Wound healing involves a complex cascade of cellular, molecular, and biochemical responses and signaling processes. It consists of successive interrelated phases, the duration of which depends on a multitude of factors. Wound treatment is a major healthcare issue that can be resolved by the development of effective and affordable wound dressings based on natural materials and biologically active substances. The proper use of modern wound dressings can significantly accelerate wound healing with minimum scar mark. Sulfated polysaccharides from seaweeds, with their unique structures and biological properties, as well as with a high potential to be used in various wound treatment methods, now undoubtedly play a major role in innovative biotechnologies of modern natural interactive dressings.Заживление ран включает сложный каскад клеточных, молекулярных и биохимических реакций и сигнальных процессов. Он состоит из последовательных взаимосвязанных фаз, продолжительность которых зависит от множества факторов. Лечение ран - серьезная проблема здравоохранения, которую можно решить путем разработки эффективных и доступных по цене перевязочных материалов на основе натуральных материалов и биологически активных веществ. Правильное использование современных перевязочных материалов может значительно ускорить заживление ран с минимальным образованием рубцов. Сульфатированные полисахариды из морских водорослей с их уникальной структурой и биологическими свойствами, а также с высоким потенциалом использования в различных методах лечения ран, несомненно, сейчас играют важную роль в инновационных биотехнологиях современных натуральных интерактивных повязок.
1. Introduction1. Введение
The skin is one of the most important organs which, besides protecting the body from external stresses and pathogenic microorganisms, is involved in respiration, thermoregulation, and communication with the environment via receptors. Each human is always exposed to all kinds of injuries and wounds while being at home, at work, or as a result of an accident. After being inflicted, they reduce the quality of life and, therefore, any skin damage must be immediately and effectively treated. The dynamic mechanism of wound healing is a process involving a complex cascade of cellular, molecular, and biochemical responses and signaling processes triggered in a certain sequence [1][2].Кожа - один из важнейших органов, который, помимо защиты организма от внешних стрессов и патогенных микроорганизмов, участвует в дыхании, терморегуляции и коммуникации с окружающей средой через рецепторы. Каждый человек всегда подвергается разного рода травмам и ранениям, находясь дома, на работе или в результате несчастного случая. После нанесения они снижают качество жизни, поэтому любое повреждение кожи необходимо немедленно и эффективно лечить. Динамический механизм заживления ран - это процесс, включающий сложный каскад клеточных, молекулярных и биохимических реакций и сигнальных процессов, запускаемых в определенной последовательности [ 1 , 2 ].
The treatment of skin wounds has always been and remains a major healthcare and social issue. Every year, an immense number of people in the world get a countless number of wounds, injuries, burns, ulcers, and surgical wounds which require substantial funds and healthcare efforts for treatment. Therefore, invention of an effective wound dressing that would be affordable and easy to apply is still an urgent problem in modern medicine [1][3][4].Лечение кожных ран всегда было и остается серьезной медицинской и социальной проблемой. Ежегодно огромное количество людей в мире получает бесчисленное количество ран, травм, ожогов, язв и хирургических ран, для лечения которых требуются значительные средства и усилия здравоохранения. Поэтому создание эффективной раневой повязки, доступной по цене и удобной в применении, по-прежнему является актуальной проблемой современной медицины [ 1 , 3 , 4 ].
The history of medicine is, to a large extent, the history of the search for the most perfect wound dressing using natural materials and substances for primary medical care and specialized treatment of skin wounds [2][5][6]. In recent decades, various approaches have been developed and implemented for this purpose, including the use of special wound dressings and coatings such as polyurethane foam films, hydrocolloids, hydrogels, and paraffin dressing, which provide moisture and exudate adsorption and also delivery of active drug molecules to wound. These dressings are now increasingly demanded in the market of medical expendable supplies [1][3][4][7].История медицины - это во многом история поиска наиболее совершенной перевязки с использованием природных материалов и веществ для оказания первичной медицинской помощи и специализированного лечения кожных ран [ 2 , 5 , 6 ]. В последние десятилетия для этой цели были разработаны и реализованы различные подходы, включая использование специальных перевязочных материалов и покрытий, таких как пленки из пенополиуретана, гидроколлоидов, гидрогелей и парафиновых повязок, которые обеспечивают адсорбцию влаги и экссудата, а также доставку активного лекарственного средства. молекулы на рану. Эти повязки сейчас все более востребованы на рынке расходных медицинских материалов [ 1 , 3 , 4 , 7 ].
To date, the most effective treatment strategies for wound healing have been multifunctional types of wound dressings (bandages). Structurally, they include synthetic or natural biologically active substances (BAS) with mechanisms of anti-inflammatory, antimicrobial, immunostimulating, analgesic, and antioxidant action
[1][5][1,5]. Modern wound bandages are designed not only for covering skin damage, but also for minimizing possible medical complications and stimulating the healing phases of various wound types. Thus, the correct choice of wound dressing type with a specific mechanism of action is crucial for the successful treatment of certain wound
[4][5][8][9][4,5,8,9].
Synthetic products and materials pose a high risk of side effects: allergic complications, toxic effects, and a probability of bacterial drug-resistance
[3][5][10][11][3,5,10,11]. Therefore, with the development of technologies for designing wound bandages, natural biopolymers, including those derived from marine organisms, become increasingly valuable
[5][6][12][5,6,12].
The world’s oceans are inhabited by an abundance of organisms that differ from terrestrial ones by a significantly higher phylogenetic diversity. Due to their evolutionary adaptation to various environmental conditions, marine organisms such as algae, mollusks, sponges, and corals have acquired the ability to synthesize unique biopolymers exhibiting extremely high biological activity
[1][3][13][14][15][16][17][1,3,13,14,15,16,17].
2. Polysaccharides from Marine Algae Used in the Development of Wound Dressings
Marine algae are among the most ancient inhabitants of the planet. These are photosynthetic organisms with complex and peculiar taxonomy
[18][19][20][21][18,19,20,42]. Currently, two main types of algae are distinguished: microalgae, consisting of a single eukaryotic cell, are widely represented in marine ecosystems as phytoplankton, and macroalgae, having large sizes, are a heterogeneous group occupying the intertidal zone
[22][23][24][25][55,56,59,70].
Over millions of years of existence in the marine ecosystem, they have developed effective mechanisms of antibiotic protection against pathogenic microorganisms and numerous strategies of survival under extreme abiotic conditions of the environment
[2][10][26][27][28][29][30][31][32][2,10,21,44,45,46,47,48,49]. During evolution, these organisms acquired the ability to synthesize a wide range of metabolites and biomolecules, many of which have a unique chemical structure that other organisms do not have
[10][33][34][22][23][35][36][10,53,54,55,56,76,77]. Polysaccharides from marine algae are of particular interest due to the high resistance, biological activity, and availability of these organisms in large numbers
[26][24][37][38][39][21,59,61,69,74].
The cultivation of marine macroalgae with subsequent extraction of polysaccharides and their use for a variety of purposes, including therapeutic, due to their antiviral, antibacterial, immunomodulatory, and antitumor activities, has been a major focus of interest since long ago
[19][22][24][37][19,55,59,61].
The unique healing properties of algae have been known and used in wound treatment for many centuries. For these properties, sailors called them “mariner’s cures”
[40][79]. Over the past few decades, rich experience has been accumulated in the use of numerous homo- and heteropolysaccharides, widely represented in the main classes of marine algae, as a therapeutic basis for wound dressings
[14][15][16][17][30][14,15,16,17,47]. In recent years, these biopolymers (consisting of monosaccharides linked via glycosidic bonds) have attracted increasing attention as biotechnological raw materials for pharmacology, food, and cosmetic additives
[14][15][14,15].
The increased technological opportunities for the isolation and purification of these polysaccharides have substantially expanded the range of their practical and potential applications as a basis for various types of wound dressings
[2][31][32][33][23][41][2,48,49,53,56,75]. Due to their chemical and physical characteristics, such as mechanical strength, emulsification, adhesive properties, the ability to form hydrocolloids, and non-toxicity, they have a more pronounced healing effect compared to that shown by their traditional natural counterparts
[32][33][23][49,53,56].
In recent decades, hydrogels, which are three-dimensional hydrophilic polymer chains consisting of 99% water, have become an example of the widespread use of seaweed-derived polysaccharides in wound dressing design and tissue engineering
[25][39][70,74]. Due to the high biocompatibility, low immunogenicity and cytotoxicity, as well as the ease of functioning, these polymer systems are now actively used in wound treatment
[10][26][27][34][38][25][42][43][10,21,44,54,69,70,71,72].
The 3D reticulate structure of hydrogels simulates the microarchitectonics of extracellular matrix of native tissue, acts as a physical barrier against bacteria, providing optimal conditions in vivo for cell survival
[26][28][22][37][21,45,55,61]. Furthermore, the attractiveness of seaweed-derived polysaccharides to be used as a material for creating hydrogels is explained by their biological activity, biocompatibility and biodegradability, as well as by the opportunity of physical and chemical modification of their structure
[2][10][38][35][36][2,10,69,76,77].
The structural attractiveness of this type of wound dressings is enhanced by including nanofillers with antimicrobial and anti-inflammatory activities (gold, silver, zinc and copper oxides, antibiotics, hormones, etc.) in their composition
[2][26][27][22][38][2,21,44,55,69].
Biocompatibility and biodegradability are particularly attractive characteristics of seaweed-derived polysaccharides, due to which they can simulate the extracellular matrix to a certain extent
[2][31][41][2,48,75]. Such properties have raised significant biotechnological interest in these biopolymers used as a therapeutic basis to design bandage materials for already several decades
[2][29][30][22][23][2,46,47,55,56].
2.1. Alginates
Alginates, being polysaccharides derived from the class of brown algae (genus
Fucus), are considered among the world’s most common marine biopolymers. They have long been effectively used as a gelling agent and stabilizer of various solutions and suspensions, as well as a valuable component in food, chemical, and biotechnological productions. These polysaccharides are an indispensable component of various products manufactured in the pharmaceutical and medical industry
[36][40][44][77,79,80]. The unique characteristics of these metabolites have found application as a therapeutic basis for nanocomposite wound dressings
[45][44][46][78,80,81].
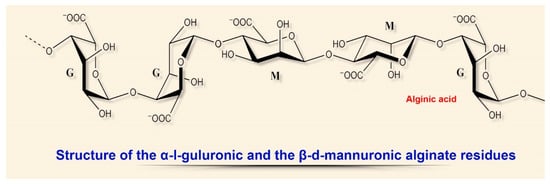
The physical and chemical properties of these linear acidic polysaccharides depend on the structural ratio of the two types of uronic acids, L-guluronic (G) and D-mannuronic (M), located in the biomolecule in the form of homo- or heteropolymer blocks (Figure
13).
Figure 13. Structural diversity of algal sulfated polysaccharides: α-I-guluronic and β-d-mannuronic alginate residues.
The most common technology for obtaining hydrogels from an aqueous alginate solution is the combination with an ionic crosslinking agent that is divalent cations (e.g., Ca
2+, Ba
2+, or Co
2+) interacting with G-fragments of polymer chains
[44][80]. Calcium alginate wound dressings with a high content of G-blocks have a lower rate of ion (Ca–Na) exchange with exudate, slowly swell, but are atraumatic and painless when removed
[47][48][82,83].
Analogous dressings with a high content of M-blocks quickly absorb wound exudate, but require special irrigation when removed
[47][48][49][82,83,84]. The ability of sodium ions to form transverse bonds with alginate makes these porous dressings an almost perfect barrier membrane for tissue engineering and targeted tissue regeneration
[50][51][52][53][23,25,29,85].
In recent reviews, the structural features and chemical properties of alginates and variants of their use in modern medicine were considered more in detail
[51][54][25,86].
When modern interactive nanocomposite wound dressings are created, the most valuable biological and pharmacological characteristics of these natural polyelectrolyte biopolymers include the biocompatibility, non-toxicity, biodegradability, as well as high hemostatic activity associated with the release of calcium ions which activate platelets and other clotting factors
[40][44][55][56][79,80,87,88]. The potent procoagulant properties of these anionic biopolymers were proposed to be used in the composition of calcium-sodium gel dressings for healing various types of wounds as long ago as in the second half of the 20th century (Kaltostat
TM, Kaltocarb
TM, Kaltoclud
TM)
[40][79].
At the same time, it was found that alginates included in wound dressings, in addition to the high hemostatic activity, provide the optimum moist environment in the wound and good absorption of wound exudate (20-fold relative to the dressing weight), stimulate the growth of granulation tissue, reduce the concentration of pro-inflammatory cytokines, inhibit the formation of free radicals, and have a pronounced antimicrobial activity
[44][55][57][80,87,89]. Clinically, this is manifested as a reduction in the healing time and longer intervals between bandagings, which also become painless and atraumatic
[44][55][56][58][80,87,88,90].
Due to their other, but no less important biotechnological properties (such as low cost, availability, and high biocompatibility), alginates are widely used in modern commercial hydrogel wound dressings in combination with metal ions for the treatment of acute and chronic wounds: diabetic ulcers, bedsores, and traumatic and surgical wounds (Algicell
TM, AlgiSite
TM M, Comfeel
TM Plus, etc.)
[56][59][60][61][62][88,91,92,93,94].
Furthermore, film and foam dressings based on sodium alginate also seem to be very promising. These types of wound dressings improve wound healing by normalizing gas exchange, protecting wounds from infection, especially in combination with other biopolymers, essential oils, or surfactants that enhance dispersion
[57][60][61][89,92,93].
The modern world pharmaceutical market offers a great variety of different types of alginate-based bandages, from traditional hydrogel dressings to innovative lyophilized sheets and nanofibers for cavity wounds
[63][29][64][65][66][27,46,95,96,97], as well as combined designs of these polysaccharides with Zn, Mn, Ag, glycerol, polyvinyl alcohol, and other marine-derived polymers
[62][67][68][69][94,98,99,100].
For example, K. Murakami with co-authors
[55][87] managed to effectively implement the wound healing properties of alginates in combination with other marine BAS (fucoidan, chitin/chitosan) and mitomycin C in a design of hydrogel-based wound dressing
[55][87]. The results of experimental studies show that this combination of marine biopolymers has a large number of properties of perfect dressing for wound healing: chemoattractant effect on fibroblasts, activation of their proliferation, as well as acceleration of tissue re-epithelialization and granulation, which began to appear on day 7.
In the analysis of the mechanisms of healing action of this wound dressing, attention is drawn, first, to the effective combined action of brown algae polysaccharides, alginates and fucoidans, whose low mechanical strength in this case is compensated by chitin and chitosan
[64][70][71][95,101,102].
Recently, polysaccharide-containing hydrogel bifunctional platforms based on a combination of alginate and hyaluronic acid, which are successfully used in cosmetology
[50][52][44][70][71][23,29,80,101,102], with hyaluronan, its derivative, have shown themselves well in wound healing. As it turned out, hyaluronan decelerates the release of Ca
2+ ions, regulates the alginate gelation, and, at the initial stages of healing, provides moisture for the wound, activates migration and proliferation of keratinocytes
[53][70][71][72][73][85,101,102,103,104].
Moreover, with the overall biological efficiency of alginate-containing hydrogel monoplatforms, the gelation process is a difficult-to-control stage, which results in heterogeneity of the gel structure and its unsatisfactory mechanical strength
[70][74][101,105]. In an experimental model, gel-like mixtures based on the alginate–hyaluronic acid combination showed a faster wound healing effect due to a positive influence on the gelation kinetics
[70][71][75][101,102,106]. In addition, alginate–hyaluronan hydrogel structures exhibit the potential to be a platform for delivering biologically active compounds directly into the wound
[71][76][102,107].
2.2. Fucoidans
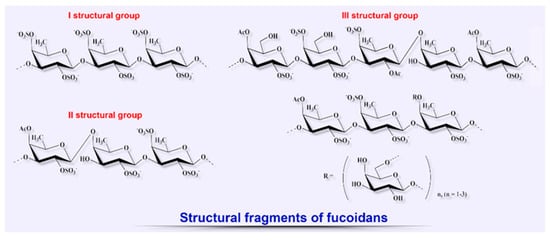
Since the late 20th century, the number of scientific studies aimed at elucidating the therapeutic potential of other biopolymers from brown algae such as fucoidans for the treatment of various diseases, including the wide range of their biological properties to be used for wound healing, has shown a tendency to increase (Figure
24)
[35][55][77][78][79][80][81][82][76,87,120,121,122,123,124,125].
Figure 24. Structural diversity of algal sulfated polysaccharides: fragments of fucoidans.
It has been established that this class of anionic sulfated heteropolysaccharides is present only in brown algae. Some marine invertebrates (sea urchins, Japanese sea cucumber) can synthesize similar polysaccharides
[15][17][15,17]. Their structure is composed only of sulfated fucose residues and is regular, which significantly distinguishes them from fucoidans
[16][17][16,17]. The chemical composition, structure, and biological properties of fucoidans are strongly dependent on the environmental conditions, season of collection, species of algae, and also on the technologies used for their fractional extraction and purification
[38][39][78][79][81][69,74,121,122,124].
Different proportions of the structural monosaccharides constituting fucoidans such as fucose (the main monomer), glucose, galactose (which is also sometimes the main monomer), xylose, mannose, as well as sulfate ester and uronic acid, determine the pattern of biological activities of these biopolymers
[38][55][82][69,87,125]. There are widely known commercial make-up products based on highly purified fucoidan extract (Maritech
® Reverse and Vita-Bright™), which exhibits pronounced regenerating, protective, and anti-aging properties for skin. However, the ability of fucans to modulate certain phases of wound healing by activating biomolecules and cellular processes has attracted the significant interest of biotechnologists in recent decades
[79][80][81][122,123,124].
For example, the presence and position of sulfate groups are important factors that determine the anti-inflammatory properties of these biopolymers, including the inhibitory activity of cell proliferation, peroxidation, and neutrophil migration, and also their properties as agents of the cell–receptor interaction and potent anticoagulants
[38][79][81][82][69,122,124,125]. In terms of the mechanism of anticoagulant action, low-molecular-weight fucoidans resemble heparin; they are a potent inducer of the production of such multifunctional cytokine as hepatocyte growth factor/scatter factor (HGF/SF), which plays an important role in the process of wound healing and re-epithelialization, stimulation of angiogenesis, and migration and proliferation of keratinocytes
[83][84][126,127].
As R. O’Leary with co-authors showed in their research
[85][128], besides the above-mentioned HGF/SF, some varieties of these polysaccharides derived from brown algae of the genus
Fucus actively interact with the transforming growth factor TGF-β, which is a potent cytokine regulating cell proliferation, differentiation, apoptosis, immune response, and remodeling of the extracellular matrix
[85][128]. In an experimental model of acute wounds caused by punch biopsy, the level of the TGF-β factor increased rapidly, which caused scars to form at the healing site. Fucoidans inhibited the antiproliferative effect of TGF-β, significantly increased the rate of repopulation of wound by fibroblasts and the rate of formation of the fibrillar collagen matrix, thus, being promoters of wound healing.
M. Kordjazi with co-authors
[38][69] were among the first to study the wound-healing effects of fucoidans in a burn wound experiment. The researchers paid attention to the anticoagulant, antithrombotic, anti-inflammatory, and antioxidant properties of these polysaccharides, whose activity depended on the degree of sulfation (from 32.6 to 19.0%). With a higher sulfate content, the wound-healing properties of fucoidans were more pronounced, which was manifested as the degree of activation of fibroblast proliferation (which is recognized as the main mechanism), collagen deposition, and an increase in the epidermis thickness.
Similar studies, conducted later, confirmed these results. A conclusion was made that low-molecular-weight fucans containing increased levels of sulfates and fucose accelerate skin wound healing through a complex and coordinated antioxidant, anti-inflammatory, and growth-dependent activity
[20][77][81][86][20,120,124,129].
The high potential of using fucoidans as the basis to create wound dressings was shown in the research of Australian scientists who used fucoidan extracted from the alga
Fucus vesiculosus to design a special polyelectrolyte multilayer assembly in combination with chitosan. According to the authors, the results obtained can contribute to the invention of promising dressing materials
[82][125].
In their recent studies, J. Cashman and A. Charboneau with co-authors revealed a high potential of fucoidans to inhibit the formation of post-operative adhesions in abdominal wounds
[86][87][129,130]. The authors used film-based wound dressings and preparations containing fucoidans derived from
F. vesiculosus in experiments with rabbit and rat models.
The potential and the identified properties of these polysaccharides are especially demanded in view of the high frequency (65–95%) of adhesions formed after surgical operations in the abdominal and pelvic regions according to medical reports
[88][63]. Therefore, the prevention and treatment of adhesion process is a public healthcare issue. Unfortunately, the search for barrier methods preventing adhesions has not been successful for many years
[88][86][63,129].
2.3. Carrageenans
Over the past few decades, the activity of studies on structurally diverse metabolites from red marine algae (Rhodophyta) having useful biological properties has substantially increased
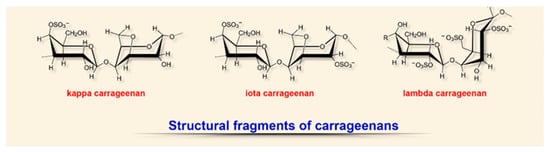
[20][89][90][91][20,135,136,137]. These algae contain chlorophylls, carotenoids, and xanthophylls, as well as pigments specific for this group: phycoerythrin and phycocyanin. The characteristic color and the name of algae are related to the presence of these pigments in different quantitative proportions (Figure
35)
[89][91][92][135,137,138].
Figure 35. Structural diversity of algal sulfated polysaccharides: fragments of carrageenans.
Carrageenans, a group of high-molecular-weight sulfated polysaccharides obtained from marine algae of the division Rhodophyta, attract particular attention as a rich and renewable source of phycocolloid polysaccharides
[18][89][18,135]. Currently, these structural components of algae membranes are considered as a promising source of biopolymers with a unique structure and specific physical and chemical properties
[20][90][91][92][20,136,137,138].
The structure of these anionic sulfated polysaccharides (polygalactans) consists of alternating linear chains of α-1,3-galactose and β-1,4,3,6-anhydrogalactose with ester sulfates (15–40%) and resembles natural glycosoaminoglycans
[18][20][89][90][91][18,20,135,136,137]. Depending on the degree of sulfation (from 15 to 40%), solubility and source of extraction, six types of carrageenans are distinguished, of which ϰ (kappa), ι (iota), and λ (lambda) are the most fully characterized and studied
[89][90][91][135,136,137]. The viscoelastic and gelling properties of these polysaccharides, as well as the presence of many functional groups in the structure (hydroxyl and sulfate), make these biopolymers a perfect material as a gelling agent in the design of hydrogel-based wound dressings with various chemical modifications
[92][93][138,139].
A few noteworthy reviews that focus on the transformation of characteristics of carrageenans depending on changes in their structure and chemical and physical properties have been published in recent years [Zhang, Shankar, Zia, Cunha]. Therefore, based on the goals of the present review, we here consider only the main trends in the use of these common and promising polysaccharides for wound healing as a basis for the design of various wound dressing types.
Among various polysaccharides from red algae, ϰ-carrageenan has certainly been studied best of all for the purpose of the development of hydrogel-based wound dressings (as the most common type of wound dressing). In addition to biocompatibility, this biopolymer type exhibits pronounced hemostasiological and immunomodulatory properties necessary for healing
[89][94][95][135,140,141].
Hydrogels are formed as a result of heat-reversible gelation, ion cross-linking, or photo-cross-linking of methacrylate modifications of the backbone of this biopolymer
[91][92][137,138]. In contrast to the simpler ionic cross-linking of polysaccharide in the presence of K
+ or Ca
2+, leading to the formation of brittle hydrogels
[92][96][138,142], the incorporation of methacrylate groups of photo-cross-linking in the main ϰ-carrageenan backbone, followed by activation with UV irradiation in the presence of a chemical photoinitiator, provided greater stability of reticulate gel
[96][97][142,143].
Gradient hydrogels based on ϰ-carrageenan and gelatin exhibit noteworthy healing properties and, therefore, have many advantages compared to conventional layered or reticulated analogues
[96][142]. The gradual and smooth variation in one of the physical properties of the material (viscosity, porosity, or density) simulates the tissue environment in vivo and has a positive effect on cell morphology
[92][96][97][138,142,143].
Promising types of ϰ-carrageenan-based hydrogels are nanogels that structurally contain medicinal nanoparticles of up to 100 nm and release them at a rate dependent on the temperature in the wound (37–45 °C), as well as hydrogels created by 3D-bioprinting with the desired shape and specified mechanical properties and chemical structure
[96][97][98][99][142,143,144,145]. These forms of carrageenan-based hydrogels are excellent excipients for the prolonged release of not only antimicrobial agents, but also bioactive molecules and growth factors
[97][143].
For example, in their recent study, H. Li with co-authors
[98][144] developed a promising strategy for three-dimensional bioprinting of a multilayered structure with strong interphase bonds using cationic (gelatin) and anionic (ϰ-carrageenan) hydrogels
[98][144]. The proposed structure was strong and also stable at 37 °C, providing high viability of cells in the wound.
The various antiviral and antibacterial activities of carrageenans, as well as their anti-inflammatory and immunomodulatory properties revealed in recent years, have raised additional interest in them from the biotechnological and pharmaceutical aspect as wound-healing biodressings
[89][90][135,136]. The insufficient mechanical strength of these polysaccharides is compensated for by the addition of various natural or synthetic polymers: polyvinylpyrrolidone, polyethylene oxide, polyvinyl alcohol, hyaluronic acid, or locust bean gum
[91][100][101][102][137,146,147,148].
For example, in a recent experimental work, A.V. Nair with co-authors
[103][149] studied the wound-healing properties of β-(1–3) (1–6) glucan/carrageenan hydrogels. The presence of carrageenan in the composition increased the porosity of gels and activated the attachment and proliferation of fibroblasts in experiments in vivo and in vitro, with a more rapid wound healing as compared to the control
[103][149].
Intact skin is known to have slightly acidic pH values (4.0–6.0); with bacterial infection, skin pH increases to an alkaline level (up to 9.0). Colonization of wounds by pathogenic bacteria negatively affects treatment, and, therefore, controlling pH as a biomarker of infection is important for assessment and monitoring of healing
[104][150].
K. Zepon with co-authors
[91][137] reported, for the first time, the development of a combined “smart” wound dressing: a pH-sensitive hydrogel film based on covalent binding of ϰ-carrageenan polysaccharide, locust bean (
Ceratonia siliqua) gum, and cranberry extract
[91][137]. In this design, locust bean gum enhanced the mechanical properties of carrageenan hydrogel. Another component of the coating, the anthocyanin-rich cranberry extract, is not only an antibacterial agent, but also acts as a sensitive pH indicator which changes its color in the case of an alkaline reaction in the wound fluid, thus indicating bacterial infection.
Thus, due to their almost perfect physical and chemical properties, carrageenans have found a wide range of applications as a basis for designing wound dressings. The presence of several functional groups in the composition, the high hydrophilicity and the strong negative charge of these polysaccharides allow the modification of their properties and enhancement of their biological activity in a wide range.
2.4. Ulvans
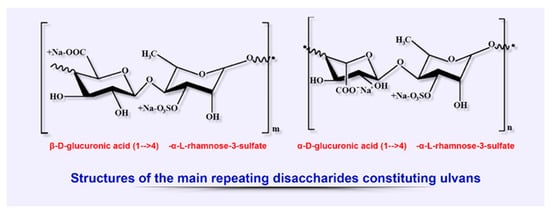
Ulvans, classified as a group of sulfated heteropolysaccharides, are among the main biopolymers extracted from cell wall of some members of green algae, the class Ulvales (species of Ulva, Enteromorpha, and Utricularia). Ulvans, being a component of the cell wall, provide osmotic stability and cell protection along with other polysaccharides of these algae (cellulose, xyloglucan, and glucoronan), constituting up to 45% of dry weight (Figure
46).
[20][105][106][20,151,152].
Figure 46. Structural diversity of algal sulfated polysaccharides: main repeating disaccharides constituting ulvans.
The chemical composition of ulvans strongly depends on the species of algae, the season of their collection, the habitat conditions during growth, and extraction methods. The typical structure of these polyanion heteropolysaccharides is represented by rhamnose, xylose, glucose, galactose, uronic acids (glucuronic and iduronic), as well as by sulfate and carboxyl groups structured as the main ulvanobiuronic (aldobiuronic) acid disaccharides designated as glucurorhamnose 3-sulfate (types A) and iduronorhamnose 3-sulfate (type B)
[18][107][18,153].
Ulvans are almost insoluble in organic solvents, which is explained by the relative hydrophobicity of rhamnose
[20][107][108][109][110][20,153,154,155,156]. This property limits the opportunities of chemical modifications of ulvans and prevents their potentially wide application in wound dressing design
[105][110][111][151,156,157]. However, in solutions with high pH, the conformation of these polysaccharides increases the intermolecular interactions in the wound, which makes it possible to obtain hydrogels with high viscosity
[106][108][109][152,154,155]. This feature allows the transformation of the gel-forming properties of polysaccharide by manipulating the structural and functional relationships
[106][107][152,153].
The presence of charged sulfate and carboxyl groups in the structure complicates obtaining mechanically stable hydrogels, which is associated with the active water absorption and development of hydrolytic degradation
[105][108][110][151,154,156]. When wound dressings are designed, these structural features of ulvans necessitate, on the one hand, solving the problem of their preliminary modification to make them insoluble and, on the other hand, increasing the mechanical properties of gels
[108][110][154,156]. The latter problem is solved by creating compound ionotropic gel complexes with cationic polymers or inorganic additives such as boric acid, copper, calcium, zinc, or magnesium
[108][112][154,158].
The presence of rare carbohydrates, iduronic acid, and sulfated rhamnose in the biochemical profile of ulvans is a feature distinguishing them from other seaweed-derived polysaccharides
[109][113][155,159]. Thus, the presence of rhamnose enhances the biological activity of ulvans, especially in the treatment of skin pathologies (by influencing the biosynthetic pathways in dermis), and also improves wound-healing properties (by reducing bacterial adhesion and stimulating cell proliferation and collagen biosynthesis)
[106][113][114][115][116][152,159,160,161,162].
The primary structure of these polysaccharides is directly related to the wide range of their macromolecular properties that determine the pharmacological attractiveness of ulvans and their potential to be used in biomedicine. Experimental and model-based studies have revealed significant antioxidant
[13][108][109][13,154,155], anticoagulant
[15][107][108][15,153,154], antitumor
[17][106][110][111][17,152,156,157], antihyperlipidemic
[13][17][106][108][13,17,152,154], and immunomodulatory
[13][16][107][113][13,16,153,159] biological activities of ulvans, both in vitro and in vivo. Moreover, ulvans, like all seaweed-derived sulfated polysaccharides, exhibit a wide range of antiviral activities. These biological features of ulvans have found application not only in the treatment of a number of diseases as a preventive anti-biofilm agent, but also in the design of bandages for wound treatment and tissue engineering
[106][109][111][115][116][152,155,157,161,162].
An example of successful application of the physical and chemical properties of this polysaccharide is the ulvan-chitosan polyion complex gel developed by K. Kanno with co-authors, which proved to be more stable than an alginic acid-chitosan gel both in acidic and basic conditions. However, under model conditions, this complex was inferior to the heparin-chitosan gel-coated vessel in terms of anticoagulant properties
[107][153].
Thus, studies on the biological properties of ulvans and their biotechnological potential for creating wound dressings are only at an initial stage, as compared to studies on other seaweed-derived polysaccharides. The structural features of these complex biopolymers require more attention to elucidate their effect on the wound process phases and subsequently propose substantiated recommendations on their use in specific types of wound dressings.Таким образом, исследования биологических свойств ульванов и их биотехнологического потенциала для создания перевязочных материалов для ран находятся только на начальной стадии по сравнению с исследованиями других полисахаридов, полученных из морских водорослей. Структурные особенности этих сложных биополимеров требуют большего внимания для выяснения их влияния на фазы раневого процесса и последующего предложения обоснованных рекомендаций по их использованию в конкретных типах раневых повязок.