Germplasm is a valuable natural resource that provides knowledge about the genetic composition of a species and is crucial for conserving plant diversity. Germplasm protection strategies not only involve rescuing plant species threatened with extinction, but also help preserve all essential plants, on which rests the survival of all organisms. The successful use of genetic resources necessitates their diligent collection, storage, analysis, documentation, and exchange. Slow growth cultures, cryopreservation, pollen and DNA banks, botanical gardens, genetic reserves, and farmers’ fields are a few germplasm conservation techniques being employed. However, the adoption of in-vitro techniques with any chance of genetic instability could lead to the destruction of the entire substance, but the improved understanding of basic regeneration biology would, in turn, undoubtedly increase the capacity to regenerate new plants, thus expanding selection possibilities. Germplasm conservation seeks to conserve endangered and vulnerable plant species worldwide for future proliferation and development; it is also the bedrock of agricultural production.
- germplasm
- plant genetic resources
- preservation
- propagation
- in vitro
1. Introduction
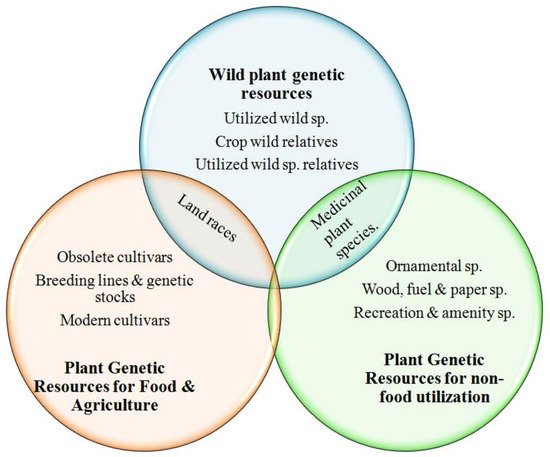
2. Conservation of Plant Genetic Resources: A Brief History
- (i)
-
The primary gene pool (GP1): Crossing among individuals is possible with normal seed set, segregation, and recombination such that gene transfer is possible through routine breeding. It includes both cultivated and wild races of a crop.
- (ii)
-
Secondary gene pool (GP2): It includes biological species which have some barriers of crossability with the crop (GP1), resulting in sterile hybrids, as chromosome pairing is not normal; hence, transfer of genes is restricted. Overcoming barriers of crossability can lead to normal seed development
- (iii)
-
Tertiary gene pool (GP3): More distant to GP2, crosses of GP3 with a crop (GP1) result in lethal or sterile hybrids due to abnormality in embryo development. Normal gene transfer is not possible but special tissue culture techniques can be deployed to produce hybrid embryos.
-
3. Need for Germplasm Conservation: Genetic Erosion and Genetic Vulnerability
-
Each crop enhancement program is aimed at increasing production which narrows down the genetic diversity. Harlan (1931) outlined the limited diversity available in barley
-
Tertiary gene pool (GP3): More distant to GP2, crosses of GP3 with a crop (GP1) result in lethal or sterile hybrids due to abnormality in embryo development. Normal gene transfer is not possible but special tissue culture techniques can be deployed to produce hybrid embryos.
-
3. Need for Germplasm Conservation: Genetic Erosion and Genetic Vulnerability
Each crop enhancement program is aimed at increasing production which narrows down the genetic diversity. Harlan (1931) outlined the limited diversity available in barley
-
- [16]
- . Similarly, Vavilov also chronicled the shrinking crop diversity due to modern agriculture breeding approaches. Since then, scientists have been concerned about the eroding genetic diversity and have realized that CWRs and landraces are a rich source of essential variability. Assessing the genetic loss in cereals
- led to the conclusion that cultivated crops have become less varied after domestication, due to selection pressures and dispersal bottlenecks
- [20]
- . Guarino refers to genetic erosion as a “loss of individual genes or combinations of genes, such as those found in locally adapted landraces, over time in a given region, and persistent reduction of common localized alleles”
- [21]
- . The definition suggests that a significant event in genetic erosion is the number and frequency of depletion of regionally adapted specific alleles. When geographical diversity reduces, the overall gene pool becomes more vulnerable to depletion and extinction, thereby reducing global equality and wealth
- [22]
- . According to the FAO, the key causes of genetic erosion are the direct replacement of local varieties, overexploitation of species, overgrazing, reduced fallow and changing agricultural systems, indirect land clearing, population pressure, environmental degradation, legislation/policy change, pest/weed/disease infestation, civil strife, and climate change making the PGRs more vulnerable to extinction. Plant species are also deemed endangered due to sudden changes in environmental conditions. They are either few in number or at risk of extinction
- [23]
- . It has been reported that about 12.5% (34,000 species) of vascular plants worldwide have been at threat (Table 1).
- Table 1.
- International Union for Conservation of Nature (IUCN) Red List for the year 2019–2020
- [24]
- .
[40]Category Status EX—Extinct 122 EW—Extinct in the wild 42 CR—Critically endangered 4674
- , the N.I. Vavilov All-Russian Science Research Institute of Plant Industry’s fruit collection (
- (accessed on 15 March 2021))
- [41]
- , and the Foreign Centre for Research in Agronomy (
- (accessed on 15 March 2021))
- . The Crop Trust’s CGIAR Gene bank Platform allows CGIAR gene banks to meet their fiduciary duties under the International Treaty on PGRFA to sustain and provide more accessions of crops and trees
- [44]
- . The 11 CGIAR gene banks are ideally situated as crop diversity hotspots, ensuring that germplasm acquisitions and distributions are global in scope, with a diverse range of partners and users
- [34]
- (Table 3) and the overall conservation trend depicted in Figure 3.
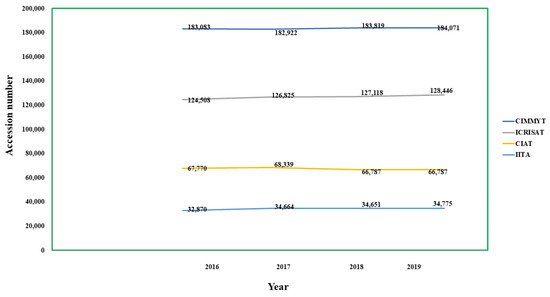
- Figure 3.
- Representation of total conserved accessions among various gene banks since 2016–2019, where years are represented by x-axis and accession numbers by the y-axis.
- Table 3.
- The CGIAR gene banks with number of accessions among respective crops as per 2019–2020.
International Institutes Number of Accessions under
Corresponding Crops as Per 2019–2020IITA- International Institute of Tropical Agriculture, Ibadan, Nigeria (my.iita.org/accession2/ (accessed on 15 March 2021))
(https://www.genebanks.org/genebanks/iita/(accessed on 15 March 2021)) [45] EN—Endangered 8593 VU—Vulnerable 8459 LR/cd—Lower risk: Conservation dependent 157 NT or LR/nt—Near threatened 3181 LC or LR/lc—Least concern 24,810 DD—Data deficient 4090
A reduction in diversity may not generally lead to genetic erosion on a comprehensive scale in a certain region. A study on Australian wheat reported no national shift in diversity, although in some countries, the genetic base of wheat has narrowed [25]. A parallel study on barley reported a decrease in allelic diversity in some of the surveyed countries in the Baltic region although overall diversity was preserved [26].
4. Methods of Germplasm Storage and Conservation
- Accessions are generally stored as different kinds of collections. Core collections
- [27]
- serve as an initial point for efficient germplasm utilization in crop breeding and refer to a subset of the base (large) collection or a limited number of accessions from an existing large collection of germplasm
- [28]
- . The core collection is used as a working collection and is closely reviewed, while reserve collections are accessions that do not form part of the core collection
- [29]
- . The vast number of base collections and a lack of accurate data on their economically important characteristics explain the underuse of genetic resources. ICARDA has made a core hybrid collection of 1000 entries of barley which reflects the genetic wealth of the entire world
- . The two fundamental storage approaches, ex situ and in situ conservation
- [32]
- , employed for germplasm storage are explained in Figure 2. PGRFAs need ex situ protection for safety from their natural environments. Samples may be stored as live plant specimens in field gene banks/botanic gardens/arboreta and can also be conserved as seed/pollen/explants/DNA in specialized artificial environments
- [33]
- . On the other hand, in situ conservation entails on-site survival of the species in its natural habitat ensuring sustainability of the environment and ecosystem, and in case of domesticated or cultivated species, storage within the ecosystem under which their distinctive characteristics developed.
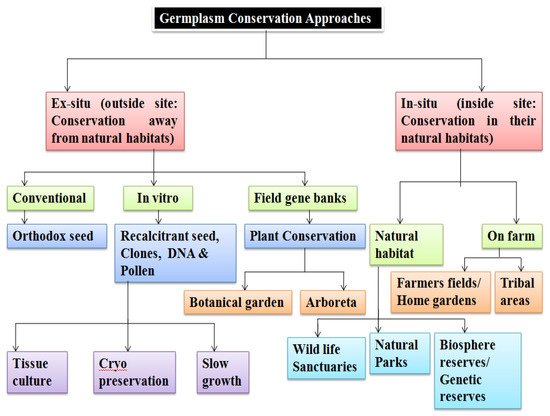
- Figure 2.
- Schematic outline of germplasm conservation approaches.
5. Status of Germplasm Conservation
- By the end of 2019, gene banks worldwide held 5.43 million accessions
- [34]
- and only 5.8% of these accessions are retained as living field collections; the rest are cryopreserved and deposited as DNA
- [35]
- . Up to December 2019, 290 gene banks across the globe managed to safeguard 96,000 of around 1700 species with a critical concern for IUCN, including wild relatives of crops that are vital for domestic and global food stability (
- (accessed on 15 March 2021))
- [36]
- . The USDA-ARS National Plant Germplasm System is the world’s largest provider of plant genetic capital, with 595,451 accessions covering 15,970 plants. However, the majority of them are annual species held as seeds, with the National Small Grains Set accounting for 25% of all accessions
- while woody perennials are less represented
- [39]
- .The USDA collections at Geneva, New York, Davis, Central America, and Riverside hold 73% of all accessions, including economically important crops like apple, grape, kiwifruit, walnut, pomegranate, mandarin, almond, and other related plants
- [39]
- . All these principal collections of annual fruit crops are conserved at institutes that include the National Fruit Collection in the United Kingdom (
- (accessed on 15 March 2021))
African Yam Bean-324, Groundnut-1890, Cassava-3184, Cowpea-15923, Maize-1561, Banana & Plantain-393, Soyabean-1575, Vigna-1878, Yam-5839 CIAT- International Centre for Tropical Agriculture, Cali, Colombia ( https://ciat.cgiar.org/ (accessed on 15 March 2021)) ( https://www.genebanks.org/genebanks/ciat/(accessed on 15 March 2021)) [46] Bean-37938, Cassava-6155, Forage-22694 CIMMYT- International Maize and Wheat Improvement Centre, Mexico City, Mexico ( https://www.genebanks.org/genebanks/cimmyt/(accessed on 15 March 2021) ) [47] Maize-28746, Wheat-155325 CIP- International Potato Centre, Lima, Peru ( https://www.genebanks.org/genebanks/international-potato-centre/ (accessed on 15 March 2021)) [48] Andean roots and tubers-2526, Potato-7224, Sweet potato-8080 ICARDA- International Centre for Agricultural Research in the Dry Areas, Aleppo, Syria ( https://www.genebanks.org/genebanks/icarda/(accessed on 15 March 2021) [49] Barley-31392, Chickpea-13299, Fababean-8736, Forages-24632, Grasspea-3992, Lentil-13128, Pea-4159, Wheat-40,843 ICRISAT- International Crops Research Institute for the Semi-Arid Tropics, Patancheru, Hyderabad ( https://www.genebanks.org/genebanks/icrisat/(accessed on 15 March 2021)) [50] Chickpea-20764, Groundnut-15699, Pearl millet-24514, Pigeon pea-13783, Small millets-11797, Sorghum-41889 AfricaRice- Africa Rice Centre, Abidjan, Côte d’Ivoire ( https://www.genebanks.org/genebanks/africarice/ (accessed on 15 March 2021)) [51] Rice- 21300 Bioversity International, Rome, Italy ( https://www.genebanks.org/genebanks/biodiversity-international/(accessed on 15 March 2021)) [52] Musa-1617 ICRAF- World Agro forestry, Nairobi, Kenya ( https://www.genebanks.org/genebanks/icraf/(accessed on 15 March 2021)) [53] Fruits-8246, Multipurpose trees-6456 ILRI- International Livestock Research Institute, Nairobi, Kenya ( https://www.genebanks.org/genebanks/ilri/(accessed on 15 March 2021)) [54] Forage grasses and legumes-18662 IRRI- International Rice Research Institute, Los Baños, Philippines ( https://www.genebanks.org/genebanks/irri/(accessed on 15 March 2021)) [55] Rice-132661
References
- Wilkes, G. In Situ Conservation of Agricultural Systems. In Biodiversity; Culture, Conservation and Eco Development; Oldfield, M., Alcorn, J., Eds.; West View Press: Boulder, CO, USA, 1991; pp. 86–101.
- Rao, N.K. Plant genetic resources: Advancing conservation and use through biotechnology. Afr. J. Biotechnol. 2004, 3, 136–145.
- Baute, G.J.; Dempewolf, H.; Rieseberg, L.H. Using genomic approaches to unlock the potential of CWR for crop adaptation to climate change. In Crop Wild Relatives and Climate Change; Redden, R., Yadav, S.S., Maxted, N., Dulloo, M.E., Guarino, L., Smith, P., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2015.
- Kell, S.; Marino, M.; Maxted, N. Bottlenecks in the PGRFA use system: Stakeholders’ perspectives. Euphytica 2017, 213, 170.
- Fao/Ipgri/Onu. Genebank standards for Plant Genetic Resources for Food and Agriculture, Rev. ed.; FAO: Rome, Italy, 2014; p. 182. ISBN 9290432365.
- Gosal, S.S.; Wani, S.H.; Kang, M.S. Biotechnology and crop improvement. J. Crop. Improv. 2010, 24, 153–217.
- De Wet, J.M.J. Cereals for the semi-arid tropics. In Plant Domestication by Induced Mutation, Proceedings of the Advisory Group Meeting on The Possible Use of Mutation Breeding for Rapid Domestication of New Crop Plants, Vienna, Austria, 17–21 November 1986; Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture: Rome, Italy, 1989; pp. 79–88.
- Wright, B.D. Crop genetic resource policy: The role of ex situ gene banks. Aust. J. Agric. Resour. Econ. 1997, 41, 81–115.
- Upadhyaya, H.D.; Gowda, C.L.L.; Buhariwalla, H.K.; Crouch, J.H. Efficient use of crop germplasm resources: Identifying useful germplasm for crop improvement through core and mini-core collections and molecular marker approaches. Plant Genet. Resour. 2006, 4, 25–35.
- Debouck, D.G. Managing Plant Genetic Diversity. Crop Sci. 2003, 43, 749–750.
- Warschefsky, E.; Penmetsa, R.V.; Cook, D.R.; Von Wettberg, E.J.B. Back to the wilds: Tapping evolutionary adaptations for resilient crops through systematic hybridization with crop wild relatives. Am. J. Bot. 2014, 101, 1791–1800.
- Foster, T.M.; Aranzana, M.J. Attention sports fans! the far-reaching contributions of bud sport mutants to horticulture and plant biology. Hortic. Res. 2018, 5.
- Vavilov, N.I. Origin and Geography of Cultivated Plant; Cambridge University Press: Cambridge, UK, 1992; ISBN 0521404274.
- Zhukovsky, P.M. Main gene centres of cultivated plants and their wild relatives within the territory of the U.S.S.R. Euphytica 1965, 14, 177–188.
- Harlan, J.R.; De Wet, J.M.J. Toward a Rational Classification of Cultivated Plants. Taxon 1971, 20, 509–517.
- Harlan, H.V. The Origin of Hooded Barley. J. Hered. 1931, 22, 265–272.
- Martos, V.; Royo, C.; Rharrabti, Y.; Garcia Del Moral, L.F. Using AFLPs to determine phylogenetic relationships and genetic erosion in durum wheat cultivars released in Italy and Spain throughout the 20th century. Fields Crop. Res. 2005, 91, 107–116.
- Rocha, F.; Bettencourt, E.; Gaspar, C. Genetic erosion assessment through the re-collecting of crop germplasm. Counties of ArcodeValdevez, Melgaço, Montalegre, Ponte da Barca and Terras de Bouro (Portugal). Plant Genet. Resour. Newsl. 2008, 154, 6–13.
- Ruiz, M.; Rodriguez-Quizano, M.; Metakovsky, E.V.; Vazquez, J.F.; Carrillo, J.M. Polymorphism, variation and genetic identity of Spanish common wheat germplasm based on gliadins alleles. Field Crops Res. 2002, 79, 185–196.
- Ross-Ibarra, J.; Morrell, P.L.; Gaut, B.S. Plant domestication, a unique opportunity to identify the genetic basis of adaptation. Proc. Acad. Natl. Sci. USA 2007, 104 (Suppl. 1), 8641–8648.
- Guarino, L. Approaches to measuring genetic erosion. PGR Documentation and Information in Europe—Towards a sustainable and user-oriented information infrastructure. In Proceedings of the EPGRIS Final Conference Combined with a Meeting of the ECP/GR Information and Documentation Network, Prague, Czech Republic, 11–13 September 2003.
- Upadhyaya, H.D.; Gowda, C.L.L. Managing and Enhancing the Use of Germplasm—Strategies and Methodologies; Technical Manual No. 10; ICRISAT: Patancheru, India, 2009.
- Brodie, J.F.; Aslan, C.E.; Rogers, H.S.; Redford, K.H.; Maron, J.L.; Bronstein, J.L.; Groves, C.R. Secondary Extinctions of Biodiversity. Trends Ecol. Evol. 2014, 29, 664–672.
- IUCN RED LIST 2019-20. Source-IUCN Official Website. Available online: (accessed on 15 March 2021).
- Brennan, J.P.; Fox, P.N. Impact of CIMMYT varieties on the genetic diversity of wheat in Australia. Aust. J. Agric. Res. 1993, 49, 175–178.
- Kolodinska, B.A.; Von Bothmer, R.; Dayeg, C.; Rashal, I.; Tuvesson, S.; Weibull, J. Inter simple sequence repeat analysis of genetic diversity and relationships in cultivated barley of Nordic and Baltic region. Hereditas 2004, 14, 186–192.
- Frankel, O.; Brown, A. Current Plant Genetic Resources: A Critical Appraisal; Oxford & IBH Publ. Co.: New Delhi, India, 1984.
- Upadhyaya, H.D.; Ortiz, R. A mini-core collection for capturing diversity and promoting utilization of chickpea genetic resources in crop improvement. Theor. Appl. Genet. 2001, 102, 1292–1298.
- Haupt, M.; Schmid, K. Combining focused identification of germplasm and core collection strategies to identify genebank accessions for central European soybean breeding. Plant Cell Environ. 2020, 43, 1421–1436.
- Furman, B.J. Methodology to establish a composite collection: Case study in lentil. Plant Genet. Resour. 2006, 4, 2–12.
- Azough, Z.; Kehel, Z.; Benomar, A.; Bellafkih, M.; Amri, A. Predictive Characterization of ICARDA Gene bank Barley Accessions Using FIGS and Machine Learning. Intell. Environ. 2019, 121–129.
- Maxted, N. Ex Situ, In Situ Conservation. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 683–695. ISBN 9780122268656.
- Ifeanyi, N.; Okorie, N.; Marshall, U.E. Germplasm Preservation and Propagation; The Foundation of Agricultural Development—A Review. IOSR J. Pharm. Biol. Sci. 2016, 11, 70–73.
- Halewood, M.; Jamora, N.; Noriega, I.L.; Anglin, N.L.; Wenzl, P.; Payne, T.; Ndjiondjop, M.N.; Guarino, L.; Lava Kumar, P.; Yazbek, M. Germplasm acquisition and distribution by cigar gen ebanks. Plants 2020, 9, 1296.
- FAO. WIEWS: World Information and Early Warning System on Plant Genetic Resources for Food and Agriculture; FAO: Rome, Italy, 2019; Available online: (accessed on 10 April 2021).
- FAO. 2020. Available online: (accessed on 13 April 2021).
- Byrne, P.F.; Volk, G.M.; Gardner, C.; Gore, M.A.; Simon, P.W.; Smith, S. Sustaining the Future of Plant Breeding: The Critical Role of the USDA-ARS National Plant Germplasm System. Crop Sci. 2018, 468, 451–468.
- GRIN-Global. U.S. National Plant Germplasm System. GRIN-Glob. Web Version 11040. 2019. Available online: (accessed on 2 April 2021).
- Migicovsky, Z.; Warschefsky, E.; Klein, L.L.; Miller, A.J. Using living germplasm collections to characterize, improve, and conserve woody perennials. Crop Sci. 2019, 59, 2365–2380.
- National Fruit Collection in the United Kingdom. Available online: (accessed on 20 March 2021).
- N.I. Vavilov All-Russian Science Research Institute of Plant Industry’s Fruit Collection. Available online: (accessed on 15 March 2021).
- Foreign Centre for Research in Agronomy. Available online: (accessed on 10 April 2021).
- CGIAR Genebank Platform. 2019 Genebank Platform Annual Report; Global Crop Diversity Trust: Bonn, Germany, 2020.
- CWR Project. Annual Report; Global Crop Diversity Trust: Bonn, Germany, 2019.
- IITA-International Institute of Tropical Agriculture. Available online: (accessed on 9 April 2021).
- CIAT-International Centre for Tropical Agriculture. Available online: (accessed on 9 April 2021).
- CIMMYT-International Maize and Wheat Improvement Centre. Available online: (accessed on 3 April 2021).
- CIP-International Potato Centre. Available online: (accessed on 9 April 2021).
- ICARDA-International Centre for Agricultural Research in the Dry Areas. Available online: (accessed on 3 April 2021).
- ICRISAT-International Crops Research Institute for the Semi-Arid Tropics. Available online: (accessed on 3 April 2021).
- AfricaRice-Africa Rice Centre. Available online: (accessed on 9 April 2021).
- Bioversity International. Available online: (accessed on 9 April 2021).
- ICRAF-World Agro Forestry. Available online: (accessed on 3 April 2021).
- ILRI-International Livestock Research Institute. Available online: (accessed on 9 April 2021).
- IRRI-International Rice Research Institute. Available online: (accessed on 3 April 2021).
