Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Silvia Fernández Martín.
Osteoarthritis is the most common progressive joint disease diagnosed in companion animals and its management continues to be a significant challenge. Nutraceuticals have been widely investigated over the years in the treatment of osteoarthritis and among them, glucosamine and chondroitin sulfate treatments are probably the most common therapies used in veterinary management.
- animal models
- biochemical markers
- cartilage
- chondroitin sulfate
- glucosamine
- nutraceuticals
- osteoarthritis
1. Introduction
Osteoarthritis (OA) is a heterogeneous chronic disease that involves all tissues in the synovial joints. It is usually characterized by progressive cartilage damage, subchondral bone changes, osteophyte formation, synovial inflammation and the secretion of inflammatory mediators [1,2][1][2]. At present, it is most common progressive joint disease diagnosed in companion animals and its management continues to be a significant challenge [3]. Lameness, stiffness and chronic pain resulting from the OA pathology have a negative impact on the quality of life of the affected animals [4]. Additionally, OA pain is frequently mishandled in animals, and consequently, some clinical cases may result in premature euthanasia [5].
At present, there is hardly any accurate epidemiological data available of this disease in the different animal species [6]. In sport horses, OA is one of the most prevalent and disabling diseases in sport horses, fundamentally affecting the metacarpophalangeal joint and causing chronic and painful lameness as well as an important economic loss in the equine industry [7]. Furthermore, a recent study has reported a noteworthy prevalence of cervical OA in jumping horses. More specifically, a moderate to severe OA was observed at C6-7 in 25% of the studied population [8]. In dogs, OA is highly prevalent with reports of around 20% of the canine population over a year-old [9]. Nevertheless, subsequent studies reported lower values, as observed by Anderson et al. [3] and O’Neill et al. [10], who estimated an OA prevalence of 2.5% or 6.6% in primary-care practices in the UK. Generally, large-breed dogs developed initial and more severe clinical signs of OA [4]. However, early symptoms may be overlooked by the owner or considered normal, thus the joint disease is usually diagnosed at a later stage [3]. In cats, it is a very common joint disease, especially in older cats. In relation to this, a previous study reported an OA prevalence of around 61% at over 6 years of age [11]. OA in cats seems to be related to behavioural changes such as decreased mobility and less grooming [11]. However, it is important to highlight the underdiagnosis of the disease associated with the lack of signs such as lameness and a lower radiographic identification. This is in addition to its difficult physical examination by clinicians [12]. Furthermore, the treatment of OA is a major challenge in this specie, related to reduced availability of drugs as well as increased adverse effects and complications [13].
For many years, the available therapeutic options for OA management were focused on inflammation relief and pain control and were basically restricted to the use of non-steroidal anti-inflammatory drugs and analgesics. However, their chronic administration was limited by their deleterious systemic side effects [6]. Currently, there is no ideal drug capable to reverse or stop the progression of the OA and for that purpose, numerous therapeutic agents have been widely researched for their potential role in targeting the underlying pathology of OA with various levels of efficacy [14]. Nutraceuticals, also classically called chondroprotectors, have been widely analysed over the years in the treatment of OA in companion animals. Among them, glucosamine and chondroitin sulfate treatments are probably the most commonly used in the veterinary management of OA [4]. These dietary supplements have been proposed to promote the cartilage and periarticular bone health status [15] and their effectiveness in the OA progression has been thoroughly tested in experimental research. However, heterogeneous results were obtained in different animal studies and their function as disease-modifying drugs is still controversial. Some published clinical trials in dogs treated with glucosamine and chondroitin sulfate, reported positive clinical effects with significant pain relief [16], whereas in other publications, no significant differences were found between treated and untreated dogs [17,18][17][18].
2. Synthesis of the Main Outcomes of the Effect of Glucosamine and/or Chondroitin Sulfate
The analysed studies were classified based on the therapy evaluated and its effects on the synovial joint tissues, osteophyte development and biochemical markers (Table 21). In the 22 studies included in this review, 26 nutraceutical effects have been evaluated, distributed as follows: Glucosamine sulfate (GS) (n = 6), glucosamine hydrochloride (GH) (n = 8), chondroitin sulfate (CS) (n = 5), CS+GH (n = 3) and CS+GS (n = 4).
Table 21. Synthesis of main outcomes of the effect of nutraceuticals.
| Nutraceutical | Reference | Initial Adminst Ration | C | SB | SM | OST | BM |
|---|---|---|---|---|---|---|---|
| Glucosamine sulfate (GS) | Abdul-Kadir et al. [33][19] | Delayed | + | x | x | x | + |
| n = 6 | Permuy et al. [36][20] | Delayed | − | − | − | x | x |
| Salman et al. [31][21] | Early | + | x | x | x | + | |
| Wang et al. [41][22] | Early | + | x | ||||
| − | |||||||
| n | |||||||
| = 3 | |||||||
| Kobayashi et al. | |||||||
| [ | |||||||
| 39 | |||||||
| ] | |||||||
| [ | |||||||
| 36 | |||||||
| ] | |||||||
| Early | |||||||
| ? | x | x | x | ? | |||
| Terencio et al. [44][37] | Pre-emptive | + | ? | ? | x | + | |
| Chondroitin sulfate + GS | Roman-Blas et al. [35] | Pre-emptive | − | − | − | x | − |
| n = 4 | Sanches et al. [43][38] | Early | + | x | x | x | ? |
| Lee et al. [28][39] | Early | x | + | x | x | x | |
| Silva et al. [48][40] | Pre-emptive | + | x | x | x x | + |
C cartilage, SB subchondral bone, SM synovial membrane, OST osteophyte, BM biochemical markers. (+) Positive effect; (−) negative effect or no effect; (?) unclear or not significantly effect; (x) not included. Therapy initial administration: Pre-emptive (before OA induction), early (OA induction- 14 days post); delayed (>14 days post-OA induction).
Most of the publications analysed the glucosamine effect (n = 14) in its hydrochloride (n = 8) or sulfate (n = 6) form. Less number of publications analysed the effect of the chondroitin sulfate, administered either alone (n = 5) or in combination with glucosamine sulfate (n = 4) or glucosamine hydrochloride (n = 3). Additionally, it should be noted that one study included the evaluation of glucosamine sulfate and chondroitin sulfate separately [36][20], another the glucosamine sulfate and the glucosamine hydrochloride [29][24], another the effect of the glucosamine hydrochloride and the chondroitin sulfate [32][31] and another the combination of chondroitin sulfate plus glucosamine hydrochloride against chondroitin sulfate plus glucosamine sulfate [35]. Consequently, as we explained before, within the 22 studies included in the present systematic review, 26 evaluations of the nutraceutical effect alone or in combination were carried out.
Regarding the parameters evaluated, the cartilage response is by far the most assessed, being included in 25 evaluations out of 26. Positive chondroprotective effects were identified in approximately half of the evaluations (14 out of 25; 56%). In the individual analyses, the results were as follows: glucosamine sulfate (4 out of 6; 67%), glucosamine hydrochloride (4 out of 8; 50%), chondroitin sulfate (3 out of 5; 60%); chondroitin sulfate plus glucosamine hydrochloride (1 out of 3; 33%) and chondroitin sulfate plus glucosamine sulfate (2 out of 3; 67%). The biochemical markers of OA were the second most studied parameter in this systematic review and was included in 20 out of 26 therapy assessments. Nutraceuticals showed a positive effect in 13 of them (13 out of 20; 65%). Specifically, in terms of glucosamine therapies, we identified positive responses in 4 out of 5 (80%) sulfate formulations and in 4 out of 6 (67%) hydrochloride ones. With respect to chondroitin sulfate, 3 out of 5 publications included biomarker evaluations and, in this case, all of them showed fewer biochemical alterations in the treated groups. The subchondral bone changes were determined in 8 out of the total number of included evaluations, identifying beneficial effects in only two of the publications (2 out of 8; 25%) [28,38][39][27]. The synovial inflammation was evaluated in 7 studies, showing supressed synovitis in only one of them (1 out of 7; 14%) [46][23]. Finally, the osteophyte development was evaluated in 3 studies, but only in one of them a reduced osteophyte formation was observed after glucosamine hydrochloride administration [29][24].
3. Therapy Duration and Initial Administration at Baseline
The nutraceutical therapy periods were shown in Table 32. The majority of the preclinical studies included in the systematic review were based on short-term therapies (n = 15). Intermediate-term therapies were employed in 6 of the selected publications, with nutraceutical treatment periods lasting 8–24 weeks. Lastly, the review only included a publication which studied the long-term therapy response in a guinea pig spontaneous OA model [32][31].
Table 32. Therapy duration of nutraceuticals.
| Animal Model | Short-term (≤8 Weeks) | Intermediate-Term (>8 to <24 Weeks) | Long-Term (≥24 Weeks) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Therapy | Duration | Reference | Therapy | Duration | Reference | Therapy | Duration | |||||||||
| Rabbit | Abdul-Kadir et al. [33][19] | GS | 8 weeks | Roman-Blas et al. [35] | GH/GS + CS | 14 weeks | |||||||||||
| Jeong et al. [34][25] | GH | 8 weeks | Torelli et al. [30][32] | CS | 12 weeks | ||||||||||||
| Permuy et al. [36][20] | GS/CS | 8 weeks | |||||||||||||||
| Ohnishi et al. [37][26] | GH | x | x | + | |||||||||||||
| 4 weeks | Wen et al. [46][23] | Delayed | + | x | + | x | + | ||||||||||
| Wang et al. [38][27 | Ivanovska et al. [29][24] | Early | - | x | x | - | - | ||||||||||
| ] | GH | 8 weeks | |||||||||||||||
| Kobayashi et al. [39][36] | GH + CS | 8 weeks | Glucosamine hydrochloride (GH) | Jeong et al. [34][25] | Early | + | |||||||||||
| Tiraloche et al. [40][ | x | 28] | GH | 8 weeks | x | x | + | ||||||||||
| n = 8 | Ohnishi et al. [37][26] | Early | + | x | x | x | − | ||||||||||
| Rat | Salman et al. [31][21] | GS | 6 weeks | Terencio et al. [44][37] | GH + CS | 12 weeks | Wang et al. [38][27] | Early | |||||||||
| Sun et al. [27][33 | ? | + | x | x | x | ||||||||||||
| ] | CS | 4 weeks | Panahafir et al. | [45][29] | GH | 12 weeks | Tiraloche et al. [40][28] | Delayed | ? | x | x | x | − | ||||
| Wang et al. [41][22] | GS | 4 weeks | Wen et al. [46][23] | GS | 10 weeks | Panahafir et al. [45][29] | Early | - | - | - | - | x | |||||
| Ren et al. [42][34] | CS | 6 weeks | Silva et al. [48][40] | GS + CS | 10 weeks | Naito et al. [47][30] | Early | ? | x | x | x | ||||||
| + | |||||||||||||||||
| Sanches et al. | [43][38] | GH + CS | 4 weeks | Ivanovska et al. [29][24] | Early | + | x | x | + | + | |||||||
| Lee et al. [28][39] | GS + CS | 7 weeks | Taniguchi et al. [32][31] | Pre-emptive | + | x | x | x | + | ||||||||
| Naito et al. [47][30] | GH | 8 weeks | Chondroitin sulfate | Permuy et al. [36][20] | Delayed | − | − | − | x | ||||||||
| Mice | Ivanovska et al. [29] | x | |||||||||||||||
| [ | 24] | GH /GS | 3 weeks | n = 5 | Torelli et al. [30 | ||||||||||||
| Guinea-Pig | ][32] | Early | − | x | x | x | x | ||||||||||
| Taniguchi et al. | [ | 32][31] | GH/CS | 18 months | Sun et al. [27][33] | Early | + | x | x | x | + | ||||||
| Ren et al. [42][34] | Delayed | + | x | x | x | + | |||||||||||
| Taniguchi et al. [32][31] | Pre-emptive | + | x | x | x | + | |||||||||||
| Chondroitin sulfate + GH | Roman-Blas et al. [35] | Pre-emptive | − | − | − | x | |||||||||||
Abbreviations: CS, Chondroitin sulfate; GH, glucosamine hydrochloride; GS, Glucosamine sulfate.
Regarding the therapy timing initiation in relation to OA induction, most of the studies applied early therapy administrations, up to only 14 days post experimental OA induction. 5 out of 22 articles studied the effect of these therapies in delayed administrations (>14 days post OA induction) and finally, 4 studies focused on the pre-emptive responses (before OA induction) (Table 21). The chondroprotective effect was observed in 7 out of the 13 publications with early treatment administrations, making up for 54%, 3 out of 5 publications with delayed initial treatments, corresponding to 60% and finally, 3 out of 4 pre-emptive protocols making up for 75%.
4. Quality and Risk-of-Bias Assessments
The quality assessments of the preclinical studies based on the essential 10 items of the ARRIVE guidelines were summarised in Figure 21. The individual analysis of the manuscripts showed that at items 4 “Randomisation” and 5 “Blinding”, information was not adequately reported in 32% and 50% of the studies, respectively. By contrast, at items 1 “Study design”, 6 “Outcome measures”, 7 “Statistical methods”, 8 “Experimental animals” and 10 “Results”, adequate and clear information was reported in the experimental studies, with percentages of 91%, 77%, 68%, 86% and 73% of the studies. Other items, such as 2 “Sample size”, 3 “Inclusion and exclusion criteria” and 9 “Experimental procedures” were graded as unclear with percentages of 82%, 68% and 82% of the studies, due to partially reported or insufficient experimental details provided in the studies.
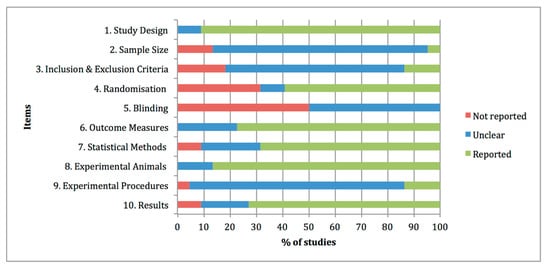

Figure 21. Quality assessments of the 22 preclinical studies included in the systematic review based on the Essential 10 items of the ARRIVE guidelines 2.0. Values are expressed by frequencies (%).
Figure 32 summarises the risk-of-bias distribution results obtained with the SYRCLE tool. The lower risk of bias was observed at items 1 “Sequence generation, 7 “Blinding of outcome assessor” and 9 “Selective outcome reporting”, with percentages of 59%, 55% and 64%, respectively. The higher risk of bias was assigned at item 3 “Allocation concealment” with a percentage of 68%, whereas high frequencies of unclear risk of bias ratings were assigned at items 4 “Random housing”, 5 “Blinding of caregivers”, 6 “Random outcome assessment”, 8 “Incomplete data outcome” and 10 “Other sources of bias”, with percentages of 100%, 91%, 91%, 73% and 63%, respectively.
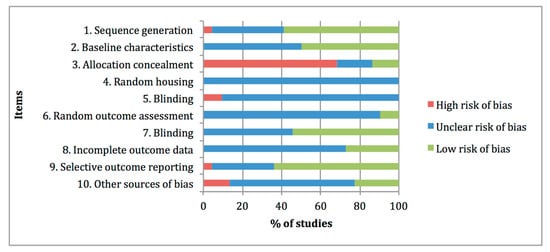

Figure 32. Risk of bias distribution graph of the 22 preclinical studies included in the systematic review according to SYRCLE tool. Values are expressed by frequencies (%).
5. Discussion
The aim of this systematic review was to examine the effect of glucosamine and chondroitin sulfate treatments in the synovial knee joint tissues and specific biomarkers of the osteoarthritic preclinical studies. A total of 22 studies with 3 different types of nutraceuticals: glucosamine sulfate, glucosamine hydrochloride and chondroitin sulfate, administered alone or in different combinations, were meticulously analysed in order to elucidate their direct influence on the main structural and biochemical elements in the OA joints.
Taking into account the experimental animal model, in the studies considered for this review, the most commonly used species were rats and rabbits, whereas only one study employed mouse as animal models [29][24]. These findings were different from those observed by other OA preclinical research, where mouse models constituted the majority of the included studies [49,50][41][42]. In regards to OA induction is concerned, in agreement with other publications, surgically induced models were one of the most selected based on the rapid OA induction, repeatability and lower costs [23,51][43][44]. Regarding spontaneous models, a slower disease progression was observed and therefore it seems closer to what naturally occurs in primary osteoarthritic disease [52][45]. However this review included only one study of spontaneous model of OA in guinea pigs [32][31].
Generally, in this systematic review we found a large inconsistency among the experimental nutraceutical protocols. As an attempt to reduce the variability among studies, we excluded the articles in which animals received intra-articular therapy injections [53,54,55,56,57][46][47][48][49][50]. Even though according to the records screened, these local therapies demonstrated a positive chondroprotective effect and anti-inflammatory activity, we decided to evaluate other administrations routes, such as oral and intraperitoneal, in order to analyse the systemic and non-local effects of glucosamine and chondroitin sulfate.
With respect to the therapeutic regimen, there are also notable differences among studies, both in the frequency and in the dosage administered. In this regard, 2 out of 22 articles included in this review examined the efficacy of chondroitin sulfate [27][33] and glucosamine sulfate [41][22] at different doses, and both of them concluded that they seemed to reduce the cartilage changes and biomarker alterations in a dose-dependent manner.
Additionally, some articles analysed the potential chondroprotective effect of different nutraceutical combinations. Furthermore, the combination of nutraceutical and other therapies and drugs was also investigated. In this context beneficial effects were observed in the association of glucosamine and risedronate [31[21][20],36], glucosamine and fish collagen peptide [37][26] and glucosamine hydrochloride and chondroitin sulfate plus fursultiamine, where only the combined group with the addition of the vitamin showed a significant chondroprotective effect [39][36]. Furthermore, in one study using an OA experimental model in rats, enhanced responses were observed with the association of glucosamine sulfate, chondroitin sulfate and photobiomodulation [43][38].
Regarding the nutraceutical combinations between glucosamine and chondroitin therapies evaluated in this review, Silva et al. [48][40] observed that the association of glucosamine sulfate and chondroitin sulfate, rather than isolated glucosamine, significantly reduced the joint pain and prevented the cartilage histology alterations. Likewise, Terencio et al. [44][37] demonstrated the chondroprotective effects of the combination chondroitin sulfate-glucosamine, as well as reduced inflammatory mediator levels. By contrast, Roman-Blas et al. [35] did not find any beneficial effects in the combined therapy with chondroitin sulfate plus glucosamine sulfate or hydrochloride. Regarding this point, a study which used a chemically induced murine model, focused on examining the effect of glucosamine on cartilage degradation and bone resorption, comparing two different pharmacological forms, sulfate and hydrochloride; the results showed less histologic effectiveness in the sulfate form when both were administered under the same conditions [29][24]. It is important to point out that some authors suggested that glucosamine hydrochloride had poorer bioavailability and less beneficial effect in relieving clinical OA symptoms [21][51]. In our review, slightly higher chondroprotective effects were determined in the glucosamine sulfate studies comparing to the studies that included the glucosamine hydrochloride formulation (67% vs. 50%, respectively). Furthermore, fewer biochemical alterations were found in the glucosamine sulfate administration compared to the hydrochloride ones (80% vs. 67%). Nevertheless, in a previous review on the use of glucosamine in the management of human OA, the authors determined that, due to the heterogeneous effects observed in the available research studies, concluding which formulation could be more effective continues to be extremely difficult [58][52].
Another point of interest in the experimental design is the therapy timing initiation in relation to the OA induction. As previously described, the articles included in this review were grouped into three distinct protocols, pre-emptive, early and delayed administrations. Among these studies, the highest chondroprotective effects were determined in the pre-emptive therapies, followed by the delayed ones, which showed slightly positive higher values than the studies with early administrations. These findings were slightly different to those observed in a recently published systematic review about the effect of bisphosphonates therapies in OA preclinical studies [59][53], where an obvious time-dependent efficacy on cartilage status was determined, showing better chondroprotective effects in pre-emptive and early therapy initiations and greater cartilage damage in the delayed ones. In our opinion, the positive values observed in the delayed administrations could be associated with an inadequate selection of the period of time determined, given that it can be established as early as 14 days after de OA experimental induction and longer periods of time may be required.
In terms of duration, attention is drawn to the lack of evidence in long-term therapy, identifying only 1 out of 22 included publications, in which glucosamine hydrochloride and chondroitin sulfate were evaluated at 8, 12 and 18 months, showing reduced cartilage degeneration and biomarker alterations in both treated groups, the animal group treated with glucosamine hydrochloride and the group treated with chondroitin sulfate [32][31]. In this context, it is important to highlight that the histological and biochemical response after long-term nutraceutical administration is basically unpredictable. The initial positive response identified in some studies may not be sustained for long periods of time. However, the opposite is also possible, and a longer duration of treatment period may be necessary to observe a beneficial effect in the synovial joint. Therefore, additional preclinical studies in OA research evaluating the effect of dietary supplements in the long term are required [19,59][54][53].
Overall, in this systematic review, we observed a high variability among the experimental designs. Consequently, making an accurate assessment of how glucosamine and chondroitin sulfate affect the OA progression continues to be a challenge. In general terms, the evaluated nutraceuticals, alone or in combination, did not seem to prevent the subchondral bone changes, the synovial inflammation or the osteophyte formation, showing poor positive responses. Nevertheless, it is true that only a few of the publications included evaluations at those levels. Cartilage continues to be the primary focus in OA research and in this sense, positive chondroprotective effects were identified in approximately half of the publications, the studies of glucosamine sulfate and the combination of chondroitin sulfate plus glucosamine sulfate showing the most promising results. There is also increasing attention on the research of biochemical markers. As it could be observed in this study, they were the second more assessed parameter. In this context, a positive response was identified in more than half of the evaluations included in this review.
Regarding the risk of bias and the quality assessments of the articles included in this review, they were similar to the previous studies [49,59][41][53]. There are essential details about the experimental design which continue to be poorly reported in the studies, such as the sample size calculation, which was only reported in one of the manuscripts [33][19]. Inclusion and exclusion criteria were also badly or incompletely reported in most of the studies as well as blinding the experimental details. More specifically, half of the studies did not report the information and the other half only specified it in the outcome assessment stage, but not in the experimental and treatment administration stages. In addition, the information concerning the acclimatisation period of the animals, the housing and husbandry was also insufficient. In this sense, a previous research evaluated the adherence to the ARRIVE checklist in 236 papers between 2009 and 2015, and unexpectedly none of the evaluated manuscripts fully reported 100% of the items [60][55]. Consequently, the improvement of the research report in animal experimentation continues to be an essential task at present [25][56].
To conclude, OA management in companion animals continues to be a challenge in veterinary medicine. As we exposed in this review, glucosamine and chondroitin sulfate seems to provide chondroprotective effects and less inflammatory biochemical response in approximately half of the evaluations. However, these effects are inconsistent between the clinical and the preclinical studies. One explanation may be related to the great variety of histological scoring evaluations, the potential assessor’s subjectivity and the possibility of intra- and inter-observer variations [61][57]. Moreover, as these therapies have a slow onset of action, long-term administrations should be required to clarify their effectiveness. Additionally, a possible caregiver placebo effect may explain some of the beneficial responses observed in clinical trials with dogs [17,62][17][58]. For all these reasons, the use of glucosamine and chondroitin sulfate should be an individual veterinary/owner decision, reached by thoroughly evaluating each particular clinical case and its symptomatic response.
References
- Felson, D.T.; Neogi, T. Osteoarthritis: Is It a Disease of Cartilage or of Bone? Arthritis Rheum. 2004, 50, 341–344.
- Karsdal, M.A.; Leeming, D.J.; Dam, E.B.; Henriksen, K.; Alexandersen, P.; Pastoureau, P.; Altman, R.D.; Christiansen, C. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthr. Cartil. 2008, 16, 638–646.
- Anderson, K.L.; O’Neill, D.G.; Brodbelt, D.C.; Church, D.B.; Meeson, R.L.; Sargan, D.; Summers, J.F.; Zulch, H.; Collins, L.M. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci. Rep. 2018, 8, 1–12.
- Rychel, J.K. Diagnosis and Treatment of Osteoarthritis. Top. Companion Anim. Med. 2010, 25, 20–25.
- Epstein, M.; Rodan, I.; Griffenhagen, G.; Kadrlik, J.; Petty, M.; Robertson, S.; Simpson, W. 2015 AAHA/AAFP pain management guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2015, 51, 67–84.
- Henrotin, Y.; Sanchez, C.; Balligand, M. Pharmaceutical and nutraceutical management of canine osteoarthritis: Present and future perspectives. Vet. J. 2005, 170, 113–123.
- Neundorf, R.H.; Lowerison, M.B.; Cruz, A.M.; Thomason, J.J.; McEwen, B.J.; Hurtig, M.B. Determination of the prevalence and severity of metacarpophalangeal joint osteoarthritis in Thoroughbred racehorses via quantitative macroscopic evaluation. Am. J. Vet. Res. 2010, 71, 1284–1293.
- Espinosa-Mur, P.; Phillips, K.L.; Galuppo, L.D.; DeRouen, A.; Benoit, P.; Anderson, E.; Shaw, K.; Puchalski, S.; Peters, D.; Kass, P.H.; et al. Radiological prevalence of osteoarthritis of the cervical region in 104 performing Warmblood jumpers. Equine Vet. J. 2020, 1–7.
- Johnston, S.A. Osteoarthritis. Joint anatomy, physiology, and pathobiology. Vet. Clin. N. Am. Small Anim. Pract. 1997, 27, 699–723.
- O′Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Prevalence of Disorders Recorded in Dogs Attending Primary-Care Veterinary Practices in England. PLoS ONE 2014, 9, e90501.
- Slingerland, L.I.; Hazewinkel, H.A.W.; Meij, B.P.; Picavet, P.; Voorhout, G. Cross-sectional study of the prevalence and clinical features of osteoarthritis in 100 cats. Vet. J. 2011, 187, 304–309.
- Bennett, D.; bt Zainal Ariffin, S.M.; Johnston, P. Osteoarthritis in the cat: 1. how common is it and how easy to recognise? J. Feline Med. Surg. 2012, 14, 65–75.
- Bennett, D.; bt Zainal Ariffin, S.M.; Johnston, P. Osteoarthritis in the cat: 2. How should it be managed and treated? J. Feline Med. Surg. 2012, 14, 76–84.
- Apostu, D.; Lucaciu, O.; Mester, A.; Oltean-Dan, D.; Baciut, M.; Baciut, G.; Bran, S.; Onisor, F.; Piciu, A.; Pasca, R.D.; et al. Systemic drugs with impact on osteoarthritis. Drug Metab. Rev. 2019, 51, 498–523.
- Henrotin, Y.; Lambert, C.; Couchourel, D.; Ripoll, C.; Chiotelli, E. Nutraceuticals: Do they represent a new era in the management of osteoarthritis? A narrative review from the lessons taken with five products. Osteoarthr. Cartil. 2011, 19, 1–21.
- McCarthy, G.; O’Donovan, J.; Jones, B.; McAllister, H.; Seed, M.; Mooney, C. Randomised double-blind, positive-controlled trial to assess the efficacy of glucosamine/chondroitin sulfate for the treatment of dogs with osteoarthritis. Vet. J. 2007, 174, 54–61.
- Scott, R.M.; Evans, R.; Conzemius, M.G. Efficacy of an oral nutraceutical for the treatment of canine osteoarthritis: A doubled-blind, randomized, placebo-controlled prospective clinical trial. Vet. Comp. Orthop. Traumatol. 2017, 30, 318–323.
- Moreau, M.; Dupuis, J.; Bonneau, N.H.; Desnoyers, M. Clinical evaluation of a nutraceutical, carprofen and meloxicam for the treatment of dogs with osteoarthritis. Vet. Rec. 2003, 152, 323–329.
- Abdul Kadir, A.; Abdul Kadir, A.; Abd Hamid, R.; Mat Jais, A.M.; Omar, J.; Sadagatullah, A.N.; Badrin, S.; Win, T.T.; Sirajudeen, K.N.S.; Salleh, A. Evaluation of Chondroprotective Activity of Channa striatus in Rabbit Osteoarthritis Model. Biomed. Res. Int. 2019, 2019.
- Permuy, M.; Guede, D.; López-Peña, M.; Muñoz, F.; Caeiro, J.R.; González-Cantalapiedra, A. Comparison of various SYSADOA for the osteoarthritis treatment: An experimental study in rabbits Clinical rheumatology and osteoporosis. BMC Musculoskelet. Disord. 2015, 16, 1–12.
- Salman, A.; Shabana, A.I.; El-ghazouly, D.E.S.; Maha, E. Protective effect of glucosamine and risedronate (alone or in combination) against osteoarthritic changes in rat experimental model of immobilized knee. Anat. Cell Biol. 2019, 52, 498–510.
- Wang, X.X.; Cai, L. Expression level of proteoglycan, collagen and type II collagen in osteoarthritis rat model is promoted and degradation of cartilage is prevented by glucosamine methyl ester. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3609–3616.
- Wen, Z.H.; Tang, C.C.; Chang, Y.C.; Huang, S.Y.; Hsieh, S.P.; Lee, C.H.; Huang, G.S.; Ng, H.F.; Neoh, C.A.; Hsieh, C.S.; et al. Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: Association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthr. Cartil. 2010, 18, 1192–1202.
- Ivanovska, N.; Dimitrova, P. Bone resorption and remodeling in murine collagenase-induced osteoarthritis after administration of glucosamine. Arthritis Res. Ther. 2011, 13.
- Jeong, D.H.; Ullah, H.M.A.; Goo, M.J.; Ghim, S.G.; Hong, I.H.; Kim, A.Y.; Jeon, S.M.; Choi, M.S.; Elfadl, A.K.; Chung, M.J.; et al. Effects of oral glucosamine hydrochloride and mucopolysaccharide protein in a rabbit model of osteoarthritis. Int. J. Rheum. Dis. 2017, 21, 620–628.
- Ohnishi, A.; Osaki, T.; Matahira, Y.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Evaluation of the chondroprotective effects of glucosamine and fish collagen peptide on a rabbit ACLT model using serum biomarkers. J. Vet. Med. Sci. 2013, 75, 421–429.
- Wang, S.X.; Laverty, S.; Dumitriu, M.; Plaas, A.; Grynpas, M.D. The effects of glucosamine hydrochloride on subchondral bone changes in an animal model of osteoarthritis. Arthritis Rheum. 2007, 56, 1537–1548.
- Tiraloche, G.; Girard, C.; Chouinard, L.; Sampalis, J.; Moquin, L.; Ionescu, M.; Reiner, A.; Poole, A.R.; Laverty, S. Effect of oral glucosamine on cartilage degradation in a rabbit model of osteoarthritis. Arthritis Rheum. 2005, 52, 1118–1128.
- Panahifar, A.; Jaremko, J.L.; Tessier, A.G.; Lambert, R.G.; Maksymowych, W.P.; Fallone, B.G.; Doschak, M.R. Development and reliability of a multi-modality scoring system for evaluation of disease progression in pre-clinical models of osteoarthritis: Celecoxib may possess disease-modifying properties. Osteoarthr. Cartil. 2014, 22, 1639–1650.
- Naito, K.; Watari, T.; Furuhata, A.; Yomogida, S.; Sakamoto, K.; Kurosawa, H.; Kaneko, K.; Nagaoka, I. Evaluation of the effect of glucosamine on an experimental rat osteoarthritis model. Life Sci. 2010, 86, 538–543.
- Taniguchi, S.; Ryu, J.; Seki, M.; Sumino, T.; Tokuhashi, Y.; Esumi, M. Long-term oral administration of glucosamine or chondroitin sulfate reduces destruction of cartilage and up-regulation of MMP-3 mRNA in a model of spontaneous osteoarthritis in Hartley guinea pigs. J. Orthop. Res. 2012, 30, 673–678.
- Torelli, S.R.; Rahal, S.C.; Volpi, R.S.; Sequeira, J.L.; Grassioto, I.Q. Histopathological evaluation of treatment with chondroitin sulphate for osteoarthritis induced by continuous immobilization in rabbits. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2005, 52, 45–51.
- Sun, Y.; Zhang, G.; Liu, Q.; Liu, X.; Wang, L.; Wang, J.; Liang, L. Chondroitin sulfate from sturgeon bone ameliorates pain of osteoarthritis induced by monosodium iodoacetate in rats. Int. J. Biol. Macromol. 2018, 117, 95–101.
- Ren, Z.; Ji, Y.; Wang, Y.; Dong, L. Chondroitin sulfate from Scophthalmus maximus for treating osteoarthritis. Int. J. Biol. Macromol. 2017, 108, 1158–1164.
- Roman-Blas, J.A.; Mediero, A.; Tardío, L.; Portal-Nuñez, S.; Gratal, P.; Herrero-Beaumont, G.; Largo, R. The combined therapy with chondroitin sulfate plus glucosamine sulfate or chondroitin sulfate plus glucosamine hydrochloride does not improve joint damage in an experimental model of knee osteoarthritis in rabbits. Eur. J. Pharmacol. 2017, 794, 8–14.
- Kobayashi, T.; Notoya, K.; Nakamura, A.; Akimoto, K. Fursultiamine, a vitamin B1 derivative, enhances chondroprotective effects of glucosamine hydrochloride and chondroitin sulfate in rabbit experimental osteoarthritis. Inflamm. Res. 2005, 54, 249–255.
- Terencio, M.C.; Ferrándiz, M.L.; Carceller, M.C.; Ruhí, R.; Dalmau, P.; Vergés, J.; Montell, E.; Torrent, A.; Alcaraz, M.J. Chondroprotective effects of the combination chondroitin sulfate-glucosamine in a model of osteoarthritis induced by anterior cruciate ligament transection in ovariectomised rats. Biomed. Pharmacother. 2016, 79, 120–128.
- Sanches, M.; Assis, L.; Criniti, C.; Fernandes, D.; Tim, C.; Renno, A.C.M. Chondroitin sulfate and glucosamine sulfate associated to photobiomodulation prevents degenerative morphological changes in an experimental model of osteoarthritis in rats. Lasers Med. Sci. 2017, 33, 549–557.
- Lee, H.; Park, Y.; Ahn, C.W.; Park, S.H.; Jung, E.Y.; Suh, H.J. Deer bone extract suppresses articular cartilage damage induced by monosodium iodoacetate in osteoarthritic rats: An in vivo micro-computed tomography study. J. Med. Food 2014, 17, 701–706.
- Silva, F.S.; Yoshinari, N.H.; Castro, R.R.; Girão, V.C.C.; Pompeu, M.M.L.; Andrade Feitosa, J.P.; Rocha, F.A.C. Combined glucosamine and chondroitin sulfate provides functional and structural benefit in the anterior cruciate ligament transection model. Clin. Rheumatol. 2009, 28, 109–117.
- Contartese, D.; Tschon, M.; De Mattei, M.; Fini, M. Molecular Sciences Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 3696.
- Shmagel, A.; Demmer, R.; Knights, D.; Butler, M.; Langsetmo, L.; Lane, N.E.; Ensrud, K. The effects of glucosamine and chondroitin sulfate on gut microbial composition: A systematic review of evidence from animal and human studies. Nutrients 2019, 11, 294.
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 1–27.
- Lampropoulou-Adamidou, K.; Lelovas, P.; Karadimas, E.V.; Liakou, C.; Triantafillopoulos, I.K.; Dontas, I.; Papaioannou, N.A. Useful animal models for the research of osteoarthritis. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 263–271.
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818.
- Fonsi, M.; El Amrani, A.I.; Gervais, F.; Vincent, P. Intra-Articular Hyaluronic Acid and Chondroitin Sulfate: Pharmacokinetic Investigation in Osteoarthritic Rat Models. Curr. Ther. Res. Clin. Exp. 2020, 92.
- Frisbie, D.D.; McIlwraith, C.W.; Kawcak, C.E.; Werpy, N.M. Evaluation of intra-articular hyaluronan, sodium chondroitin sulfate and N-acetyl-d-glucosamine combination versus saline (0.9% NaCl) for osteoarthritis using an equine model. Vet. J. 2013, 197, 824–829.
- Gibson, M.; Li, H.; Coburn, J.; Moroni, L.; Nahas, Z.; Bingham, C.; Yarema, K.; Elisseeff, J. Intra-articular delivery of glucosamine for treatment of experimental osteoarthritis created by a medial meniscectomy in a rat model. J. Orthop. Res. 2014, 32, 302–309.
- Scotto D’Abusco, A.; Corsi, A.; Grillo, M.G.; Cicione, C.; Calamia, V.; Panzini, G.; Sansone, A.; Giordano, C.; Politi, L.; Scandurra, R. Effects of intra-articular administration of glucosamine and a peptidyl-glucosamine derivative in a rabbit model of experimental osteoarthritis: A pilot study. Rheumatol. Int. 2008, 28, 437–443.
- Shikhman, A.R.; Amiel, D.; D’Lima, D.; Hwang, S.B.; Hu, C.; Xu, A.; Hashimoto, S.; Kobayashi, K.; Sasho, T.; Lotz, M.K. Chondroprotective activity of N-acetylglucosamine in rabbits with experimental osteoarthritis. Ann. Rheum. Dis. 2005, 64, 89–94.
- Bhathal, A.; Spryszak, M.; Louizos, C.; Frankel, G. Glucosamine and chondroitin use in canines for osteoarthritis: A review. Open Vet. J. 2017, 7, 36–49.
- Henrotin, Y.; Mobasheri, A.; Marty, M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res. Ther. 2012.
- Fernández-Martín, S.; López-Peña, M.; Muñoz, F.; Permuy, M.; González-Cantalapiedra, A. Bisphosphonates as disease-modifying drugs in osteoarthritis preclinical studies: A systematic review from 2000 to 2020. Arthritis Res. Ther. 2021, 23, 60.
- Comblain, F.; Serisier, S.; Barthelemy, N.; Balligand, M.H. Review of dietary supplements for the management of osteoarthritis indogs in studies from 2004 to 2014. J. Vet. Pharmacol. Ther. 2016, 39, 1–15.
- Leung, V.; Rousseau-Blass, F.; Beauchamp, G.; Pang, D.S.J. ARRIVE has not ARRIVEd: Support for the ARRIVE (Animal Research: Reporting of in vivo Experiments) guidelines does not improve the reporting quality of papers in animal welfare, analgesia or anesthesia. PLoS ONE 2018, 13, e0197882.
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLOS Biol. 2020, 18, e3000410.
- Pastoureau, P.C.; Hunziker, E.B.; Pelletier, J.P. Cartilage, bone and synovial histomorphometry in animal models of osteoarthritis. Osteoarthr. Cartil. 2010, 18, S106–S112.
- Malek, S.; Sample, S.J.; Schwartz, Z.; Nemke, B.; Jacobson, P.B.; Cozzi, E.M.; Schaefer, S.L.; Bleedorn, J.A.; Holzman, G.; Muir, P. Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client-owned dogs with hip osteoarthritis. BMC Vet. Res. 2012, 8, 185.
More
