Weed management is an arduous undertaking in crop production. Integrated weed management, inclusive of the application of bioherbicides, is an emerging weed control strategy toward sustainable agriculture. In general, bioherbicides are derived either from plants containing phytotoxic allelochemicals or certain disease-carrying microbes that can suppress weed populations.
- bioherbicides
- plant-based
- physiological response
- biochemical activity
- efficacy
1. Introduction
The growth factors of crops are challenged by weeds and cause on average 15 to 66% yield losses in direct-seeded rice, 18 to 65% in maize, 50 to 76% in soybean, and 45 to 71% in groundnut [2][1]. Crop yield losses due to weeds vary considerably depending on the crop, weed management strategies, weed composition, infestation period, and abiotic factors (e.g., climate and soil edaphic factors) Owing to the labor shortage in the agriculture sector, the practice of using herbicides to control weed densities is increasing around the world [4][2]. Inevitably, the constant use of herbicides on the same field to control weeds over a prolonged period has been shown to cause herbicide resistance, residue in crops, ecological imbalance between harmful and beneficial organisms, and environmental pollution.
Recently, there has been a growing interest in organic fruits, vegetables, dairy products, and beverages all over the world, particularly in developed countries [5][3]. In 2013, there were almost two million produces, and 36% of global organic farmers are in Asia, followed by Africa (29%) and Europe (17%) [5][3]. The fundamental philosophy of sustainable weed management is based on the idea of preventing the spread of weeds rather than controlling them until they have developed and started to cause harm [7][4]. Sustainable weed management comprises a suite of weed management options such as crop rotation, intercropping, crop competitiveness tillage, mulching, biological control agents, and green/bioherbicides which preclude the use of chemical herbicides.
Biological weed control is a mechanism to suppress the germination and growth of weed populations to an economic threshold level by utilizing natural enemies, natural substances, or biotic agents. Bioherbicide and conventional herbicide application methods are similar, although for mycoherbicides the pathogenic fungi are ‘inoculated’ by spraying the pathogens onto target weeds. Recently, bioherbicides have been regarded as a crucial weed control element [8][5], albeit not as a total replacement but rather as an alternative to chemical herbicides [9][6]. Sustainable weed management does not rely on any single weed management technique, unlike synthetic herbicides in conventional agriculture; therefore, bioherbicides should be used concurrently with other weed management techniques to control weeds.
Bioherbicides that are thought to be safer and ‘greener’ have drawn attention, as scientific reports provide increasing evidence of their efficacy. However, their commercial presence in comparison to conventional herbicides is relatively new. Therefore, rigorous testing and validation is necessary to evaluate their efficacy and reliability for weed control. This review explores the impact of bioherbicides, particularly plant-based, on weed physiology and the factors that influence their efficacy as well as the limitations of their use.
2. Effects of Plant-Based Bioherbicides on Weed Growth
Seed germination is considered as an important factor in plant development and productivity. The inhibition of the germination process by plant extracts involves osmotic effects on imbibition rates, which eventually inhibit germination and, in particular, cell elongation [102][7]. The phytotoxicity of plant extracts, residue, or mulch may affect weed germination and growth. Likewise, the extracts ofS. nigrum,C. album,andMatricaria chamomillaL. exhibited suppressive action on the germination and seedling growth ofH. vulgare,Phaseolus vulgarisL.,Cicer arietinumL.,Zea maysL.,Allium cepaL.,Capsicum annuumL.,S. lycopersicum,andTriticum aestivumL. [104][8].
Generally, shoot growth is less sensitive to phytotoxic plant extracts compared to radicle growth [106][9]. The greater sensitivity of radicle growth to plant extracts is due to the radicle being the first organ to be exposed to the phytotoxic substances and having a more permeable tissue than other organs and/or a low mitotic division in the root apical meristem [24][10]. Moreover, phytotoxic substances can affect genes responsible for the cellular characterization of radicle tissues and the endoderm, inhibiting their development. showed a promising inhibitory effect in the shoot and root growth
It is a fundamentally essential tool used in scientific disciplines such as agronomy, plant physiology, entomology, ecology, plant pathology, and many others. In wheat, the leaf area index was significantly affected by sorghum extract at different application times [108][11]. The leaf area index decreased with each increase in concentration, and a lower leaf area index (3.00) was recorded with 1:5 concentrations. Sorghum water extract applied at tillering gave a higher leaf area index, whereas sorghum water extract applied 50% at emergence + 50% at tillering resulted in a reduced leaf area index [108][11].
Stomata are small pores that absorb CO2and remove water vapor from the top and/or bottom of a leaf. When plant stomata open, water escapes through transpiration, and carbon dioxide is taken up via photosynthesis. The measurement of the gaseous exchange is important, as it is linked to photosynthesis and transpiration, which may be manipulated by environmental elements such as carbon dioxide, light, humidity, temperature, and wind speed [110][12]. The rhizome yield of Zingiber officinale Roscoe was significantly low when treated with Tamarindus indica L. (512 g/plant) leaves and mulched Mangifera indica L. (521 g/plant), attributed to drastic decreases in stomatal conductance, leaf production, rhizome thickness, rhizome spread, root length and spread, tiller production, and plant growth [111][13].
Chlorophyll molecules play an important role in photosynthesis and serve as a green pigment embedded in photosynthetic membranes, which is why a chlorophyll reduction normally results in a decrease in photosynthesis. Phenolic compounds such asp-coumarin, ferulic, ando-hydroxyphenyl acetic acids have been shown to inhibit chlorophyll biosynthesis and stimulate its degradation within 1 h after treatment. A crude powder ofM. polymorphasignificantly decreased the chlorophyll content and total photosynthetic pigments of the recipient species [112][14]. Plant extracts inhibit certain enzymes associated with chlorophyll and ultimately affect the integrity of chloroplasts and thylakoid membranes.
Plant extracts affect protein metabolism in plants, with the result that protein binding in chlorophyll a/b is reduced by two-fold. They also affect the photosynthesis by suppressing chlorophyll synthesis. Bioherbicides from plant extracts have the ability to reduce the Mg concentration in weed species, which has a major effect on chlorophyll synthesis [114][15]. [115][16] reported that photosynthesis-inhibiting herbicides like bentazon can limit electron transfer and carbohydrate synthesis.
Plant hormones are essential to plant growth through different metabolic activities. Gibberellin (GA) is a plant hormone that assists in the development of plant shoot growth [116][17]. Increased ABA production causes stomatal closing, catalyzes plant senescence, and lowers the rate of photosynthesis, further inhibiting plant growth and development [117][18]. Moreover, higher accumulations of JA could greatly reduce photosynthesis.
3. Effects of Plant-Based Bioherbicides on Weed Biochemistry
One of the important characteristics of plant tissues is their ability to respond to variable changes in the environment, which allows them to effectively manage and adjust to the altered ecosystem. Abiotic stress conditions, which include drought, heat, cold, salinity, nutrient deficiency, and oxidative stress, are among the environmental changes that greatly influence plant productivity, leading to morphological, physiological, and biochemical responses in plants [119,120][19][20]. Oxidative stress is one of the main consequences of biotic and abiotic stresses which affect physiological and biochemical metabolism in plants; hence, a balanced amount of reactive oxygen species (ROS) scavenging through proteins and antioxidant enzymes is important [121][21]. These oxidative molecules are typically formed when oxygen is reduced by 1, 2, or 3 electron transfer to form hydrogen peroxide, superoxide, and hydroxyl radicles, which are highly reactive species that are cytotoxic to biomolecules such as nucleic acids, proteins, and lipids, thereby causing protein denaturation and lipid peroxidation [124][22].
The plasma membrane is the primary site of cellular and organelle injury [125][23] by ROS, since they can react with unsaturated fatty acids to cause the peroxidation of the lipid bilayer in both cellular and intercellular structures [123][24]. Cellular damage consequently leads to the leakage of cellular contents, rapid desiccation, and, inevitably, cell death [118][25], while intercellular damage affects the respiratory activity in mitochondria and causes pigment breakdown in chloroplasts (Figure 1) [126,127][26][27]. ROS are equally produced by normal cell metabolism in organelles such as chloroplasts (photosynthesis), mitochondria (photorespiration), and peroxisomes (respiration), all powerful generators of ROS [126][26]. Generally, ROS function as an oxidant of proteins and lipids by changing their functions through the release of single active compounds that regulate photosynthesis, flower senescence, pollen growth, root formation, and root hairs [126][26].
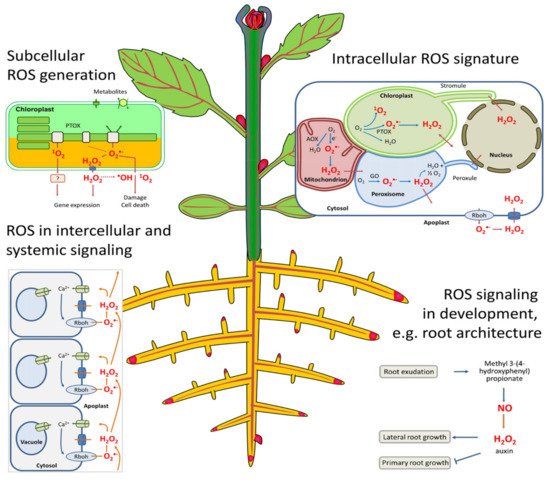
SOD is one of the first protection lines for ROS and stomatal reactions involved in the O2metabolism in plants [120][20]. It regulates lipid peroxidation and inhibits ROS damage to the membrane system, while hydrogen peroxide (H2O2) could be a strong oxidizing agent, producing highly reactive OH-that may decompose into H2O and O2by POD and CAT, thereby preventing possible ROS oxidative damage in plants [65][28]. SOD, POD, and CAT highly suppressed physiological activity in rice that was exposed to volatile allelochemicals (octane and undecane), and this activity was gradually enhanced as their concentrations increased [129][29]. This shows that the SOD and POD activity of a plant can be decreased by allelochemicals, thereby increasing the number of free radicals in the cells, which in turn leads to lipid peroxidation and the damaging of cellular and intercellular membranes [130][30].
To avoid self-destruction or necrosis in the cells, plants generally respond to oxidative stress by maintaining antioxidant defense compounds and continuously engage in removing the ROS at levels that reflect safety. Similarly, the antioxidant assay provides an insight into the metabolic phenomena that reflect physiological and biochemical responses in the plant’s ecosystem. The physiological and biochemical mechanisms underlying the inhibition effect of allelochemicals are of paramount importance to understand the mode of action and allelopathic response in the plant. For example, allelochemicals restrained seed germination through the inhibition of radicle development and hypocotyl elongation, hindered seed germination by the synthesis of ROS, and damaged sub-cellular structures, protein metabolism, and phytohormones [131][31].
Peroxidases bind to cellular polymers through covalent interactions, and these bindings are thought to be involved in lignin biosynthesis and the development of the cross-linking within the cell membrane. Sometimes, their activity in seedlings initiates mitochondrial respiration, and this accounts for the oxygen molecules that are converted into hydrogen peroxides [132][32]. A marked increase in POD production in lettuce seedlings, from 13 to 15%, was evident at both chloroformic and methanolic fraction concentrations [132][32].Brassica napusextract increased POD, SOD, and lipid peroxidation in the radicle and hypocotyl of rice, sorghum, and rape compared to control [133][33].
Catalases are important regulators of ROS in plant homeostasis. Although their regulatory activity is not fully understood [135][34], their reported functions include relieving cells from oxidative stress and improving organelles’ integrity. The enzymes have been documented to play an important role in plant detoxification and anti-oxidative processes that are closely linked to ROS generation throughout photorespiration and photosynthesis [135,136][34][35]. The inhibitory effect ofMentha piperitaL. caused an increase in catalase activity from 6 to 10% in the aerial part of radish and tomato, which is an indication of the induction of stress [137,138][36][37].
Proline is the amino acid that has been linked to different plant stresses. Under abiotic stress, an accumulation of proline in the plant may be an adaptive and metabolic measure of stress, an inhibitor of lipid peroxidation, and a defense against toxicity [123][24]. Proline is used by numerous organisms against the cellular imbalance caused by environmental stress. It is an inhibitor of lipid peroxidation and is usually synthesized by the plant in a high amount under stress conditions, where it serves as a scavenging molecule that protects the plant against abiotic stresses [139][38].
Malondialdehyde (MDA) content serves as an important indicator of membrane damage due to oxidative pressure. ROS induce the oxidation of unsaturated fatty acids through the overproduction of MDA in the cells [145][39]. The excessive secretion of ROS leads to the peroxidation of unsaturated lipid components, resulting in the damage of membrane rigidity and, eventually, cell dehydration. [148][40] reported that the application of an aqueous extract ofArtemisia absinthiumL. andPsidium guajavaL. resulted in an increased MDA content inP. hysterophorusseedlings which support free radical production and the occurrence of lipid peroxidation.
4. Factors That Influence Bioherbicide Efficacy
The efficacy of bioherbicides is the key restrictive aspect for their implementation. It is easy to see that bioherbicides play a key role in weed management, driving farmer incomes and feeding a growing population; however, applying bioherbicides is not as straightforward as its sounds. There are many elements that can influence the efficacy of bioherbicides, such as the bioactive compound/allelochemical content, plant growth stage, formulation type, spray preparation, application method, type of soil, and environmental factors (light, CO2, temperature, humidity).
They are non-nutritional compounds that can be synthesized from plant parts as secondary metabolites and also reflect the relationship between plants and the surrounding environment. The allelochemicals produced by plants are composed of various compounds, such as organic acids, aldehydes, lactones, ketones, fatty acids, amino acids, quinines, flavonoids, phenolics, coumarins, tannins, terpenoids, alkaloids, purines, and others [149][41]. Allelochemical type, concentration, and target plant are the major factors responsible for allelopathic interactions. Conversely, allelochemicals’ effectiveness depends on the type and age of the target plant.
Plant growth can be suppressed by the presence of an ample amount of amino acids. Consequently, selecting fungal strains based on their ability to produce amino acids is proving to be an effective weed control strategy [152][42]. Trichothecenes are bioherbicidal compounds prepared fromFusarium tumidum, which are effective againstCytisus scoparius(L.) Link. In another study,M. sativaextracts inhibited the germination ofA. vulgarisby up to 83% in petri dish assays and by up to 89% under field conditions [77][43].
Bioherbicide compounds are generally applied to weeds in the form of an emulsion, which can increase weed control stability and effectiveness [155][44]. Additionally, a careful selection of surfactants must be taken into consideration, since certain ingredients used in herbicide formulations can be toxic to humans [157][45]. For example, while pathogens can efficiently control a variety of weeds, they can also create some undesired toxins that could harm mammals and avians [158][46]. The vegetable oil and surfactants increased the adsorption ability of polar molecules, dissolved cuticular fatty acids, and thereby improved the penetration of hydrophilic active substances [161][47].
Water is one of the main inputs of spray preparation. The amount of water applied per hectare is closely connected to spray coverage and bioherbicide performance. The water quality used for bioherbicide mixing can influence the effectiveness of bioherbicide usage [10][48]. Water of poor quality can reduce agricultural chemicals’ activity, increase chemical breakdown in the spray water (hydrolysis), block spray lines or nozzles, as well as reduce the uniformity of chemical application [162][49].
Application methods have a significant impact on bioherbicide efficacy. Spray droplet size, distribution, retention, volume, and types of equipment are all factors involved in the application method of bioherbicides which influence their efficacy [163][50]. Weeds’ morphological characteristics, leaf surface characteristics, and the type of adjuvants are also important factors that can influence the efficacy of a bioherbicide. Other factors to be considered that influence bioherbicide efficacy include bioherbicide host spectrum, which may be broad or targeted to specific species, and also the nature of the formulation.
The efficacy of pathogens in bioherbicide formulation to control weeds can be affected by soil moisture. However, the soil must be covered with jute fabric following the bioherbicide application [169][51]. Under greenhouse conditions, the conidial suspensions ofColletotricum truncatumwith the addition of an invert oil emulsion decreased the moisture, yielding a 100% control [171][52] reported that the use of the bioherbicideP. macrostomain combination with nitrogen fertilizers to manage broadleaf weed species significantly enhanced the effectiveness of the bioherbicide, by 10 to 20%, againstT. officinale.
An effective use of bioherbicides relies upon environmental factors While several environmental factors impact foliar or post-emergence herbicides, The efficacy of a bioherbicide is influenced by environmental factors such as light, CO2, temperature, soil moisture, relative humidity, rainfall, and wind. These factors may impact the effectiveness of a bioherbicide directly, by altering its plant penetration and translocation mechanism, or indirectly, by changing plant growth and physiological characteristics [173][53].
References
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18.
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12.
- Somasundram, C.; Razali, Z.; Santhirasegaram, V. A review on organic food production in Malaysia. Horticulturae 2016, 2, 12.
- Sims, B.; Corsi, S.; Gbehounou, G.; Kienzle, J.; Taguchi, M.; Friedrich, T. Sustainable weed management for conservation agriculture: Options for smallholder farmers. Agriculture 2018, 8, 118.
- Hoagland, R.E.; Boyette, C.D.; Weaver, M.A.; Abbas, H.K. Bioherbicides: Research and risks. Toxin Rev. 2007, 26, 313–342.
- Singh, S.; Chhokar, R.S.; Gopal, R.; Ladha, J.K.; Gupta, R.K.; Kumar, V.; Singh, M. Integrated Weed Management: A key to Success for Direct-seeded Rice in the Indo-Gangetic Plains. In Integrated Crop and Resource Management in the Rice—Wheat System of South Asia; International Rice Research Institute: Los Banos, The Philippines, 2009; pp. 261–278.
- El-Mergawi, R.A.; Al-Humaid, A.I. Searching for natural herbicides in methanol extracts of eight plant species. Bull. Natl. Res. Cent. 2019, 43, 22.
- Kadioglu, I.; Yanar, Y.; Asav, U. Allelopathic effects of weeds extracts against seed germination of some plants. J. Environ. Biol. 2005, 26, 169–173.
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.; Hasan, M. Allelopathic potential of Malaysian invasive weed species on Weedy rice (Oryza sativa f. spontanea Roshev). Allelopathy J 2021, 53, 53–68.
- Motmainna, M.; Juraimi, A.S.; Uddin, M.; Asib, N.B.; Islam, A.K.M.; Hasan, M. Bioherbicidal properties of Parthenium hysterophorus, Cleome rutidosperma and Borreria alata extracts on selected crop and weed species. Agronomy 2021, 11, 643.
- Rab, A.; Khalil, S.K.; Asim, M.; Mehmood, N.; Fayyaz, H.; Khan, I.; Nawaz, H. Response of sorghum (Sorghum bicolor L.) extract type, concentration and application time to weeds weight, grain and biomass yield of wheat. Pure Appl. Biol. 2016, 5, 1.
- Tardieu, F. Plant Response to environmental conditions: Assessing potential production, water demand, and negative effects of water deficit. Front. Physiol. 2013, 4, 17.
- Abraham, E.; John, J.; Pillai, P.S. Allelopathic effect of leaf loppings of homestead trees on ginger (Zingiber officinale Roscoe). J. Trop. Agric. 2016, 54, 60.
- Najafpour, M.M. Oxygen evolving complex in photosystem II: Better than excellent. Dalton Trans. 2011, 40, 9076–9084.
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 309–323.
- Sousa, C.P.D.; Farias, M.E.D.; Shock, A.A.; Bacarin, M.A. Photosynthesis of soybean under the action of a photosystem II-inhibiting herbicide. Acta Plant Physiol. 2014, 36, 3051–3062.
- Radhakrishnan, R.; Khan, A.L.; Lee, I.J. Endophytic fungal pre-treatments to seeds alleviate salinity stress effects in soybean plants. J. Microbiol. 2013, 51, 850–857.
- Grossmann, K. Mediation of herbicide effects by hormone interactions. J. Plant Growth Regul. 2003, 2, 109–122.
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167.
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 163, 2–12.
- Grene, R. Oxidative stress and acclimation mechanisms in plants. Arab. Book Am. Soc. Plant Biol. 2002, 1, e0036.
- Quiles, M.J.; López, N.I. Photoinhibition of photosystems I and II induced by exposure to high light intensity during oat plant growth: Effects on the chloroplast nadh dehydrogenase complex. Plant Sci. 2004, 166, 815–823.
- Candan, N.; Tarhan, L. The correlation between antioxidant enzyme activities and lipid peroxidation levels in Mentha pulegium organs grown in Ca2+, Mg2+, Cu2+, Zn2+ and Mn2+ stress conditions. Plant Sci. 2003, 165, 769–776.
- Esfandiari, E.; Shekari, F.; Shekari, F.; Esfandiari, M. The Effect of salt stress on antioxidant enzymes’ activity and lipid peroxidation on the wheat seedling. Not. Bot. Hortiagrobot. Cluj. 2007, 35, 48–56.
- Janda, K.; Hidega, E.; Szalai, G.; Kovacs, L.; Janda, T. Salicylic acid may in-directly influences the photosynthetic electron transport. J. Plant Physiol. 2012, 169, 971–978.
- Dietz, K.J.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016, 171, 1535–1539.
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559.
- Ben Kaab, S.; Lins, L.; Hanafi, M.; BettaiebRebey, I.; Deleu, M.; Fauconnier, M.L.; Clerck, C.D. Cynara cardunculus crude extract as a powerful natural herbicide and insight into the mode of action of its bioactive molecules. Biomolecules 2020, 10, 209.
- Zhiqun, T.; Jian, Z.; Junli, Y.; Chunzi, W.; Danju, Z. Allelopathic effects of volatile organic compounds from Eucalyptus grandis rhizosphere soil on Eisenia fetida assessed using avoidance bioassays, enzyme activity, and comet assays. Chemosphere 2017, 173, 307–317.
- Cheema, Z.A.; Farooq, M.; Wahid, A. (Eds.) Allelopathy: Current Trends and Future Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012.
- Chen, F.; Meng, Y.; Shuai, H.; Luo, X.; Zhou, W.; Liu, J.; Shu, K. Effect of plant allelochemicals on seed germination and its ecological significance. Chin. J. Eco Agric. 2017, 25, 36–46.
- Nunes, P.M.P.; Silva, C.B.D.; Paula, C.D.S.; Smolarek, F.F.; Zeviani, W.M.; Chaves, S.C.; Miguel, M.D. Residues of Citrus sinensis (L.) osbeck as agents that cause a change in antioxidant defense in plants. Braz. J. Pharm. Sci. 2015, 51, 479–493.
- Haddadchi, G.R.; Gerivani, Z. Effects of phenolic extracts of canola (Brassica napuse L.) on germination and physiological responses of soybean (Glycin max L.) seedlings. Int. J. Plant Prod. 2009, 3, 63–74.
- Li, J.; Liu, J.; Wang, G.; Cha, J.Y.; Li, G.; Chen, S.; Kim, W.Y. A chaperone function of no catalase activity1 is required to maintain catalase activity and for multiple stress responses in Arabidopsis. Plant Cell 2015, 27, 908–925.
- Mhamdi, A.; Noctor, G.; Baker, A. Plant catalases: Peroxisomal redox guardians. Arch. Biochem. Biophys. 2012, 525, 181–194.
- Mahdavikia, F.; Saharkhiz, M.J. Phytotoxic activity of essential oil and water extract of peppermint (Mentha × piperita L. CV. Mitcham). J. Appl. Res. Med. Aromat. Plants 2015, 2, 146–153.
- Mahdavikia, F.; Saharkhiz, M.J.; Karami, A. Defensive response of radish seedlings to the oxidative stress arising from phenolic compounds in the extract of peppermint (Mentha × piperita L.). Sci. Hortic. 2017, 214, 133–140.
- El-Shora, H.M.; Abd El-Gawad, A.M. Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresenius Environ. Bull. J. 2015, 24, 386–393.
- El-Khatib, A.A.; Barakat, N.A.; Nazeir, H. Growth and physiological response of some cultivated species under allelopathic stress of Calotropis procera (Aiton) WT. Appl. Sci. Report. 2016, 14, 237–246.
- Kapoor, D.; Tiwari, A.; Sehgal, A.; Landi, M.; Brestic, M.; Sharma, A. Exploiting the allelopathic potential of aqueous leaf extracts of Artemisia absinthium and Psidium guajava against Parthenium hysterophorus, a widespread weed in India. Plants 2019, 8, 552.
- Cotruţ, R. Allelopathy and allelochemical interactions among plants. Sci. Pap. 2018, 1, 188–193.
- Pilgeram, A.L.; Sands, D.C. Molecular biology of the biological control of plant bacterial diseases. Molecular Biology of the Biological Control of Pests and Diseases of Plants; CRC Press: Boca Raton, FL, USA, 2020; pp. 39–56.
- Dudai, N.; Poljakoff-Mayber, A.; Mayer, A.M.; Putievsky, E.; Lerner, H.R. Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 1999, 25, 1079–1089.
- Boyette, C.D.; Hoagland, R.E.; Stetina, K.C. Efficacy Improvement of a bioherbicidal fungus using a formulation-based approach. Am. J. Plant Sci. 2016, 7, 2349–2358.
- Castro, M.J.; Ojeda, C.; Cirelli, A.F. Surfactants in agriculture. In Green Materials for Energy, Products and Depollution; Springer: Dordrecht, Germany, 2013; pp. 287–334.
- Kremer, R.J. The role of bioherbicides in weed management. Biopestic. Int. 2005, 1, 127–141.
- Hazrati, H.; Saharkhiz, M.J.; Niakousari, M.; Moein, M. Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol. Environ. Saf. 2017, 142, 423–430.
- Bailey, K.L. The bioherbicide approach to weed control using plant pathogens. In Integrated Pest Management; Academic Press: Cambridge, MA, USA, 2014; pp. 245–266.
- Schilder, A. Effect of Water pH on the Stability of Pesticides; Michigan State University Extension: East Lansing, MI, USA, 2008.
- Peng, G.; Wolf, T.M. Spray retention and its potential impact on bioherbicide efficacy. Pest Technol. 2008, 2, 70–80.
- Abu-Dieyeh, M.H.; Watson, A.K. Increasing the efficacy and extending the effective application period of a granular turf bioherbicide by covering with jute fabric. Weed Technol. 2009, 23, 524–530.
- Bailey, K.L.; Falk, S.; Derby, J.; Melzer, M.; Boland, G.J. The effect of fertilizers on the efficacy of the bioherbicide, Phomamacrostoma, to control dandelions in turfgrass. Biol. Control 2013, 65, 147–151.
- Cobb, A.H.; Reade, J.P. Herbicides and Plant Physiology; John Wiley & Sons: Hoboken, NJ, USA, 2011.
