of precipitating polysaccharides and glycoproteins or agglutinating cells
[48][49][50][51][82,83,84,85]. Overall, these changes are mostly induced by hydrophobic effects, covalent binding and hydrogen binding of their phenolic compounds
[35][69]. The multitarget action of plant extracts, unlikely to induce resistance
[52][86], has the potential to surpass the current clinical failures posed by traditional antibiotics
[32][66]. For instance, gallic acid (a phenolic acid), while loaded into CS-based NPs and dispersed within collagen and fibrin hydrogels
[53][87], has shown an excellent DPPH (2,2-Diphenyl-2-picryl hydrazyl hydrate) radical scavenging activity even at the lowermost concentration of 0.05 mg/mL, strongly contributing for a faster re-epithelialization and wound contraction, qualities that are highly valued for wound dressing applications. In another study
[54][88], thyme-essential-oil-loaded CS NPs and nanocapsules, rich in thymol and carvacrol (simple phenols), exhibited an antibacterial action dependent on thymol and carvacrol release rate, with 100% phenol release in 5 h (rather than 10 h) evoking 50% larger ZoIs, thus reinforcing their importance in the field. Authors indicated that studies related to mechanism of action on bacteria were ongoing. A final example described cinnamaldehyde combination with CS in the form of NPs via Schiff reaction between the free amine groups of CS and the aldehyde group of the phenylpropanoid
[55][89]. It substantially enhanced CS’s antibacterial capacity, additionally improving the stability of the CS NPs. The bacterial growth inhibition was 33–34% higher for grafted CS than for the unmodified polysaccharide-based NPs. Lectins and polypeptides were excluded from the table, given that they have more complex structures than the other cited classes. Regardless, these proteins or glycoproteins are often positively charged, with disulphide bonds. Concanavalin A and galectin-1 are well-known examples, having as ligands Manα1-OCH
3 and Gal(β1→4)Glc, respectively
[48][49][50][51][56][82,83,84,85,90].