Biogenic amines produced during fermentation may be harmful when ingested in high concentrations. As current regulations remain insufficient to ensure the safety of fermented vegetable products, the current study determined the risks associated with the consumption of kimchi by evaluating the biogenic amine concentrations reported by various studies.
- kimchi
- Jeotgal
- Aekjeot
- Myeolchi-jeot
- Myeolchi-aekjeot
- biogenic amines
- recommended limits
- occurrence
- reduction
- starter cultures
1. Introduction
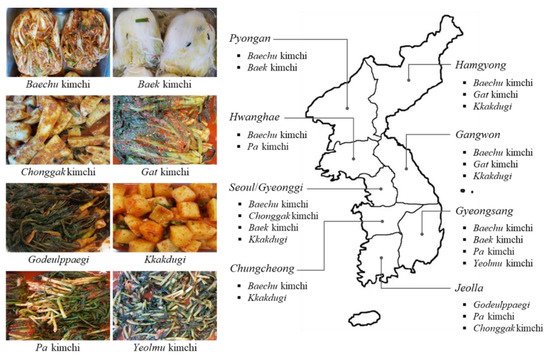
2. Biogenic Amine Content in Kimchi Products
Korean Fermented Vegetable Products | N 1 | Biogenic Amines (mg/kg) 2 | Ref. | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
TRP | PHE | PUT | CAD | HIS | TYR | SPD | SPM | |||||||||||||||||||||||||
Baechu kimchi (Napa cabbage kimchi) | 3 | NT 3 | NT | 11.2–89.0 4 | ND 5-151.8 | ND-5.1 | ND-28.2 | ND | ND |
[29] |
||||||||||||||||||||||
20 | 2.3–22.6 | ND-6.8 | 15.1–240.4 | 3.6–44.9 | 0.6–142.3 | 9.7–118.2 | 7.7–16.5 | ND-3.7 |
[25] |
|||||||||||||||||||||||
37 | ND-114 | ND | ND-73 | ND-1550 | ND-5350 | ND-42 | ND-88 | ND-121 |
[27] |
|||||||||||||||||||||||
18 | ND-43.9 | NT | ND-245.9 | ND-63.3 | NT | ND-103.6 | ND-74.8 | NT |
[26] |
|||||||||||||||||||||||
20 | ND-74.8 | ND-2.0 | 2.3–148.6 | 0.9–39.8 | ND-21.8 | 1.1–27.9 | ND-6.7 | ND-5.1 |
[28] |
|||||||||||||||||||||||
Baek kimchi (Napa cabbage kimchi prepared without red pepper powder) | 3 | NT | NT | ND-54.7 | ND-94.8 | ND | ND | ND | ND |
[29] |
||||||||||||||||||||||
3 | tr 6 | NT | 1.9–39.6 | 11.5–25.6 | NT | 7.8–64.9 | ND-1.7 | NT |
[26] |
|||||||||||||||||||||||
Chonggak kimchi (ponytail radish kimchi) | 3 | NT | NT | ND-11.2 | ND-70.7 | ND | ND | ND | ND |
[29] |
||||||||||||||||||||||
3 | 2.3–15.2 | NT | ND-20.3 | ND-85.7 | NT | 20.2–58.1 | ND | NT |
[26] |
|||||||||||||||||||||||
5 | ND-23.70 | ND-2.80 | 3.89–853.70 | 2.00–148.50 | 8.24–131.20 | 0.79–18.70 | 6.10–14.00 | ND-20.74 |
[30] |
|||||||||||||||||||||||
Gat kimchi (mustard leaf kimchi) | 3 | NT | NT | ND-10.4 | ND-11.6 | ND | ND | ND | ND |
[29] |
||||||||||||||||||||||
13 | ND-26.74 | ND-15.75 | 1.89–720.82 | 2.12–52.43 | 3.30–232.10 | 1.28–149.77 | 12.26–32.62 | ND-61.94 |
[31] |
|||||||||||||||||||||||
Godeulppaegi (Korean lettuce kimchi) | 3 | NT | NT | ND-6.4 | ND-26.7 | ND | ND | ND | ND |
[29] |
||||||||||||||||||||||
Kkakdugi (diced radish kimchi) | 3 | NT | NT | ND-15.4 | ND-55.1 | ND | ND-9.0 | ND | ND |
[29] |
||||||||||||||||||||||
5 | 5.5–18.6 | NT | ND-51.6 | ND-56.2 | NT | ND-10.8 | ND-21.8 | NT |
[26] |
|||||||||||||||||||||||
5 | ND | ND-15.24 | 10.85–982.32 | ND-124.60 | 18.75–127.78 | 2.97–76.95 | ND-16.76 | ND-3.10 |
[30] |
|||||||||||||||||||||||
Pa kimchi (green onion kimchi) | 3 | NT | NT | ND-7.8 | ND-15.9 | ND-21.7 | ND | ND | ND |
[29] |
||||||||||||||||||||||
13 | ND-15.95 | ND-5.97 | ND-254.47 | ND-123.29 | 8.67–386.03 | ND-181.10 | 2.32–18.74 | ND-33.84 |
[31] |
|||||||||||||||||||||||
Yeolmu kimchi (young radish kimchi) | 3 | NT | NT | ND | ND | ND | ND | ND | ND |
[29] |
||||||||||||||||||||||
3. Biogenic Amine Content of Other Vegetable Products
4. Strategies to Reduce Biogenic Amine Content in Kimchi Products
References
- Food Information Statistics System. Available online: (accessed on 3 October 2019).
- Lee, C.-H.; Ahn, B.-S. Literature review on Kimchi, Korean fermented vegetable foods I. History of Kimchi making. Korean J. Food Cult. 1995, 10, 311–319.
- Cheigh, H.-S. Kimchi Culture and Dietary Life in Korea, 1st ed.; Hyoil Publishing Co.: Seoul, Korea, 2002; pp. 304–312.
- Choi, S.-K.; Hwang, S.-Y.; Jo, J.-S. Standardization of kimchi and related products (3). Korean J. Food Cult. 1997, 12, 531–548.
- Sim, S.G.; Shon, H.S.; Sim, C.H.; Yoon, W.H. Fermented Foods, 1st ed.; Jin Ro Publishing Co.: Seoul, Korea, 2001; pp. 233–286.
- Codex Alimentarius Commission. Codex Standard for Kimchi, Codex Stan 223–2001; Food and Agriculture Organization of the United Nations: Rome, Italy, 2001.
- Park, K.-Y. The nutritional evaluation, and antimutagenic and anticancer effects of Kimchi. Korean J. Food Nutr. 1995, 24, 169–182.
- Park, K.-Y. Increased health functionality of fermented foods. Korean J. Food Nutr. 2012, 17, 1–8.
- Jung, E.H.; Ryu, J.P.; Lee, S.-I. A study on foreigner preferences and sensory characteristics of kimchi fermented for different periods. Korean J. Food Cult. 2012, 27, 346–353.
- Jung, J.Y.; Lee, S.H.; Jeon, C.O. Kimchi microflora: History, current status, and perspectives for industrial kimchi production. Appl. Microbiol. Biotechnol. 2014, 98, 2385–2393.
- Cho, E.-J.; Rhee, S.-H.; Lee, S.-M.; Park, K.-Y. In vitro antimutagenic and anticancer effects of kimchi fractions. J. Cancer Prev. 1997, 2, 113–121.
- Kim, J.-H.; Ryu, J.-D.; Song, Y.-O. The effect of kimchi intake on free radical production and the inhibition of oxidation in young adults and the elderly people. Korean J. Community Nutr. 2002, 7, 257–265.
- Askar, A.; Treptow, H. Biogene Amine in Lebensmitteln: Vorkommen, Bedeutung und Bestimmung, 1st ed.; Verlag Eugen Ulmer: Stuttgart, Germany, 1986; pp. 21–74.
- Silla Santos, M.H. Biogenic amines: Their importance in foods. Int. J. Food Microbiol. 1996, 29, 213–231.
- Ten Brink, B.; Damink, C.; Joosten, H.M.L.J.; Huis in’t Veld, J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990, 11, 73–84.
- Gilbert, R.J.; Hobbs, G.; Murray, C.K.; Cruickshank, J.G.; Young, S.E.J. Scombrotoxic fish poisoning: Features of the first 50 incidents to be reported in Britain (1976–9). Br. Med. J. 1980, 281, 71–72.
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Fernandez, M.; Martin, M.C.; Ruas-Madiedo, P.; Alvarez, M.A. The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem. 2017, 218, 249–255.
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Spermine and spermidine are cytotoxic towards intestinal cell cultures, but are they a health hazard at concentrations found in foods? Food Chem. 2018, 269, 321–326.
- Del Rio, B.; Redruello, B.; Linares, D.M.; Ladero, V.; Ruas-Madiedo, P.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods. Sci. Rep. 2019, 9, 120.
- Ender, F.; Čeh, L. Conditions and chemical reaction mechanisms by which nitrosamines may be formed in biological products with reference to their possible occurrence in food products. Z. Lebensm. Unters. Forsch. 1971, 145, 133–142.
- Mah, J.-H.; Yoon, M.-Y.; Cha, G.-S.; Byun, M.-W.; Hwang, H.-J. Influence of curing and heating on formation of N-nitrosamines from biogenic amines in food model system using Korean traditional fermented fish product. Food Sci. Biotechnol. 2005, 14, 168–170.
- Taylor, S.L. Histamine food poisoning: Toxicology and clinical aspects. Crit. Rev. Toxicol. 1986, 17, 91–128.
- Smith, T.A. Amines in food. Food Chem. 1981, 6, 169–200.
- Mah, J.-H.; Park, Y.K.; Jin, Y.H.; Lee, J.-H.; Hwang, H.J. Bacterial production and control of biogenic amines in Asian fermented soybean foods. Foods 2019, 8, 85.
- Cho, T.-Y.; Han, G.-H.; Bahn, K.-N.; Son, Y.-W.; Jang, M.-R.; Lee, C.-H.; Kim, S.-H.; Kim, D.-B.; Kim, S.-B. Evaluation of biogenic amines in Korean commercial fermented foods. Korean J. Food Sci. Technol. 2006, 38, 730–737.
- Kang, K.H.; Kim, S.H.; Kim, S.-H.; Kim, J.G.; Sung, N.-J.; Lim, H.; Chung, M.J. Analysis and risk assessment of N-nitrosodimethylamine and its precursor concentrations in Korean commercial kimchi. J. Korean Soc. Food Sci. Nutr. 2017, 46, 244–250.
- Tsai, Y.-H.; Kung, H.-F.; Lin, Q.-L.; Hwang, J.-H.; Cheng, S.-H.; Wei, C.-I.; Hwang, D.-F. Occurrence of histamine and histamine-forming bacteria in kimchi products in Taiwan. Food Chem. 2005, 90, 635–641.
- Shin, S.-W.; Kim, Y.-S.; Kim, Y.-H.; Kim, H.-T.; Eum, K.-S.; Hong, S.-R.; Kang, H.-J.; Park, K.-H.; Yoon, M.-H. Biogenic-amine contents of Korean commercial salted fishes and cabbage kimchi. Korean J. Fish. Aquat. Sci. 2019, 52, 13–18.
- Mah, J.-H.; Kim, Y.J.; No, H.-K.; Hwang, H.-J. Determination of biogenic amines in kimchi, Korean traditional fermented vegetable products. Food Sci. Biotechnol. 2004, 13, 826–829.
- Jin, Y.H.; Lee, J.H.; Park, Y.K.; Lee, J.-H.; Mah, J.-H. The occurrence of biogenic amines and determination of biogenic amine-producing lactic acid bacteria in Kkakdugi and Chonggak kimchi. Foods 2019, 8, 73.
- Lee, J.-H.; Jin, Y.H.; Park, Y.K.; Yun, S.J.; Mah, J.-H. Formation of biogenic amines in Pa (green onion) kimchi and Gat (mustard leaf) kimchi. Foods 2019, 8, 109.
- Halász, A.; Baráth, Á.; Holzapfel, W.H. The influence of starter culture selection on sauerkraut fermentation. Z. Lebensm. Unters. Forsch. 1999, 208, 434–438.
- Kalač, P.; Špička, J.; Křížek, M.; Steidlová, Š.; Pelikánová, T. Concentrations of seven biogenic amines in sauerkraut. Food Chem. 1999, 67, 275–280.
- Lee, K.J. Westerner’s view of Korean food in modern period-centering on analyzing Westerners’ books. Korean J. Food Cult. 2013, 28, 356–370.
- Taylor, S.L.; Leatherwood, M.; Lieber, E.R. Histamine in sauerkraut. J. Food Sci. 1978, 43, 1030–1032.
- Mouritsen, O.G. Tsukemono—Crunchy pickled foods from Japan: A case study of food design by gastrophysics and nature. Int. J. Food Des. 2018, 3, 103–124.
- Handa, A.; Kawanabe, H.; Ibe, A. Content and origin of nonvolatile amines in various commercial pickles. J. Food Hyg. Soc. Jpn. 2018, 59, 36–44.
- Kung, H.-F.; Lee, Y.-H.; Teng, D.-F.; Hsieh, P.-C.; Wei, C.-I.; Tsai, Y.-H. Histamine formation by histamine-forming bacteria and yeast in mustard pickle products in Taiwan. Food Chem. 2006, 99, 579–585.
- Kim, S.-H.; Kang, K.H.; Kim, S.H.; Lee, S.; Lee, S.-H.; Ha, E.-S.; Sung, N.-J.; Kim, J.G.; Chung, M.J. Lactic acid bacteria directly degrade N-nitrosodimethylamine and increase the nitrite-scavenging ability in kimchi. Food Control 2017, 71, 101–109.
- Lee, G.-I.; Lee, H.-M.; Lee, C.-H. Food safety issues in industrialization of traditional Korean foods. Food Control 2012, 24, 1–5.
- Kang, H.-W. Characteristics of kimchi added with anchovy sauce from heat and non-heat treatments. Culin. Sci. Hosp. Res. 2013, 19, 49–58.
- Becker, K.; Southwick, K.; Reardon, J.; Berg, R.; MacCormack, J.N. Histamine poisoning associated with eating tuna burgers. JAMA 2001, 285, 1327–1330.
- Mah, J.-H.; Han, H.-K.; Oh, Y.-J.; Kim, M.-G.; Hwang, H.-J. Biogenic amines in Jeotkals, Korean salted and fermented fish products. Food Chem. 2002, 79, 239–243.
- Mah, J.-H.; Kim, Y.J.; Hwang, H.-J. Inhibitory effects of garlic and other spices on biogenic amine production in Myeolchi-jeot, Korean salted and fermented anchovy product. Food Control 2009, 20, 449–454.
- Mah, J.-H.; Hwang, H.-J. Effects of food additives on biogenic amine formation in Myeolchi-jeot, a salted and fermented anchovy (Engraulis japonicus). Food Chem. 2009, 114, 168–173.
- Mah, J.-H.; Hwang, H.-J. Inhibition of biogenic amine formation in a salted and fermented anchovy by Staphylococcus xylosus as a protective culture. Food Control 2009, 20, 796–801.
- Jeong, D.-W.; Han, S.; Lee, J.-H. Safety and technological characterization of Staphylococcus equorum isolates from jeotgal, a Korean high-salt-fermented seafood, for starter development. Int. J. Food Microbiol. 2014, 188, 108–115.
- Kim, S.-H.; Kim, S.H.; Kang, K.H.; Lee, S.; Kim, S.J.; Kim, J.G.; Chung, M.J. Kimchi probiotic bacteria contribute to reduced amounts of N-nitrosodimethylamine in lactic acid bacteria-fortified kimchi. LWT-Food Sci. Technol. 2017, 84, 196–203.
- Ku, K.H.; Sunwoo, J.Y.; Park, W.S. Effects of ingredients on the its quality characteristics during kimchi fermentation. J. Korean Soc. Food Sci. Nutr. 2005, 34, 267–276.
- Jin, H.S.; Kim, J.B.; Yun, Y.J.; Lee, K.J. Selection of kimchi starters based on the microbial composition of kimchi and their effects. J. Korean Soc. Food Sci. Nutr. 2008, 37, 671–675.
