Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Roberto Grosso.
Thiomers (or thiolated polymers) have broken through as avant-garde approaches in anticancer therapy. Their distinguished reactivity and properties, closely linked to their final applications, justify the extensive research conducted on their preparation and use as smart drug-delivery systems (DDSs).
- thiomers
- thiolated polymers
- chitosan
- hyaluronic acid
- anticancer therapy
- nanoformulations
- nanotechnology
- siRNA
- taxanes
1. Cancer in today's society
Cancer is a major public health problem worldwide and is the second-leading cause of death in developed countries—only exceeded by cardiovascular diseases—which accounts for more than 8.8 million deaths per year. The lifetime probability of developing an invasive cancer is currently above 40% for men and close to that for women; in 2020, 1,806,590 new cancer cases and 606,520 cancer deaths were projected to occur in the United States (Figure 1) [1]. Cancer therapy is dictated by the cancer type, stage at diagnosis, and the patient’s tolerance to the prescribed therapy [2]. Chemotherapy serves as a method to block the expansion of growing neoplasm. Its results are enhanced when combined with other classical anticancer approaches [3]. However, the active pharmaceutical ingredients (APIs) for the treatment of cancer do not usually differentiate between healthy cells and cancer cells, as both of them are exposed to the cytotoxic effects of chemotherapeutic drugs [4]. In addition, cancer treatments generally involve the administration of relatively high doses of the drug in the hope that a portion, although minor, will go to damaged tissues [5]. Therefore, it is necessary to explore other therapeutic options that allow the drug of interest to accumulate in the cancerous tissue while decreasing the adverse reactions associated with its presence in healthy niches.
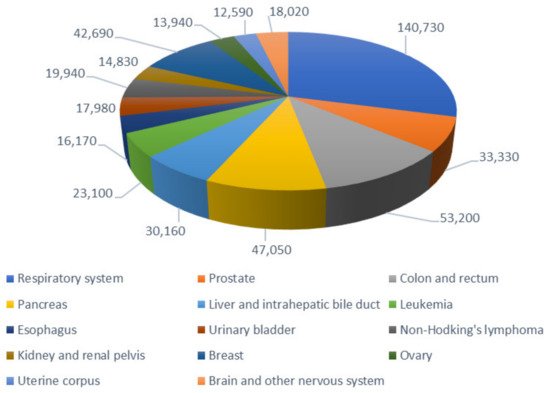
Figure 1. Estimated number of deaths by the main types of cancer in the United States, for both sexes, in 2020. Data obtained from [1].
2. Thiolated polymers as potencially efficient DDS
Nanotechnology has been extensively investigated for potential applications in the diagnosis and treatment of cancer, since it offers a suitable means of site-specific and/or time-controlled delivery of small- or large-molecular-weight drugs and other bioactive agents [1,2,3,4,5,6][1][2][3][4][5][6]. Drug-delivery systems (DDSs) based on polymer NPs have the potential to improve current disease therapies because of their ability to go through multiple biological barriers, overcoming the drawbacks of insoluble drugs, whereas their renal clearance will be reduced [4]. Additional benefits such as an increase in half-life, payload, and suppression of the side effects of toxic drugs are of great interest. Therefore, nanometric-sized systems are being studied for their use in either passive or active targeted cancer therapy. In cancer tissues, there are two relevant parameters that can act as internal stimuli in smart DDSs: the acidic pH [7,8][7][8] and the reductive environment found in tumors [9]. Both have been vastly explored as internal trigger for the release of payloads.
Thiolated polymers (thiomers) are biocompatible polymers that bear free or activated thiol groups covalently attached to them. They began to be explored in 1999 [10], and have since been used in multiple biomedical and pharmaceutical applications due to the close interest in their study among the research community [11,12,13][11][12][13]. These applications are connected with the presence of their thiol groups and their capability of conducting thiol–disulfide exchange reactions and oxidizing to disulfide bridges: from soft-tissue engineering [14] as biomaterial support for cartilage repair [15] and 3D bioscaffolds for cell culture [16,17][16][17] to antibacterial activity [18,19,20,21][18][19][20][21]. They are also prominent candidates for the formulation of controlled delivery carriers of APIs, whether for topical use [22], as dry powder inhalers [23], or in oral administration [24], among others. Their formulations can enclose small molecules such as methotrexate [25], sodium naproxen [26], isoniazid [23] docetaxel [27], and paclitaxel [28], as well as hydrophilic macromolecular drugs [29], but also peptides, RNA, and biomacromolecules like insulin [24], basic fibroblast growth factors [30], and siRNA [31].
3. Molecular aspects of cancer
Cancer has been one of the most studied pathologies in modern history. Molecularly, it is interesting to mention that, as of the year 2000, it was proposed that neoplastic diseases shared six characteristics that constitute their basic organizing principles, thus providing a logical framework to understand them even when the diversity among this group of pathologies is enormous. These peculiarities were baptized as “The Six Hallmarks of Cancer”, and the consensus reached at that moment was that as healthy cells evolve gradually to a cancerous state, they acquire a succession of these hallmark abilities that allow them to behave as a prototypical tumor. The acquisition of such malignant properties during multistep tumorigenesis has been concluded to be favored by two enabling characteristics; that is, phenomena that create the necessary breeding ground for cancer cells to start developing: genome instability and mutation, and tumor-promoting inflammation [33][32].
Therefore, the six hallmarks of cancer can be defined as distinctive and complementary capabilities that enable tumor growth and metastatic dissemination [33][32]. They include: (1) sustaining proliferative signaling, (2) evading growth suppressors, (3) activating invasion and metastasis, (4) enabling replicative immortality, (5) inducing angiogenesis, and (6) resisting cell death. Nonetheless, an increased body of research during the past decades has suggested that there are not six hallmarks of cancer, but in fact eight, the list being completed with (7) deregulating cellular energetics and (8) avoiding immune destruction [33][32].
4. Antitumoral therapy. Drug delivery concerns
As far as cancer therapy is concerned, treatment is dictated by the cancer type, stage at diagnosis, and the patient’s tolerance to the prescribed therapy [2]. While surgery and radiotherapy are the primary treatment used for local and nonmetastatic cancers, other treatments can be employed such as chemotherapy, and hormone and biological therapies. Thus, for example, in the case of solid tumors, surgery is the local treatment of choice, as the damage is confined to a limited area of the body. However, most patients require the combination of two or more therapeutic treatments due to the potential spread of the disease, as well as to effectively prevent the evolution of the disease from early to advanced stages. Chemotherapy works by inhibiting the division of rapidly growing cells, and combined with surgery or radiotherapy, the effectiveness of these treatment modalities are increased [3].
Although chemotherapy is the main treatment for cancer patients, the active pharmaceutical ingredients (APIs) used do not differentiate between healthy cells and cancer cells. Both of them are exposed to the cytotoxic effects of chemotherapeutic drugs, and consequently, the drugs interfere the growing pattern of normal cells with fast proliferation rates, such as the hair follicles, and bone marrow and gastrointestinal tract cells, and provoke long-term toxic effects on the heart, lungs, and kidneys [4]. Typical harmful side effects associated with chemotherapy, such as nausea, vomiting, immune suppression, hepatotoxicity, nephrotoxicity, memory loss, anemia, and even death, are rooted in the use of such nonspecific therapeutic systems.
Therefore, and regarding drug administration, the methods used traditionally have been limited to making the drug accessible to the bloodstream, relying on the irrigation and the drug affinity for the tissues for the access to the target. In fact, bioavailability is still measured from drug levels in the bloodstream, not in the target surroundings. In many cases, only a small portion of the administered drug reaches the tumor site [6]. As a consequence, cancer treatments generally involve the administration of relatively high doses of the drug in the hope that a portion, although minor, will go to damaged tissues [5]. Thus, there is a need to increase the drug concentration in the cancerous tissue while reducing the side effects associated with chemotherapeutic molecules. This requirement is even more compelling in the case of highly toxic anticancer drugs, which may also present physicochemical and stability features that are too deficient. In addition, this becomes complicated when considering that even when the drug reaches the tumor, cancer cells can develop drug resistance. For instance, P-glycoprotein (P-gp, where “P” refers to permeability) has been documented to be overexpressed in various drug-resistant tumors, thereby enabling direct drug efflux (meaning it works as a drug efflux pump) and limiting intracellular accumulation of several anticancer agents [34][33]. Thus, the indiscriminate destruction of normal cells, the toxicity of conventional chemotherapeutic drugs, as well as the development of multidrug resistance, support the need of finding new effective targeted treatments based on the differences found in the molecular biology of the tumor cells.
Significant efforts have been devoted to take advantage of the potentials of nanotechnology in drug delivery, since it offers a suitable means of site-specific and/or time-controlled delivery of small- or large-molecular-weight drugs and other bioactive agents. Nanotechnology has also been extensively studied for potential applications in the diagnosis and treatment of cancer [1,2,3,4,5,6][1][2][3][4][5][6].
5. Exploitation of polymeric nanoforms as DDS
Pharmaceutical nanotechnology focuses on formulating APIs into biocompatible nanoforms in which the drug is dissolved, entrapped, encapsulated, or attached to a nanoparticle matrix. The nanoscale size of these delivery systems is the basis for many of these advantages. Several types of nanoparticulate systems have been attempted as potential DDSs, including biodegradable polymeric nanoparticles (NPs), polymeric micelles, solid nanoparticles, lipid-based nanoparticles (e.g., solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), and lipid drug conjugates (LDCs)), nanoliposomes, inorganic nanoparticles, dendrimers, magnetic nanoparticles, ferro-fluids, and quantum dots [35][34]. Among the most widely investigated nanocarriers in cancer detection and cancer therapy are carbon nanotubes, micelles, dendrimers, polymeric nanoparticles, liposomes, nanoshells, and polymer–drug conjugates/proteins.
Polymer NPs have gained significant attention among the numerous nanotechnology approaches, as is stressed by the fact that over 90% of the scientific articles published on cancer therapeutics in the last decade were based on the use of such systems [4]. DDSs based on polymer NPs have the potential to improve current disease therapies because of their ability to overcome multiple biological barriers and release a therapeutic molecule within the optimal dosage range. They are also capable of overcoming the drawbacks of insoluble drugs. This is the case for anticancer molecules such as camptothecin [7], doxorubicin [36][35], and the taxanes paclitaxel [37][36] and docetaxel [27]. Additional benefits such as an increase in half-life, payload, and solubility of APIs can be attained. Being embedded into the nanoplatform conjugate, chemotherapeutic drugs can be transported to tumors without damaging healthy tissues and, therefore, a drop in their toxicity profile in the human body is expected to occur.
Consequently, nanometric-sized systems have become the option of choice, and they are being investigated for their use in either passive (by enhanced permeability and retention) or active (by the functionalization of the surface of the carriers) targeted cancer therapies. Angiogenesis during tumor growth results in a defective hypervascularization and a deficient lymphatic drainage system, which has given rise to the concept of passive targeting of NPs to tumors through the “enhanced permeability and retention” (EPR) effect [4,38][4][37]. Passive tissue targeting uses the increased permeability of tumor vasculature and the poor lymphatic drainage of tumors (EPR effect) [39][38], allowing drug-delivery nanocarriers (cutoff size of >400 nm) to accumulate and diffuse preferentially in the vicinity of tumors, with the desirable release of the chemotherapeutic agents in the tumor.
6. Glutathione-mediated drug release
However, not only the encapsulation, but also the release of drugs at the target tissue is vital in drug delivery. By introducing responsive groups, the carrier could release the drug under specific stimuli. In order to ensure controllable and optimal releasing at desirable sites, a variety of “stimulus-responsive” nanoparticulated DDSs have been designed [40,41][39][40]. Internal (or endogenous) stimulus arises from variation in a target site parameter, such as relative changes in pH; different expression of a specific enzyme, factors, or other molecules; and abnormal redox balance [42][41]. In cancer tissues, there are two relevant parameters that can act as internal stimuli: the acidic pH and the reductive environment found in tumors. Thus, compared with healthy tissues, lower pHs (6.2–6.9) of the extracellular matrix have been found in cancerous cells because of the Warburg effect [43][42]. The differences in pH have been vastly explored as an internal trigger for the release of payloads [7,8][7][8]. In addition, pH differences between intracellular endosomes/lysosomes (with pH of 4.0–6.0) of normal and cancer cells is extraordinary [44][43]. Redox potential is another internal stimulus for responsive release of drugs [9].
Glutathione (GSH), a tripeptide constituted by L-γ-glutamyl-L-cysteinyl-glycine, is involved in the formation and lysis of disulfide bridges; it serves as a general reductant for cells, and its intracellular concentration is 1–10 mM in mammalian cells [45][44]. Glutathione exists in reduced (GSH) and oxidized (glutathione disulfide, GSSG) states, where the GSH/GSSG system is the major redox couple in animal cells [14]. GSH plays several vital roles in maintaining the bioactivity of cells, including antioxidation, maintenance of the redox state, modulation of the immune response, and detoxification of xenobiotics [42][41]. The ratio of the GSSG/GSH couple can serve as an important indicator of the cellular redox environment [46][45]. Intracellular concentration of GSH is hundreds to thousands of times higher than that in ECM [47][46], and more importantly, elevated levels of GSH are found in tumor cells; for example, in bone marrow, breast, colon, larynx, and lung cancers, to minimize radical damage from oxidative stress [46][45]. Thus, the disulfide crosslinking reaction has an advantage in the development of cancer therapies over other chemistries, since redox potential is an internal stimulus for responsive releasing of delivery system.
With all of this in mind, it is no surprise that thiomers (that is, polymers that have undergone modifications regarding the addition of thiol groups) have broken though as avant-garde approaches in this field. Several studies agreed that their multiple properties make them perfect candidates to take part in nanoformulations that act as drug-delivery systems for compounds with major difficulties in administration, especially in anticancer therapy.
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26.
- Gavhane, Y.; Shete, A.; Bhagat, A.; Shinde, V.; Bhong, K.; Khairnar, G.; Yadav, A. Solid Tumors: Facts, Challenges and Solutions. Int. J. Pharma Sci. Res. 2011, 2, 1–12.
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79.
- Alvarez-Lorenzo, C.; Cocheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765.
- Muller, R.H.; Keck, C.M. Challenges and solutions for the delivery of biotech drugs—A review of drug nanocrystal technology and lipid nanoparticles. J. Biotechnol. 2004, 113, 151–170.
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; de-Paz, M.V.; Galbis, J.A. Loading studies of the anticancer drug camptothecin into dual stimuli-sensitive nanoparticles. Stability scrutiny. Int. J. Pharm. 2018, 550, 429–438.
- Galbis, E.; De-Paz, M.-V.; Iglesias, N.; Lacroix, B.; Alcudia, A.; Galbis, J.A. Core cross-linked nanoparticles from self-assembling polyfma-based micelles. Encapsulation of lipophilic molecules. Eur. Polym. J. 2017, 89, 406–418.
- Romero-Azogil, L.; Benito, E.; Iglesias, N.; Galbis, E.; De-Paz, M.-V.; García-Martín, M.G. Chapter 11. Redox polymers for drug delivery. In Redox Polymers for Energy and Nanomedicine; Casado, N., Mecerreyes, D., Eds.; Polymer Chemistry Series; The Royal Society of Chemistry: London, UK, 2020; ISBN 978-1-78801-871-5.
- Bernkop-Schnürch, A.; Schwarz, V.; Steininger, S. Polymers with thiol groups: A new generation of mucoadhesive polymers? Pharm. Res. 1999, 16, 876–881.
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N.; Bernkop-Schnürch, A. Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms. J. Control. Release 2021, 330, 470–482.
- Federer, C.; Kurpiers, M.; Bernkop-Schnürch, A. Thiolated Chitosans: A Multi-talented Class of Polymers for Various Applications. Biomacromolecules 2021, 22, 24–56.
- Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 191–221.
- Pérez-Madrigal, M.M.; Shaw, J.E.; Arno, M.C.; Hoyland, J.A.; Richardson, S.M.; Dove, A.P. Robust alginate/hyaluronic acid thiol-yne click-hydrogel scaffolds with superior mechanical performance and stability for load-bearing soft tissue engineering. Biomater. Sci. 2020, 8, 405–412.
- Jin, R.; Teixeira, L.S.M.; Krouwels, A.; Dijkstra, P.J.; Van Blitterswijk, C.A.; Karperien, M.; Feijen, J. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: An injectable biomaterial for cartilage repair. Acta Biomater. 2010, 6, 1968–1977.
- Bian, S.; He, M.; Sui, J.; Cai, H.; Sun, Y.; Liang, J.; Fan, Y.; Zhang, X. The self-crosslinking smart hyaluronic acid hydrogels as injectable three-dimensional scaffolds for cells culture. Colloids Surf. B Biointerfaces 2016, 140, 392–402.
- Asim, M.H.; Silberhumer, S.; Shahzadi, I.; Jalil, A.; Matuszczak, B.; Bernkop-Schnürch, A. S-protected thiolated hyaluronic acid: In-situ crosslinking hydrogels for 3D cell culture scaffold. Carbohydr. Polym. 2020, 237.
- Croce, M.; Conti, S.; Maake, C.; Patzke, G.R. Synthesis and screening of N-acyl thiolated chitosans for antibacterial applications. Carbohydr. Polym. 2016, 151, 1184–1192.
- Carvalho, I.C.; Medeiros Borsagli, F.G.L.; Mansur, A.A.P.; Caldeira, C.L.; Haas, D.J.; Lage, A.P.; Ciminelli, V.S.T.; Mansur, H.S. 3D sponges of chemically functionalized chitosan for potential environmental pollution remediation: Biosorbents for anionic dye adsorption and ‘antibiotic-free’ antibacterial activity. Environ. Technol. 2021, 42, 2046–2066.
- Luo, Q.; Han, Q.; Wang, Y.; Zhang, H.; Fei, Z.; Wang, Y. The thiolated chitosan: Synthesis, gelling and antibacterial capability. Int. J. Biol. Macromol. 2019, 139, 521–530.
- Geisberger, G.; Gyenge, E.B.; Hinger, D.; Käch, A.; Maake, C.; Patzke, G.R. Chitosan-thioglycolic acid as a versatile antimicrobial agent. Biomacromolecules 2013, 14, 1010–1017.
- Laffleur, F. Evaluation of chemical modified hydrogel formulation for topical suitability. Int. J. Biol. Macromol. 2017, 105, 1310–1314.
- Mukhtar, M.; Pallagi, E.; Csóka, I.; Benke, E.; Farkas, Á.; Zeeshan, M.; Burián, K.; Kókai, D.; Ambrus, R. Aerodynamic properties and in silico deposition of isoniazid loaded chitosan/thiolated chitosan and hyaluronic acid hybrid nanoplex DPIs as a potential TB treatment. Int. J. Biol. Macromol. 2020, 165, 3007–3019.
- Rekha, M.R.; Sharma, C.P. Simultaneous Effect of Thiolation and Carboxylation of Chitosan Particles towards Mucoadhesive Oral Insulin Delivery Applications: An In Vitro and In Vivo Evaluation. J. Biomed. Nanotechnol. 2015, 11, 165–176.
- Gao, C.; Liu, T.; Dang, Y.; Yu, Z.; Wang, W.; Guo, J.; Zhang, X.; He, G.; Zheng, H.; Yin, Y.; et al. PH/redox responsive core cross-linked nanoparticles from thiolated carboxymethyl chitosan for in vitro release study of methotrexate. Carbohydr. Polym. 2014, 111, 964–970.
- Perrone, M.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Laquintana, V.; Franco, M.; Bernkop-Schnürch, A.; Denora, N. S-preactivated thiolated glycol chitosan useful to combine mucoadhesion and drug delivery. Eur. J. Pharm. Biopharm. 2018, 132, 103–111.
- Saremi, S.; Atyabi, F.; Akhlaghi, S.P.; Ostad, S.N.; Dinarvand, R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluation. Int. J. Nanomed. 2011, 6, 119–128.
- Guo, Y.; Liu, R.; Zhou, L.; Zhao, H.; Lv, F.; Liu, L.; Huang, Y.; Zhang, H.W.; Yu, C.; Wang, S. Blood-brain-barrier penetrable thiolated paclitaxel-oligo (p-phenylene vinylene) nanomedicine with increased drug efficiency for glioblastoma treatment. Nano Today 2020, 35, 100969.
- Dünnhaupt, S.; Barthelmes, J.; Köllner, S.; Sakloetsakun, D.; Shahnaz, G.; Düregger, A.; Bernkop-Schnürch, A. Thiolated nanocarriers for oral delivery of hydrophilic macromolecular drugs. Carbohydr. Polym. 2015, 117, 577–584.
- Ho, Y.C.; Wu, S.J.; Mi, F.L.; Chiu, Y.L.; Yu, S.H.; Panda, N.; Sung, H.W. Thiol-modified chitosan sulfate nanoparticles for protection and release of basic fibroblast growth factor. Bioconjug. Chem. 2010, 21, 28–38.
- Zhou, Z.; Li, H.; Wang, K.; Guo, Q.; Li, C.; Jiang, H.; Hu, Y.; Oupicky, D.; Sun, M. Bioreducible Cross-Linked Hyaluronic Acid/Calcium Phosphate Hybrid Nanoparticles for Specific Delivery of siRNA in Melanoma Tumor Therapy. ACS Appl. Mater. Interfaces 2017, 9, 14576–14589.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Turner, A.P.; Alam, C.; Bendayan, R. Efflux Transporters in Cancer Resistance: Molecular and Functional Characterization of P-glycoprotein; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128164341.
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649.
- Benito, E.; Romero-Azogil, L.; Galbis, E.; De-Paz, M.V.; García-Martín, M.G. Structurally simple redox polymersomes for doxorubicin delivery. Eur. Polym. J. 2020, 137, 109952.
- Akhlaghi, S.P.; Saremi, S.; Ostad, S.N.; Dinarvand, R.; Atyabi, F. Discriminated effects of thiolated chitosan-coated pMMA paclitaxel-loaded nanoparticles on different normal and cancer cell lines. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 689–697.
- Vivek, R.; Nipun Babu, V.; Thangam, R.; Subramanian, K.S.; Kannan, S. PH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf. B Biointerfaces 2013, 111, 117–123.
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121.
- Manouras, T.; Vamvakaki, M. Field responsive materials: Photo-, electro-, magnetic- and ultrasound-sensitive polymers. Polym. Chem. 2017, 8, 74–96.
- Wong, P.T.; Choi, S.K. Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chem. Rev. 2015, 115, 3388–3432.
- Zhou, L.; Qiu, T.; Lv, F.; Liu, L.; Ying, J.; Wang, S. Self-Assembled Nanomedicines for Anticancer and Antibacterial Applications. Adv. Healthc. Mater. 2018, 1800670, 1–29.
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009.
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 190, 485–499.
- Ortega, A.L.; Mena, S.; Estrela, J.M. Glutathione in Cancer Cell Death. Cancers 2011, 3, 1285–1310.
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352.
- López-Mirabal, H.R.; Winther, J.R. Redox characteristics of the eukaryotic cytosol. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2008, 1783, 629–640.
More
