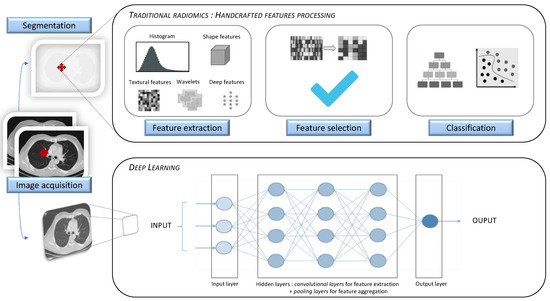
Artificial intelligence (AI) has increasingly been serving the field of radiology over the last 50 years. As modern medicine is evolving towards precision medicine, offering personalized patient care and treatment, the requirement for robust imaging biomarkers has gradually increased. Radiomics, a specific method generating high-throughput extraction of a tremendous amount of quantitative imaging data using data-characterization algorithms, has shown great potential in individuating imaging biomarkers. Radiomic analysis can be implemented through the following two methods: hand-crafted radiomic features extraction or deep learning algorithm. Its application in lung diseases can be used in clinical decision support systems, regarding its ability to develop descriptive and predictive models in many respiratory pathologies.
- radiomics
- artificial intelligence
- lung diseases
- precision medicine
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
| Terminology Used in Radiomics and AI | |
|---|---|
| Artificial intelligence | |
| Study | Description | Cohort | Performance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Wide-ranging branch of computer science, generating complex software that perform tasks that would typically have required human intelligence, by sensing and responding to a feature of their environment. | |||||||||
| Chen et al. (2018) [7] |
| 33 benign CT 42 malignant CT |
Benign vs. malignant Accuracy 84% Sensitivity 92.85% Specificity 72.73% |
||||||
| CAD (Computer Aided Detection or Diagnosis) | |||||||||
| De Koning et al. (2020) | Technology combining elements of artificial intelligence with radiological and pathology image processing. Its aim is to assist in the detection and/or diagnosis of diseases, improving the accuracy of radiologists with a reduction in time in the interpretation of images. | ||||||||
| [8] | De Koning et al. (2020) [9] |
| 15,792 patients | Benign vs. malignant: impact on mortality At 10 years, cancer mortality = 2.5 deaths/100,000 persons/years (screening group) vs. 3.3 deaths/100,000 (no-screening group) Cumulative ratio 0.76 ( | p | = 0.01) | Radiomics | ||
| Ma et al. (2016) [9 | Method that extracts a large number of quantitative features from radiographic medical images using data-characterization algorithms, to help in disease diagnosis and prognosis. | ||||||||
| ] | Ma et al. (2016) [10] |
| Machine Learning | Hawkins et al. (2016) [10] | Hawkins et al. (2016) [11Field in artificial intelligence studying computer algorithms that improve automatically through experience, by building a model based on sample data, known as “training data”, in order to make predictions or decisions. Supervised learning: The computer receives example inputs and their foreseen outputs. Its goal is to learn a general and reproducible function that links inputs to outputs. Unsupervised learning: The computer receives no labels to the learning algorithm for previously undetected patterns in a data set, leaving it on its own to find structure in its input. |
||||
| 36 benign CT | 94 malignant CT | Benign vs. malignant Accuracy 82.7% Sensitivity 80% Specificity 85.5% |
] |
| 328 benign CT 170 malignant CT |
Benign vs. malignant Accuracy 80% |
|||
| |||||||||
| Transient PSNs 39 benign CT Persistent PSNs 17 benign CT 30 malignant CT |
Prediction of persistent part-solid nodules AUC 0.93 if texture analysis was combined to clinical and CT features |
|||||||
| Autrusseau et al. (2021) [18] | Autrusseau et al. (2021) [17] |
| 99 lung nodules |
| Concordance between FD and ULD chest CT in radiomic-guided nodule risk assessment ICC of 0.82, displaying a good agreement in malignancy similarity index between ULD and FD chest CT |
2.2. Cancer
| Study | Description | Cohort | Performance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wu et al. (2016) [24] | Wu et al. (2016) [23] |
| Training cohort 198 malignant CT Validation cohort 152 malignant CT |
Tumor histology correlation AUC 0.72 |
||||||||
| Yu et al. (2019) [31] | Yu et al. (2019) [34] |
| Training cohort 87 NSCLC CT Validation cohort 58 NSCLC CT |
Diagnosis and staging in NSCLC AUC > 0.70, with predictive accuracy higher in lung adenocarcinoma than in lung squamous cell carcinoma |
||||||||
| Liu et al. (2016) [32] | Liu et al. (2016) [36] |
| 298 malignant CT | Prediction of mutation status AUC EGFR+ status prediction 0.647, improved to 0.709 when adding a clinical model |
||||||||
| Rios Velasquez et al. (2017) [33] | Rios Velasquez et al. (2017) [37] |
| ||||||||||
| Convolutional neural networks | Class of deep neural networks, which have the particularity of being fully connected networks. It gives them the advantage of understanding the hierarchical pattern in data and assembling more complex patterns using smaller and simpler patterns. | |||||||||||
| Training cohort 353 malignant CT Validation cohort |
Huang et al. (2018) [11] | Huang et al. (2018) [12] |
| Training cohort 70 benign CT 70 malignant CT Validation cohort 26 benign CT 20 malignant CT |
Benign vs. malignant Accuracy 91% |
Voxel | Single sample, or data point, on a regularly spaced, three-dimensional grid. In CT scans, the values of voxels are Hounsfield units. A voxel is a 3D pixel. |
||||
| ROI (Region of Interest) | Image areas containing the information relevant to image processing. | |||||||||||
| Skew of histogram | Measure of the asymmetry of attenuation distribution. The lung normal attenuation histogram is skewed to the left. There is a decreased leftward skewness in IPF. |
|||||||||||
| Kurtosis of histogram | Measurement of how sharp an attenuation distribution curve is. Kurtosis is abnormally low in idiopathic pulmonary fibrosis (IPF). |
|||||||||||
| Threshold measurement | Total count of pixels/voxels above or below a specific attenuation value that determines a relative volume. Threshold measures in emphysema quantifies the extent of emphysema according to a specific index of −950 Hounsfield units (HU). |
|||||||||||
| Texture analysis | Statistical methods that evaluate spatial relationship between voxels in an ROI, in order to characterize textural features of the parenchyma and give information about heterogeneity. |
2. The Role of Radiomics in Lung Diseases
2.1. Lung Nodules
| 352 malignant CT | |||||||||
| Prediction of mutation status | AUC EGFR + versus EGFR− status 0.70 | AUC KRAS + versus KRAS− status 0.63 AUC EGFR+ versus KRAS+ status 0.80 |
|||||||
| Tang et al. (2018) [34] | Tang et al. (2018) [39] |
| Sensitivity 95% Specificity 88% |
||||||
| Training cohort 114 malignant CT Validation cohort 176 malignant CT |
Prediction of immune modulator status Favorable outcome in low CT intensity and high heterogeneity with low PDL 1 and high CD3 |
Uthoff et al. (2020) [12] | Uthoff et al. (2020) [13] | |||||
| Wu et al. (2020) [35] | Wu et al. (2020) [40] |
|
|
Training cohort 289 benign CT 74 malignant CT Validation cohort 50 benign CT 50 malignant CT |
Benign vs. malignant | Training cohort 229 NSCLC Validation cohort 68 NSCLC Accuracy 98% Sensitivity 100% Specificity 96% |
|||
| Prediction of invasiveness | AUC 0.98 for the model combining ground-glass and solid features | Improvement of 0.14 in AUC when adding ground-glass radiomic features to solid features |
Mao et al. (2019) [13] | Mao et al. (2019) [14] | |||||
| Coroller et al. (2015) [36] | Coroller et al. (2015) [41] |
|
| Training cohort 156 benign CT 40 malignant CT Validation cohort 75 benign CT 23 malignant CT |
Training cohort 98 malignant CT Validation cohort 84 malignant CTBenign vs. malignant Accuracy 89.8% Sensitivity 81% Specificity 92.2% |
||||
| Prediction of distant metastasis | A multivariate radiomic signature (3 features) yielded a high prognostic performance for distant metastasis (CI 0.61) | Maldonado et al. (2021) [14] | Maldonado et al. (2021) [18] | ||||||
| He et al. (2019) [37] | He et al. (2019) [43] |
|
|
|
Validation cohort 91 malignant CT 79 benign CT |
Benign vs. malignant | Training cohort 423 NSCLC CT Validation cohort 294 NSCLC CT AUC 0.90 Sensitivity 92.3% Specificity 62% |
||
| Prediction of lymph node metastasis | Good discrimination for the model defining a radiomics-based predictive score (C index 0.785) | Mehta et al. (2021) [15] | Mehta et al. (2021) [22] | ||||||
| Ferreira et al. (2018) [38] | Ferreira et al. (2018) [45] |
|
|
| 1018 CTs Malignancy rating from 1 to 5 |
Training cohort 52 malignant CT Validation cohort 16 malignant CTBenign vs. malignant AUC 0.87 on fully supervised 3D CNN + random forest model (images, biomarkers and volumetric features) AUC 0.93 on semi-supervised random forest (biomarkers only) |
|||
| Histology and distant metastasis | AUC lymph nodal metastasis 0.89 | AUC distant metastasis 0.97 AUC histopathology 0.92 |
Digumarthy et al. (2019) [16] | Digumarthy et al. (2019) [24] |
| ||||
| Mattonen et al. (2016) [39] | Mattonen et al. (2016) [46] |
| 182 malignant CT | Prediction of recurrence after SBRT AUC 0.85 (radiomic signature of 5 features predicting local recurrence) |
|||||
| Coroller et al. (2016) [40] | Coroller et al. (2016) [49] |
| 127 malignant CT Training cohort 80% Validation cohort 20% |
Prediction of response after NCT AUC for pathologic gross residual disease prediction (7 features) > 0.6 AUC for pathologic complete response (1 feature) 0.63 AUC for poor response 0.63 (spherical disproportionality) or 0.61 (heterogeneous texture) |
|||||
| Kim et al. (2017) [41] | Kim et al. (2017) [50] |
| 48 malignant CT (NSCLC, EGFR mutant) | Prediction of response to TKI |
| ||||
| Lafata et al. (2019) [42] | Lafata et al. (2019) [52] |
| 64 malignant CT (NSCLC) | Prediction of PFTs |
|
2.3. Obstructive Lung Diseases
2.3.1. COPD
2.3.2. Asthma
2.4. Interstitial Lung Diseases
| Study | Description | Cohort | Performance | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Schniering et al. (2019) [73] | Schniering et al. (2019) [74] |
| 66 ILD CT (20 mild ILD and 46 advanced ILD) | Staging of ILD (proof of concept) AUC 0.929 |
||||||
| Stefano et al. (2020) [74] | Stefano et al. (2020) [75] |
| 32 IPF CT | Severity of IPF NL (normally attenuated lung) at -200 HU demonstrated the strongest correlation with disease severity ( | p | = 0.009) | ||||
| Martini et al. (2020) [75] | Martini et al. (2020) [76] |
| 66 SSc CT Training cohort 70% Validation cohort 30% |
Severity and staging of SSc-ILD |
| |||||
| Ungprasert et al. (2017) [76] | Ungprasert et al. (2017) [77] |
| 110 ILD CT |
| Correlation with PFTs in IIM associated ILD |
| ||||
| Kim et al. (2015) [77] | Kim et al. (2015) [78] |
| 57 IPF patients |
| Correlation with baseline lung function and prediction of evolution in IPF |
| ||||
| De Giacomi et al. (2017) [78] | De Giacomi et al. (2017) [79] |
| 40 biopsy-confirmed UIP 20 biopsy-confirmed NSIP |
Differentiation NSIP vs. IPF |
| |||||
| Lee et al. (2018) [79] | Lee et al. (2018) [80] |
| 26 CT from tubrcolosis patients, 21 CT from sarcoidosis patients. | Differentiation between tuberculosis and sarcoidosis LN |
| |||||
| Best et al. (2008) [80] | Best et al. (2008) [81] |
| 167 IPF patients |
| Prediction of mortality and progression in IPF |
| ||||
| Maldonado et al. (2014) [81] | Maldonado et al. (2014) [82] |
| 55 IPF patients | Correlation between CT changes and mortality in IPF |
| |||||
| ||||||||||
| Jacob et al. (2017) [82] | Jacob et al. (2017) [83] |
|
|
| 31 benign CT 77 malignant CT |
Benign vs. malignant according to temporal changes AUC 0.741 |
||||
| 283 IPF CT | Prediction of mortality in IPF |
| Lee et al. (2014) [17] | Lee et al. (2014) [16] |
||||||
| Kim et al. (2011) [83] | Kim et al. (2011) [84] |
| 83 SSc-ILD CT |
| Evaluate the effectiveness of cyclophosphamide in SSc-ILD |
|
