SARS-CoV-2 has been the biggest pandemic since the influenza outbreak of 1918-1919. One of the biggest differences during the COVID-19 pandemic, in comparison to 1918-1919, has been the ability to rapidly test and diagnose the presence of the virus within patients and the general public. As with every testing regime, there is always going to be false negatives and misdiagnoses. The aim of this critical review was to assess the factors contributing to misdiagnosis of COVID-19 by examining sample types, diagnostic methods and by looking at asymptomatic versus symptomatic patients. It was found that a combination of detection methods such as the additional use of a computer tomography scan may help in reducing the level of false negatives in both symptomatic and asymptomatic patients. It was concluded that sputum and oral throat-washing samples should take precedence over swabbing where possible, while sample pooling should be used for widespread screening within the general population. The novel Oxford antibody assay was found to have the highest sensitivity and specificity of all commercially available kits, but should only be used within a specific timeframe to avoid misdiagnosis. Similarly, sample collection time and test method can greatly affect the outcome of the diagnostic method being conducted.
1. Introduction
Real-time polymerase chain reaction (Rt-PCR)-based testing is currently the most common form of sample testing for the presence of SARS-CoV-2 for both symptomatic and asymptomatic patients and is seen as a reliable method for the detection of virus nucleic acid
[1]. A 2015 meta-analysis carried out in Shanghai to evaluate the effectiveness of Rt-PCR for diagnosing novel coronavirus infections, based on past pandemics, concluded that PCR is an effective tool in disease diagnosis
[1].
A Canadian study by LeBlanc et al. (2020) in a comparison of 23 PCR laboratory-developed tests (LDTs) used for the detection of SARS-CoV-2 and some commercially available real-time reverse transcriptase PCR (RT-PCR) assays has found that, overall, there was little variation in the limit of detection between the LDTs (which tended to be between 3.4 and 4.5 log10 copies/mL) and the commercial assays and that few of the assays showed a reduced sensitivity
[2].
Unfortunately, the use of cycle threshold values for positive qualitative Rt-PCR tests for denoting viral load in the patient is not helpful as this method depends on the sensitivity of the assay being performed and does not give an absolute value for the viral copy number in the sample. There is a scarcity of reports of the expected viral load in respiratory samples of patients with COVID-19 disease during the course of the infection. One study published by Pan et al. (2020) in the Lancet, examining (only) two patients, has shown when using a PCR assay that amplified the N-gene in a quantitative RT-PCR that viral loads in throat swab and sputum samples peaked at approximately 5–6 days after symptom onset and contained approximately 104 to 107 copies per mL during that period.
Notwithstanding the scarcity of quantitative assays, another approach towards assessing the accuracy of testing methods for COVID-19 was a systematic review of the accuracy of COVID-19 diagnostic tests consisting of 34 case studies and 12,057 COVID-19-confirmed cases, reported false negatives between 2% and 29% (equating to a sensitivity of 71–98%)
[3], thus signalling the need for a comprehensive analysis focusing on the reasons contributing to misdiagnosis.
2. Symptomatic and Asymptomatic Patients
Asymptomatic patients are those who are actively carrying the live replicating virus, yet not presenting with any of the typical characteristic symptoms, in contrast to symptomatic patients who do. Due to the lack of physical indicators which may alert a carrier to the presence of the virus within their system, these asymptomatic patients are a cause for concern with regard to the continual, unknown transmission of the virus. In some instances, methods used to detect the presence of the virus may even be less sensitive for asymptomatic patients than those who are symptomatic
[4,5][4][5].
As shown in , it is evident that across both single-case studies and meta-analyses, a sizeable proportion of all cases are asymptomatic. In the study carried out by Sayampanathan (2021), 3790 close contacts were also observed for the development of COVID-19. Overall, 89 people tested positive. Of those, 50 people (56%) were asymptomatic, while 39 people (44%) were symptomatic. In general, this study concluded that symptomatic cases were 3.85-fold more likely to transmit the virus in comparison to asymptomatic cases
[6]. It must be noted that there is a lack of published literature surrounding comparative transmission models in asymptomatic and symptomatic patients, but it has been deduced that viral shedding of patients with laboratory-confirmed COVID-19 has shown to peak, on or before symptom onset, and a substantial proportion of transmission likely occurs before the first symptoms are present
[7]. It can be recommended that robust comparative experimentation should be conducted on the differences in transmission of both asymptomatic and symptomatic patients.
Table 1.
Collation of numerous studies showcasing the number and percentage of patients presenting asymptomatic and symptomatic for diagnosis with SARS-CoV-2 infection.
| No. of Patients and Case Studies |
No. of Asymptomatic
Patients (%) |
No. of Symptomatic
Patients (%) |
Reference |
628 patients
1 study |
576 (92%) |
52 (8%) |
[6] |
303 patients
1 study |
110 (36.3%) |
193 (63.7%) |
[8] |
50,155 patients
41 studies |
7818 (15.6%) |
42,297 (84.4%) |
[7] |
N/A patients
94 studies |
* N/A (20%) |
* N/A (80%) |
[9] |
213 patients
1 study |
41 (19.2%) |
172 (80.8%) |
[10] |
The development of symptoms in asymptomatic carriers was found to be a common occurrence in most studies. The occurrence of symptoms occurred in the following studies as shown: 21/110 (19.1%)
[8]; 88/180 (48.9%)
[7] across 10 studies and in 36% of asymptomatic obstetric patients
[11].
Interestingly, across 11 case studies focused on 1152 COVID-19-positive children, the amount of asymptomatic cases was found to be 27.7%, which was noted to be much higher than patients from all other age groups [7]. Worryingly, there appears to be an informed mindset that children are less susceptible to acquiring and transmitting COVID-19 infection due to numerous physiological and socio-economic reasons [12,13,14], yet evidence suggests that children are the most likely age group to be asymptomatic carriers. Therefore, it can be recommended that all scientific outreach surrounding children and COVID-19 should not perpetuate an idea of age-based ‘safety’ from infection and transmission of the virus. Interestingly, across 11 case studies focused on 1152 COVID-19-positive children, the amount of asymptomatic cases was found to be 27.7%, which was noted to be much higher than patients from all other age groups [7]. Worryingly, there appears to be an informed mindset that children are less susceptible to acquiring and transmitting COVID-19 infection due to numerous physiological and socio-economic reasons [12][13][14], yet evidence suggests that children are the most likely age group to be asymptomatic carriers. Therefore, it can be recommended that all scientific outreach surrounding children andCOVID-19 should not perpetuate an idea of age-based ‘safety’ from infection and transmission of the virus.
3. Examination of Sample Types Efficacy Used for Diagnosing COVID-19 Disease
3. Examination of Sample Types Efficacy Used for Diagnosing COVID-19 Disease
3.1. Nasopharyngeal Swabs and Oropharyngeal Swabs
A study from China examined 410 COVID-19 patients across 213 hospitals in China as they were subjected to intermittent NP, OP swabbing, and sputum sampling
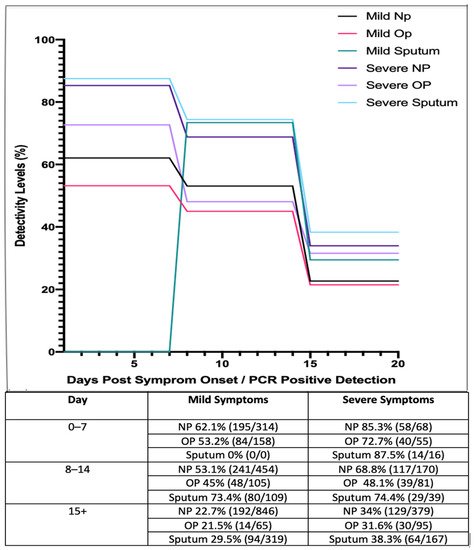
[73][15]. The results of these studies are presented in below.
Figure 21. Longitudinal analysis of COVID-19 percentage positivity rates when sampled across 213 hospitals in China, over 15+ days, using nasopharyngeal (NP), oropharyngeal (OP) swabs and sputum samples (adapted from [73]). Longitudinal analysis of COVID-19 percentage positivity rates when sampled across 213 hospitals in China, over 15+ days, using nasopharyngeal (NP), oropharyngeal (OP) swabs and sputum samples (adapted from [15]).
These studies suggest that those with mild symptoms are less detectable as the disease progresses, while detectability rates in general appear to decrease as the disease progresses and viral shedding occurs. These results suggest that NP and OP swabs are most reliable during early onset of the virus. In accordance with this, it has been reported that patients who have received negative results for both NP and OP swab tests have tested positive by other means such as bronchiolar lavage, as detailed in a research letter by Winichankoon, whereby a man in Thailand presented with the symptoms of COVID-19 and was repeatedly deemed negative
via NP and OP swabs. After careful examination, a bronchoalveolar lavage was conducted and the man was found to be positive for COVID-19
[74][16]. Overall, it is evident from this study that NP tests are more reliable than OP tests but are subject to inaccuracy on both accounts in respect to detection and disease stage.
In another study carried out in Wuhan, 4480 suspected cases were tested using nucleic acid (Rt-PCR) detection methods. Of those 4480 cases, 1875 (38.42%) tested positive for SARS-CoV-2. Among these cases NP swabs exhibited a 39.80% positivity rate, while 40.98% of OP swabs were found to be positive
[75][17]. While in contrast to the detectivity rates observed by Yang (2020), the range in observed differences is not as great. It is important to note that it is unclear what stage of the disease the patients were in as RT-qPCR is at its most sensitive within 7 days post-symptom onset
[42][18]; and while the first study carried out by Yang (2020) shows high detectivity rates, the sample size used was smaller. A higher sample size allows for a more accurate observation into how well these methods work.
Upon review, it would suggest that general swabbing alone, by means of testing the NP and OP cavities, may prove to be inefficient in comparison to other methods, both individually and in tandem. It is important to reiterate that NP tests are still the recommended testing method by the CDC. Swabbing is considered invasive to the patient, it requires care and precision upon collection by the front-line staff and is open to shortages and PCR-interference issues based upon material. Swabbing also carries a level of risk to front-line staff of aerosolised transmission upon collection, and they are not easily self-collected by the patient. When compared and contrasted to other sampling methods, it can be said that having NP and OP swabs listed as a preferred collection method must be reassessed, along with the recommendation that multiple sample types should be collected if relying on the swabbing method. If patients are presenting with upper-respiratory symptoms such as a sore throat, runny nose or nasal congestion, then OTW, NP and OP swabs would be a suitable method of collection, as seen in .
3.2. Bronchoalveolar Lavage Liquid Sample
Based on several studies, BLL is one of the most reliable samples to test for COVID-19, due to the nature of this disease. BLL samples were also collected during the same study conducted by Liu (2020) and Yang (2020). Firstly, within a hospital in Wuhan, China, only 5 patients within a cohort tested using BLL sampling methods (while not stated as to why, it can be speculated only 5 patients were intubated, making a BLL sample accessible) and an 80% positivity rate was observed
[75][17]. Secondly, daily samples were taken from infected patients in Shenzhen Third Peoples hospital across 4 weeks. It was noted that BLL showed 100% positivity in all patients upon admission, followed by 95%, 82%, 72% and 54% detection over weeks 1, 2, 3 and 4, respectively
[73][15].
Another set of samples was taken from 3 hospitals across the Hubei and Shandong provinces in China; 15 of these were BLL samples. It was observed how a 93% positivity rate was recorded in BLL samples in comparison to sputum, NP and OP samples (72%, 63% and 32%, respectively)
[76][19].
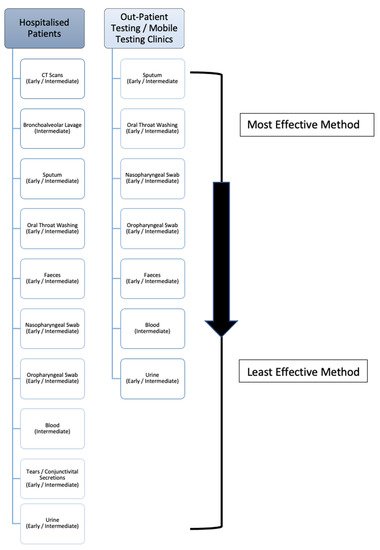
Upon review it must be noted, while the recorded percentage positivity rates of these BLL samples is high, the reported sample sizes are low in comparison to the other test methods due to the fact bronchoalveolar lavage testing is cumbersome and not routinely carried out unless a patient has typically been previously intubated. However, due to having direct access to the lungs, BLL has a high level of viral detection, and as seen in is the second best sampling method in hospitalised patients. Nonetheless, if patients are showing symptoms such as haemoptysis (a significant indicator of lung disease), as seen in , a bronchoalveolar lavage sample should be taken to both confirm the presence of COVID-19 and rule out the presence of any other disease/infection.
Figure 32. The deduced hierarchy in sample type and CT scan efficacy for diagnosis of COVID-19 disease, in both hospitalised patients and outpatient/mobile clinics along with the recommended disease-stage progression time-point to test at.
Table 32. The prevalence of COVID-19 within patients presenting with defined symptoms, contrasted against the percentage of those who experience these symptoms, all matched to the appropriate sample type to test for the disease
[42,77][18][20].
Examination or
Sample Type |
Symptom(s)
Associated with Choosing This Sample Type |
Number of Studies |
Number of People
Sampled |
% Prevalence of COVID-19 in Sampled Patients with
Associated Symptoms |
% Prevalence of
Symptoms in the
General Public Reporting to Be COVID-19 Positive |
| Chest-CT scan |
Dry Cough |
136 |
17,380 |
58 |
69.15 |
| Chest Pain |
30 |
3510 |
7 |
N/A |
| Bronchoalveolar lavage |
Haemoptysis |
21 |
4698 |
2 |
0.48 |
| Sputum |
Productive Cough |
136 |
17,380 |
58 |
27.88 |
| Faeces |
Diarrhoea |
93 |
11,707 |
10 |
9.96 |
Oral throat wash, nasopharyngeal swab and
oropharyngeal swab |
Sore Throat |
78 |
11,721 |
12 |
9.55 |
| Runny Nose |
36 |
10,656 |
8 |
N/A |
| Nasal Congestion |
10 |
2584 |
5 |
3.36 |
| Conjunctivital secretions |
Conjunctivitis |
9 |
2715 |
2 |
0.55 |
3.3. Sputum Samples
According to the WHO–China Join Mission Report on COVID-19, sputum production is observed in 33.7% of all patients who acquire the disease
[78][21], suggesting that this sampling method has testing limitations. However, in patients that are actively producing the specimen, studies have shown that this sampling technique is successful in diagnosing patients
[75][17]. In particular, 1875 patients from a hospital in Wuhan, China, were diagnosed with COVID-19. Different sampling methods were used and it was found that sputum samples exhibited a 49.12% positivity rate, in comparison to NP swabs which exhibited a 39.64% positivity rate
[75][17]. Furthermore, a study by Yang et al. (2020) collected 866 samples from 213 infected patients in Shenzhen Third Peoples hospital. Samples were divided into groups based on stage of infection ranging from days 0 to 7, 8 to 14 and 15+ (). Based on these results, sputum samples showed a high level of viral detection with a percentage positivity of 87.5%, while NP swabs showed a positivity rate of 72.7%. Even after 15+ days, sputum samples still exhibited a high level of positivity, ranging from 29.5 to 38.3%
[73][15].
Upon review, despite only being produced in 33.7% (according to the WHO) of patients, sputum comes directly from the lungs, thus yielding a high level of detectivity. Based on the evidence provided, it can be said that sputum may be a more accurate specimen to collect in comparison to NP and OP swabs, when the patient is actively producing sputum. When compared to oral throat wash (OTW) samples, more studies must be carried out on OTW methods in order to accurately make a considerate statement when contrasting these upper-respiratory collection methods. As a result, sputum should be the primary sample collected in outpatient centres/mobile testing units, and is the third most effective sample for hospitalised patients (), as it is relatively easy to collect and non-invasive. The nature of this method, unlike the previous, allows for self-collection which can help protect front-line workers and prevent viral transmission. Consequently, this is the most effective sample that outpatient and mobile clinic front-line workers with limited resources can collect. If patients are actively producing sputum via a productive cough, as reflected in , it could be recommended to sample the sputum instead of nasopharyngeal swabs or bronchoalveolar lavage liquid
3.4. Oral Throat Washing
While NP and OP swabbing is the preferred sampling method of the CDC and the World Health Organisation (WHO), it can be argued that OTW is another safer, less invasive and more efficient means of sample collection. Firstly, the production of live aerosolised virus during NP and OP sampling places front-line workers at risk; conversely, the self-collection of gargled solution prevents aerosol transmission. Secondly, the quality of swab sampling and stage-of-illness is open to significant variation, thus creating the possibility of false negatives; saline solution is standardised and throat washing covers a large surface area. Thirdly, swabbing is highly invasive and leads to patient discomfort; throat washing is a comfortable, non-invasive experience
[79][22].
The OTW technique was carried out on 11 COVID-19-positive patients in China comprising 5 discharged patients and 6 hospitalised patients, with a median sample collection date of 53 days post-symptom onset (range: 48–57 days). A total of 24 paired (taken at the same time) OTW samples and NP samples were taken. While most of the paired samples came back as negative, 5 pairs had shown inconsistent results, whereby the OTW came back as positive, and the NP swabs came back as negative. Using statistical analysis, a statistically significant difference was observed between using OTW samples as opposed to NP swabs. While the sample size was small, the OTW sampling method was able to detect viral nucleic acid 48 days PSO
[79][22].
The OTW method was observed to be a significant way of detecting SARS-CoV virus in 2004, with large recorded viral titre (9.58 × 10
2 to 5.93 × 10
6 copies/mL) yields. As a result, it was recommended by the study’s authors that OTW should be included in the collection guidelines for SARS diagnosis
[80][23].
Upon review, the next most effective collection method is the oral throat washing sample. Again, this is non-invasive, allows for self-collection to prevent transmission and has a high level of detection due to the large surface area that the wash can cover within the oral cavity as it collects the sample. The oscillation of the cricopharyngeal muscle over posterior pharyngeal wall allows for the virus-containing mucus to be dislodged to an extent and efficiently gathered within the saline rinse. As a result, it has previously been recommended to be included in collection guidelines as outlined by Wang (2004). As seen in , this is the second most effective sample collection method for mobile clinics and outpatient staff, and the fourth most effective sample collection method within hospitalised patients.
3.5. Faecal Sampling
For the purpose of uniformity, anal swabs will be included under the subsection of faecal sample (FS) collection. A study involved nine patients (noted as a limitation within the study) confirmed to have COVID-19, who were subjected to a range of sampling methods at different days PSO (post-symptoms onset) including anal swabs. Of the 9 patients, two showed viral titres from their anal swabs, both at day 3. Interestingly, not all patients that tested positive
via anal swabs had diarrhoea, indicating that COVID-19 can invade the digestive system. Viral components that have been shed through the digestive tract have been detected in faeces and as a result may be transmitted via faecal-oral transmission, with evidence of viral nucleic acid being found within clinical stool samples
[81,82][24][25].
Furthermore, a study by Wang, 2020, with 1014 patients, in Wuhan, had been confirmed to have the virus by Rt-PCR. A total of 153 of these patients had their faeces examined and 44 people (29%) had detectable levels of the virus within their stool samples
[76][19].
Another study followed the detection of viral nucleic acid in patient stools upon admission and over the course of 4 weeks, across mild and severe symptom patients. Out of a total of 93 samples, upon admission, 55 (59%) tested positive for the virus. There was no observed difference between mild and severe cases in viral loads. Upon review of the cases, in both mild and severe cases, the amount of viral load present within the stool peaked upon admission, reduced over the first week, peaked again over weeks 2 and 3, and declined at week 4. While the administration of therapeutic care could affect these results, it appears that the shedding of viral load is high at the initial stages of infection, and again at weeks 2 and 3, which would indicate the best times for sample collection
[83][26]. Similar sentiments were observed with the 2004 SARS virus, whereby a 13% detectivity of the virus was found upon admission, with peak levels of detectivity (70%) occurring at days 9–11, with a steady decline in the days thereafter
[84][27].
Upon review, while the length and duration of the presence of the virus within the GI (gastro-intestinal) tract is unknown, some studies suggest it may be present longer than found within the respiratory system. Indeed, a study detected viral RNA in stool samples from paediatric patients for longer than 4 weeks
[85][28]. While less sensitive than the previously listed sample types, evidence suggesting that viral RNA can be detected within faecal matter long after it has left the lungs is a highly relevant piece of information for two reasons. Firstly, it shows that the virus could still be transmitted via the faecal-oral route, even after the initial 2 week quarantine period, and secondly, it has a potential to be a highly useful diagnostic tool within a clinical setting during post-disease analysis of a patient. As a sampling method for detection, FS appears to be more conclusive than blood, urine and conjunctivital secretions for the detection of COVID-19, but less conclusive than some upper-respiratory samples. Upon examination of a total population of results, FS appear to be only slightly better than both NP and OP swabs in terms of the percentage of positive detection within the sample. Therefore, upon admission to hospital (as seen in ) patients should be sampled collectively
via faecal sampling and both NP and OP swabs. For outpatient/mobile clinics, both NP and OP swabs are more efficient, followed by faecal samples. That being said, the persistent shedding of viral RNA allows for the possibility of longer term detection of the virus, should the necessity arise, which is not commonly seen in the other sample types and may be useful information within a clinical diagnostic setting. It should also be noted that carers of patients should be careful of possible faecal transmission of the virus on approximately days 9–14, where shedding is at its highest.
3.6. Other Potential Sample Types
In a study by Wang (2020), both urine and blood samples were taken from infected patients from 3 hospitals across the Hubei and Shandong provinces in China; 72 of which were urine samples (US), and 307 of which were blood samples (BS). Of the samples taken, none of the US came back as positive, and 3/307 (1%) of the BS came back positive
[76][19].
Conversely, there is only one study carried out in which a patient tested positive via a US
[81][24]. Nine patients, confirmed to have COVID-19, were subjected to a range of sampling methods at different days from the onset of their symptoms. These samples were subjected to Rt-PCR testing. Of the 9 patients, one showed viral titres (3.22 × 10
2) in their urine at day 7. While the sample size is low, this may be due to a sampling error, but it must be noted that the effects of COVID-19 on the urinary system is not fully understood. In a second study by Peng et al. (2020), 2/9 patients showed viral titres within their blood samples. Interestingly, both patients’ samples were collected on day 3, yet the severity of their symptoms was not reported
[81][24].
Upon review, blood, conjunctivital secretions and urine (in that order as seen in ), are the least effective samples to collect for diagnostic purposes within a hospital setting.
Though the research carried out is limited, from the given evidence, it appears that urine is the least useful sample (11% detection) from those examined to date while blood is also a highly unreliable source of testing for the virus (22% detection). As a result, blood and urine should not, in any setting, be used as a primary sampling method and should only be considered as an additional post hoc test having employed more effective methods as listed above.
According to the WHO–China joint mission on COVID-19 report, conjunctivital congestion is observed in 0.8% of all COVID-19 patients. While the lack of specimen production deems this method a highly unsuitable testing technique, it carries a level of caution as CS and tears can transmit the virus. At the First Affiliated Hospital of Zhejiang University, China, 30 COVID-19-confirmed patients had their tears and CS collected and subjected to RT-PCR analysis, over 2–3 day intervals. Of these patients, 21 had mild symptoms and 9 had severe symptoms. Only a single patient’s (1 out of 30) tears and CS tested positive for the virus while the rest tested negative. It was noted that all patients without CS tested negative
[86][29]. A meta-analysis examining the levels of confirmed positivity via ocular swabs for the detection of COVID-19 found that the proportion of patients reporting conjunctivitis/red eye was 3.175% (95% CI 1.165–6.127). However, only 0.703% patients (95% CI 0.0358–3.269) reported conjunctivitis as the first symptom of the disease. Among all COVID-19 patients, the proportion of conjunctival/tear sample that was positive for the virus (RT-PCR detection of SARS-CoV-2) was found to be 1.949% (95% CI 0.743–4.113)
[87][30]. Upon review, while the occurrence of this specimen is low and an unsuitable sampling technique, based upon statistical reliability when compared to the other sampling techniques, it carries a level of caution as a form of viral transmission.