Polyamines (PAs), such as putrescine (PUT), spermine (SPE), and spermidine (SPD), are organic polycationic alkylamines, which are synthesized from L-ornithine or by the decarboxylation of amino acids. They are found in all living cells and mammalian cells contain a millimolar concentration of PAs. In 1678, the SPE was first identified by Van Leeuwenhoek as crystals in dried semen but not in fresh ones.
- polyamines
- biosynthesis
- nutritional role
- human health
- disease prevention
1. Introduction
Polyamines (PAs), such as putrescine (PUT), spermine (SPE), and spermidine (SPD), are organic polycationic alkylamines, which are synthesized from L-ornithine or by the decarboxylation of amino acids [1,2,3][1][2][3]. They are found in all living cells and mammalian cells contain a millimolar concentration of PAs [4]. In 1678, the SPE was first identified by Van Leeuwenhoek as crystals in dried semen but not in fresh ones. In 1791, Vauquelin identified these crystals as an unknown phosphate-derived compound [5]. Further, Schreiner reported SPE as a basic compound in 1878, while Ladenburg and Abel proposed its name “spermine” in 1888 [6,7][6][7]. After one decade (1898), Poehl suggested the use of SPE for the treatment of several diseases [8], and finally, in 1924, SPE, SPD, and PUT were synthesized by Rosenheim, which led to the foundation of the modern science of PAs [9]. Moreover, the PUT was discovered in the microorganisms in ~1800s, and SPD was identified in the 20th century [10].
Therefore, PAs regulate the electronic equilibrium, electric excitation, and cardiac activity by facilitating K+movement into K+(Kir) channels of different cell types [19,20][11][12]. SPE and SPD work as the substrates for different biological enzymes to form cytotoxic metabolites by the activity of spermine oxidase (SMO), monoamine oxidase (MAO), copper amine oxidase (CuAOs), and polyamine oxidases (PAO) [30,31][13][14]. H2O2and aldehyde(s) cross the inner mitochondrial membrane and react with endogenous structures and molecules in order to induce the death of tumor cells [32][15]. In vitro cytotoxicity can be induced in the presence of internal PAs or external SPE in various tumor cell lines of humans using CuAOs, i.e., bovine serum amine oxidase [31,32][14][15].
There are three ways to maintain the PA pool in the body: intestinal microorganisms, de novo biosynthesis (endogenous), and supply through diet (exogenous). Hence, PAs in nutrition (dietary polyamines) play a crucial role in maintaining the biosynthesis of PAs because distortion in the metabolism of PAs may lead to several health disorders [34][16]. Various food items contain the required amounts of PAs, i.e., plant-derived foods have mostly PUT and SPD, and meat products mainly contain SPE, while dairy products are rich in SPD and PUT [34][16]. A controlled diet, solely or with clinical applications, can be used as an effective treatment against various cancer, cardiovascular diseases, Huntington’s disease, Alzheimer’s disease, and Parkinson’s disease.
2. Types, Structures, and Functions of PAs
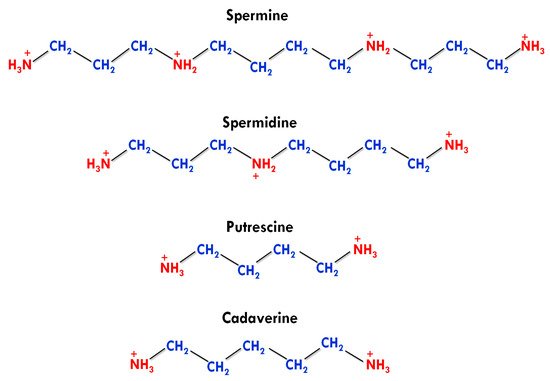
Apart from this, agmatine was also detected in human tissues in a trace amount, but it has no active physiological role [4]. The number and presence of amino groups impart different physiological and biochemical roles to biogenic PAs. PUT and cadaverine have two amino groups in their structures and are known as diamines. SPD contains three amino groups and classified as triamine, while having four amino groups, SPE is generally referred to as tetramine (Figure 1) [38][17].
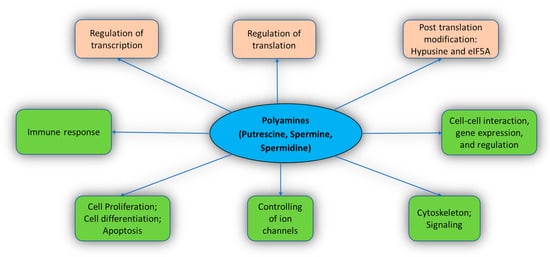
The functions of PAs include cell differentiation, cell proliferation, gene regulation, cell signaling, and apoptosis [4,18,39,40][4][18][19][20]. PAs also stimulate post-translation modification with the help of eIF5A (a translation factor) [41][21]. PAs interact extensively with the cellular molecules and perform various crucial functions in the body (Figure 2). Important known functions of PAs are described below.
PAs are necessary for cell proliferation and differentiation. The depletion of PA by α-difluoromethylornithine (DFMO) leads to an enhancement in the expression of p53, which consequently inhibits cell growth both in vivo and in vitro conditions [17][22]. It helps in the migration of vascular smooth muscle cells after endothelial injury [43][23]. It consequently halted cell growth [29,47][24][25].
PAs are highly positive molecules that bind on the acidic sites of different macromolecules, such as nucleic acids, proteins, and membrane phospholipids [39][19]. PAs govern various gene expression activities and their regulation as well [17,39][22][19]. Moreover, PAs’ interaction with RNA has also been illustrated in the presence of physiological Mg2+ions PAs act at different levels during protein expression, i.e., initiation of 30S subunit of the ribosome for assembly, protein expression at the cellular level, and initiation to form Ile-ANRt
The transcription of many genes, including c-Jun and c-Myc, are regulated by PAs [39][19]. Likewise, selective PAs are responsible for the regulation of AdoMetDC, AZ, and SSAT for the translation of various mRNA sections [51,52,53][26][27][28]. Additionally, several studies showed the effect of PAs on the cell signaling pathways by affecting the status and levels of main regulatory proteins such as CDK-4, GSK-3β, p53, p27Kip, p21Cip1, Src, EGFR, Mdm2, Akt/protein kinase B, and importin-α1 [54,55,56,57][29][30][31][32].
The interaction of PAs with RNA affects the level of individual cell proteins in several ways such as facilitating initiation complexes formation, change in the structures of ribosomes, and enhancing frameshifting [58,59][33][34]. PAs can also influence the protein structure by the direct or indirect effect on the degradation and processing of post-translational protein [60,61][35][36].
During post-translation modification, SPD donates the aminobutyl group to the translation factor (eIF5A) with the help of deoxyhypusine synthase enzyme, which consequently results in the formation of hypusine (Nϵ-(4-amino-2-hydroxybutyl) lysine) [62][37]. Proteins having these proline stretches regulate several functions, such as DNA binding, transcription, RNA splicing, cell signaling, and cytoskeleton-related functions for the development and growth of the cells [16,68][38][39]. Vertebrates possess another gene encoding eIF5A2, which expresses less and is not crucial for the body; however, eIF5A2 has been found in various cancer cells, responsible for poor prognosis and rapid growth [14,16][40][38]. Moreover, PAs’ depletion in mammalian cells using an inhibitor of SPE synthase, i.e., difluoromethylornithine plusN1-(3-aminopropyl)-cyclohexylamine showed an inhibitory effect on cell growth by impacting hypusine level [41][21].
Kir channels represent a superfamily of K+ion channels, such as voltage-gated, two-pore, cyclic nucleotide-gated, and calcium-gated channels [4]. The potassium flux via Kir channels maintains the electrolyte equilibrium, membrane potential, and electron activity of neurons and cardiac muscles. A study ofXenopusoocytes revealed that polyamine binding initiated the rectification in the HRK1 Kir channel, which was observed subsequently in the large family of such Kir channels [71][41]. This brought a small change in the concentration of PAs because of the higher potency of SPE than SPD, which was responsible for a significant change in the activity of Kir channels [71,72][41][42].
In a structural study of Kir1 to Kir7 subfamilies, it was observed that the PAs first bind to cytoplasmic pore at a shallow binding site with low voltage dependence and then move toward a deep position through a long pore. This position is called the rectification controller or acidic residue, which interacts with PAs to initiate steep voltage dependence [73,74][43][44].
TRPC channels are comprised of a seven-member family (TRPC-1, 2, 3, 4, 5, 6, and 7) in the mammalian cells. Additionally, they act as second messenger-operated and store-operated channels, responsible for contractility and extractability of smooth gastrointestinal muscle [4]. On the other hand, intracellular SPE increases the communication between astrocytes and also in gap junctions [77][45]. It also helps in coupling connexin Cx43
Synaptic plasticity and synaptic transmission that determine learning and memory occur in the cellular membrane by the binding of glutamate (ligand). There are three classes of these receptors and each class has many members on the basis of their active agents, such as AMPA, NMDA, and kainate. Few NMDA receptors work as voltage-dependent and ligand-gated channels to control synaptic plasticity [80,81][46][47]. PA effects include inhibition and stimulation of a voltage-dependent channel, which depicts an open-channel block.
PAs also impact the AMPA receptors family, which do not have glutamate subunits [84][48]. AMPA receptors act as neurotransmitters to regulate synaptic power and enhancing neurotransmission in the central nervous system (CNS). Intracellular PAs, potently SPE, have the capacity to block these channels, which bind on the pore region of the channels [85][49]. Notably, PAs can regulate the excitability limit of synapses and the concentration of Ca2+flux.
It has been reported that autoreactive B cells and T cells along with cancerous cells contain a higher concentration of PAs during autoimmune diseases [12][50]. The L-arginine catabolism in suppressive myeloid and tumor cells decreases the functions of cytotoxic T cell, which suggests a link between T cell suppression and PAs [12][50]. It has been observed that the higher concentration of PAs in an autoimmune patient form a nuclear cluster that reacts with RNA, DNA, and other molecules for stabilizing autoantigens [86][51]. The formation of single-stranded or double-stranded DNA is the predominant response of autoimmune B cell [87][52].
PAs were reported to regulate the activity of TGase in many functions, including cell differentiation, post-translational protein modification, kinase activity, wound healing, and signal transduction [88,89,90][53][54][55]. (TG2) catalyzes protein post-translational change by adding PAs into protein or forming epsilon lysine bonds in an inter- or intramolecular cross-link manner [91,92][56][57]. On the other hand, a higher enzyme activity of TG2 was found to be associated with various neuropathological conditions (acute and chronic) such as amyotrophic lateral sclerosis (ALS), Huntington’s disease, Alzheimer’s disease, and Parkinson’s disease [93,94][58][59]. For instance, primary astrocytes (cultured) were exposed to glutamate (excitotoxic) that led to oxidative stress with TG2 up-regulation.
In a study, the activation of nuclear factor-κB was found to be involved in the development of ROS and the activation of TG2 upregulation when the cultured astrocytes of rat hippocampus were exposed to lipopolysaccharide (LP) [97][60]. LP is commonly used to stimulate iNOS induction. They reported a suppressed level of LP-induced effect after the treatment of ammonium pyrrolidine-1-carbodithioate (nuclear factor-κB inhibitor) in the astrocytes [97][60].
3. Metabolic and Transport Pathway of Polyamines in Humans
The homeostasis of PAs in the mammalian species can be understood through three steps that can be broadly classified as synthesis, catabolism, and transport. PAs are produced in the cell cytoplasm. In vivo production of polyamine begins with the intake of amino acids (arginine, lysine, and methionine) through food, serving as substrates for polyamine synthesis through the action of micro-organisms/enzymes [2] (Figure 3).
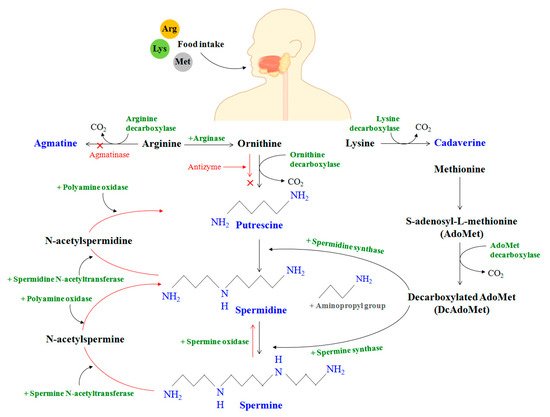
In the mammalian gut, the enzyme arginase first decomposes the amino acid arginine to produce ornithine. Meanwhile, methionine is transformed to S-adenosyl-L-methionine (AdoMet), which is further converted to decarboxylated AdoMet or DcAdoMet in the presence of the AdoMet decarboxylase enzyme. The DcAdoMet thus produced serves as an aminopropyl group donor to putrescine for the synthesis of spermidine in the presence of spermidine synthase. It should be noted that both ODC and AdoMet decarboxylase are PA-rate-limiting enzymes that are strictly controlled at the transcriptional and post-transcriptional stages.
/spermine N-acetyltransferase (SSAT) and polyamine oxidase (PAO) are jointly responsible for the mechanism of polyamine catabolism. A higher degree of regulation involves the action of other oxidases, along with their cofactors, within the body to generate permanently polyamine derivatives from amino acids that cannot be recycled back to PAs. AZIn can therefore bind to AZ, releasing ODC in the process that accelerates polyamine formation. It was concocted that the increase in spermine oxidase expression with age could cause oxidative degradation in the levels of spermine.
Polyamine regulation in the body is also facilitated by polyamine transport and cellular uptake. In a later investigation, Abdulhussein and Wallace [105][61] studied the PA transport mediating potential of ABC and eight SLC transporters (SLC22A1, SLC22A2, SLC22A3, SLC47A1, SLC7A1, SLC3A2, SLC12A8A, SLC22A16). Hamouda et al. also reported an unexplored gene, ATP13A3, as a potential candidate for PA transport that complemented PUT transport deficiency [106][62]. In another recent study, the gene SLC18B1 of the vesicular amine transporter family was identified as a transporter of spermine and spermidine.
It has been demonstrated that cancer cell proliferations have high polyamine transport activity, and the transport system holds relevance as a target site for selective drug delivery. Taking advantage of the intrinsic needs of cancer cells to utilize polyamine metabolites for growth, Muth et al. [110][63] showed that the novel compoundN1,N1-Naphthalene-1,4-diylbis(methylene)]bis{N4-4-(methylamino)butyl])butane-1,4-diamine}, 3b, had excellent polyamine transport system selectivity and was stable to amine oxidases, making it a candidate for targeting breast cancer cells and melanomas. It was established that the rates of cell growth and polyamine depletion were associated with polyamine transport.
References
- Sharma, S.; Pareek, S.; Sagar, N.A.; Valero, D.; Serrano, M. Modulatory effects of exogenously applied polyamines on postharvest physiology, antioxidant system and shelf life of fruits: A review. Int. J. Mol. Sci. 2017, 18, 1789.
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018, 6, 1–18.
- Firpo, M.R.; Mounce, B.C. Diverse functions of polyamines in virus infection. Biomolecules 2020, 10, 628.
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912.
- Vauquelin, L.N. Experiences sur le sperme humain. Ann. Chim. 1791, 9, 64–80.
- Schreiner, P. Ueber eine neue organische Basis in thierischen Organismen. Justus Lieb. Annal. Chem. 1878, 194, 68–84.
- Ladenburg, A.; Abel, J. Ueber das aethylenimin (Spermin?). Berichte Deutschen Chemischen Gesellschaft 1888, 21, 758–766.
- Poehl, A.V.E. Die Physiologisch-Chemischen Grundlagen der Spermintheorie Nebst Klinischem Material zur Therapeutischen Verwendung des Sperminum-Poehl; Wienecke: Sain Petersburg, Russia, 1898.
- Rosenheim, O. The isolation of spermine phosphate from semen and testis. Biochem. J. 1924, 18, 1253.
- Gerner, E.W.; Meyskens, F.L. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792.
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.A.N.; Kurachi, Y. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol. Rev. 2010, 90, 291–366.
- Baronas, V.A.; Kurata, H.T. Inward rectifiers and their regulation by endogenous polyamines. Front. Physiol. 2014, 5, 325.
- Ohkubo, S.; Mancinelli, R.; Miglietta, S.; Cona, A.; Angelini, R.; Canettieri, G.; Agostinelli, E. Maize polyamine oxidase in the presence of spermine/spermidine induces the apoptosis of LoVo human colon adenocarcinoma cells. Int. J. Oncol. 2019, 54, 2080–2094.
- Agostinelli, E. Biochemical and pathophysiological properties of polyamines. Amino Acids 2020, 52, 111–117.
- Agostinelli, E.; Condello, M.; Tempera, G.; Macone, A.; Bozzuto, G.; Ohkubo, S.; Molinari, A. The combined treatment with chloroquine and the enzymatic oxidation products of spermine overcomes multidrug resistance of melanoma M14 ADR2 cells: A new therapeutic approach. Int. J. Oncol. 2014, 45, 1109–1122.
- Büyükuslu, N. Dietary polyamines and diseases: Reducing polyamine intake can be beneficial in cancer treatment. J. Nutr. 2015, 2, 27–38.
- Kabir, A.; Kumar, G.S. Binding of the biogenic polyamines to deoxyribonucleic acids of varying base composition: Base specificity and the associated energetics of the interaction. PLoS ONE 2013, 8, e70510.
- Lenis, Y.Y.; Elmetwally, M.A.; Maldonado-Estrada, J.G.; Bazer, F.W. Physiological importance of polyamines. Zygote 2017, 25, 244.
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894.
- Uemura, T.; Akasaka, Y.; Ikegaya, H. Correlation of polyamines, acrolein-conjugated lysine and polyamine metabolic enzyme levels with age in human liver. Heliyon 2020, 6, e05031.
- Dever, T.E.; Ivanov, I.P. Roles of polyamines in translation. J. Biol. Chem. 2018, 293, 18719–18729.
- Moinard, C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197.
- Liang, M.; Ekblad, E.; Hellstrand, P.; Nilsson, B.O. Polyamine synthesis inhibition attenuates vascular smooth muscle cell migration. J. Vasc. Res. 2004, 41, 141–147.
- Jain, V. Role of polyamines in asthma pathophysiology. Med. Sci. 2018, 6, 4.
- Pegg, A.E. Spermidine/spermine-N1-acetyltransferase: A key metabolic regulator. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E995–E1010.
- Nilsson, J.A.; Keller, U.B.; Baudino, T.A.; Yang, C.; Norton, S.; Old, J.A.; Nilsson, L.M.; Neale, G.; Kramer, D.L.; Porter, C.W.; et al. Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell 2005, 7, 433–444.
- Xiao, L.; Rao, J.N.; Zou, T.; Liu, L.; Marasa, B.S.; Chen, J.; Turner, D.J.; Passaniti, A.; Wang, J.Y. Induced JunD in intestinal epithelial cells represses CDK4 transcription through its proximal promoter region following polyamine depletion. Biochem. J. 2007, 403, 573–581.
- Vaidya, R.J.; Ray, R.M.; Johnson, L.R. Akt-mediated GSK-3β inhibition prevents migration of polyamine-depleted intestinal epithelial cells via Rac1. Cell Mol. Life Sci. 2006, 63, 2871–2879.
- Zou, T.; Liu, L.; Rao, J.N.; Marasa, B.S.; Chen, J.; Xiao, L.; Zhou, H.; Gorospe, M.; Wang, J.Y. Polyamines modulate the subcellular localization of RNA-binding protein HuR through AMP-activated protein kinase-regulated phosphorylation and acetylation of importin alpha1. Biochem. J. 2008, 409, 389–398.
- Bhattacharya, S.; Ray, R.M.; Johnson, L.R. Role of polyamines in p53-dependent apoptosis of intestinal epithelial cells. Cell Signal. 2009, 21, 509–522.
- Kucharzewska, P.; Welch, J.E.; Svensson, K.J.; Belting, M. The polyamines regulate endothelial cell survival during hypoxic stress through PI3K/AKT and MCL-1. Biochem. Biophys. Res. Commun. 2009, 380, 413–418.
- Ramos-Molina, B.; Lambertos, A.; Peñafiel, R. Antizyme inhibitors in polyamine metabolism and beyond: Physiopathological implications. Med. Sci. 2018, 6, 89.
- Sakamoto, A.; Terui, Y.; Yoshida, T.; Yamamoto, T.; Suzuki, H.; Yamamoto, K.; Suzuji, H.; Yamamoto, K.; Ishihama, A.; Igarashi, K.; et al. Three members of polyamine modulon under oxidative stress conditions: Two transcription factors (SoxR and EmrR) and a glutathione synthetic enzyme (GshA). PLoS ONE 2015, 10, e0124883.
- Yamashita, T.; Nishimura, K.; Saiki, R.; Okudaira, H.; Tome, M.; Higashi, K.; Nakamura, M.; Terui, Y.; Fuziwara, K.; Kashiwagi, K.; et al. Role of polyamines at the G1/S boundary and G2/M phase of the cell cycle. Int. J. Biochem. Cell Boil. 2013, 45, 1042–1050.
- Tolbert, W.D.; Zhang, Y.; Cottet, S.E.; Bennett, E.M.; Ekstrom, J.L.; Pegg, A.E.; Ealick, S.E. Mechanism of human S-adenosylmethionine decarboxylase proenzyme processing as revealed by the structure of the S68A mutant. Biochemistry 2003, 42, 2386–2395.
- Pegg, A.E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532.
- Caraglia, M.; Park, M.H.; Wolff, E.C.; Marra, M.; Abbruzzese, A. eIF5A isoforms and cancer: Two brothers for two functions? Amino Acids 2013, 44, 103–109.
- Pällmann, N.; Braig, M.; Sievert, H.; Preukschas, M.; Hermans-Borgmeyer, I.; Schweizer, M.; Balabanov, S. Biological relevance and therapeutic potential of the hypusine modification system. J. Biol. Chem. 2015, 290, 18343–18360.
- Mandal, A.; Mandal, S.; Park, M.H. Genome-wide analyses and functional classification of proline repeat-rich proteins: Potential role of eIF5A in eukaryotic evolution. PLoS ONE 2014, 9, e111800.
- Mathews, M.B.; Hershey, J.W. The translation factor eIF5A and human cancer. Biochimica Biophysica Acta (BBA)-Gene Regul. Mech. 2015, 1849, 836–844.
- Lopatin, A.N.; Makhina, E.N.; Nichols, C.G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 1994, 372, 366–369.
- Stanfield, P.R.; Michael, J.S. Spermine is fit to block inward rectifier (Kir) channels. J. Gen. Physiol. 2003, 122, 481–484.
- Kurata, H.T.; Zhu, E.A.; Nichols, C.G. Locale and chemistry of spermine binding in the archetypal inward rectifier Kir2. 1. J. Gen. Physiol. 2010, 135, 495–508.
- Kurata, H.T.; Akrouh, A.; Li, J.W.; Marton, L.J.; Nichols, C.G. Scanning the topography of polyamine blocker binding in an inwardly rectifying potassium channel. J. Biol. Chem. 2013, 288, 6591–6601.
- Benedikt, J.; Inyushin, M.; Kucheryavykh, Y.V.; Rivera, Y.; Kucheryavykh, L.Y.; Nichols, C.G.; Skatchkov, S.N. Intracellular polyamines enhance astrocytic coupling. Neuroreport 2012, 23, 1021.
- Bowie, D.; Mayer, M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 1995, 15, 453–462.
- Williams, K. Modulation and block of ion channels: A new biology of polyamines. Cell. Signal. 1997, 9, 1–13.
- Bowie, D. Redefining the classification of AMPA-selective ionotropic glutamate receptors. J. Physiol. 2012, 590, 49–61.
- Shin, J.; Shen, F.; Huguenard, J. PKC and polyamine modulation of GluR2-deficient AMPA receptors in immature neocortical pyramidal neurons of the rat. J. Physiol. 2007, 581, 679–691.
- Hesterberg, R.S.; Cleveland, J.L.; Epling-Burnette, P.K. Role of polyamines in immune cell functions. Med. Sci. 2018, 6, 22.
- Riboldi, P.; Gerosa, M.; Moroni, G.; Radice, A.; Allegri, F.; Sinico, A.; Meroni, P.L. Anti-DNA antibodies: A diagnostic and prognostic tool for systemic lupus erythematosus? Autoimmunity 2005, 38, 39–45.
- Fineschi, S.; Borghi, M.O.; Riboldi, P.; Gariglio, M.; Buzio, C.; Landolfo, S.; Meroni, P.L. Prevalence of autoantibodies against structure specific recognition protein 1 in systemic lupus erythematosus. Lupus 2004, 13, 463–468.
- Agostinelli, E. Polyamines and transglutaminases: Biological, clinical, and biotechnological perspectives. Amino Acids 2014, 46, 475–485.
- Folk, J.E.; Cole, P.W. Mechanism of action of guinea pig liver transglutaminase: I. Purification and properties of the enzyme: Identification of a functional cysteine essential for activity. J. Biol. Chem. 1966, 241, 5518–5525.
- Fesus, L.; Piacentini, M. Transglutaminase 2: An enigmatic enzyme with diverse functions. Trends Biochem. Sci. 2002, 27, 534–539.
- Telci, D.; Griffin, M. Tissue transglutaminase (TG2)-a wound response enzyme. Front. Biosci. 2006, 11, 867–882.
- Folk, J.E.; Park, M.H.; Chung, S.I.; Schrode, J.; Lester, E.P.; Cooper, H.L. Polyamines as physiological substrates for transglutaminases. J. Biol. Chem. 1980, 255, 3695–3700.
- Ruan, Q.; Johnson, G.V. Transglutaminase 2 in neurodegenerative disorders. Front. Biosci. 2007, 12, 891–904.
- Caccamo, D.; Currò, M.; Ferlazzo, N.; Condello, S.; Ientile, R. Monitoring of transglutaminase2 under different oxidative stress conditions. Amino Acids 2012, 42, 1037–1043.
- Takano, K.; Shiraiwa, K.; Moriyama, M.; Nakamura, Y. Transglutaminase 2 expression induced by lipopolysaccharide stimulation together with NO synthase induction in cultured astrocytes. Neurochem. Int. 2010, 57, 812–818.
- Abdulhussein, A.A.; Wallace, H.M. Polyamines and membrane transporters. Amino Acids 2014, 46, 655–660.
- Hamouda, N.N.; Van den Haute, C.; Vanhoutte, R.; Sannerud, R.; Azfar, M.; Mayer, R.; Calabuig, Á.C.; Swinnen, J.V.; Agostinis, P.; Baekelandt, V.; et al. ATP13A3 is a major component of the enigmatic mammalian polyamine transport system. J. Biol. Chem. 2021, 296, 100182.
- Muth, A.; Kamel, J.; Kaur, N.; Shicora, A.C.; Ayene, I.S.; Gilmour, S.K.; Phanstiel IV, O. Development of polyamine transport ligands with improved metabolic stability and selectivity against specific human cancers. J. Med. Chem. 2013, 56, 5819–5828.
