Intracellular protein tyrosine kinases, including Abelson (Abl), Src, JNK and many others, play a pivotal role in signal transduction pathways and cancer development, being highly activated in malignant tumor cells, but having very low activity and expression in normal cells. Consequently, in the last thirty years, many small molecule tyrosine kinase inhibitors (TKIs) have entered in clinical trials and were approved to treat hematologic and non-hematologic tumors, thus improving cancer treatment. In particular, the greatest progress has been made with the use of TKIs in the treatment of chronic myeloid leukemia (CML).
- tyrosine kinase inhibitors
- nanoparticles
- drug delivery
- EPR
1. Introduction of Tyrosine Kinase Inhibitors
Intracellular protein tyrosine kinases, including Abelson (Abl), Src, JNK and many others, play a pivotal role in signal transduction pathways and cancer development, being highly activated in malignant tumor cells, but having very low activity and expression in normal cells [1]. Consequently, in the last thirty years, many small molecule tyrosine kinase inhibitors (TKIs) have entered in clinical trials and were approved to treat hematologic and non-hematologic tumors, thus improving cancer treatment. The majority of these molecules are ATP-competitive inhibitors and are not selective, acting also on receptor tyrosine kinases (in particular, platelet-derived growth factor receptor, PDGFR, and vascular endothelial growth receptor, VEGFR) or other intracellular kinases with different selectivity and potency. Unfortunately, all of these new compounds presented sub-optimal properties such as poor solubility (very high pH-dependent solubility), low oral bioavailability and severe adverse effects, which limited their clinical application; in addition, the onset of resistance became the biggest obstacle in clinical application for some of the new molecules (in particular for Imatinib,Figure 1).
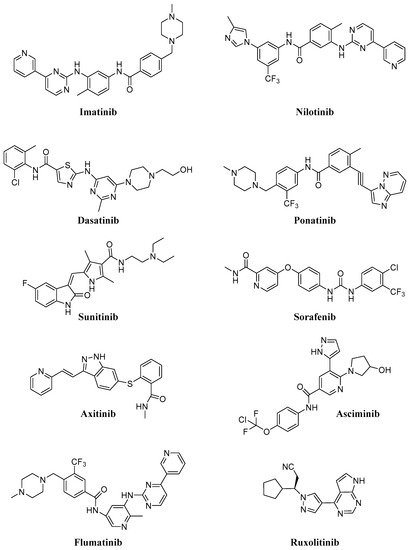
Consequently, in recent years many efforts have been made to find new molecules (e.g., Asciminib, Flumatinib; Figure 1) active on resistant CML (in particular on T315I mutation), for the treatment of different diseases (e.g., Ruxolitinib; Figure 1) currently without an effective therapy. In Table 1, selected TKIs (the most important for CML treatment and more recent and innovative than other ones) and their applications are reported. Issues related to solubility and resistance onset could be solved using safe and efficient delivery vehicles, that could improve the therapeutic efficacy, minimize toxicity, ameliorate tumor targetability and decrease drug resistance [2][3].
Consequently, in recent years many efforts have been made to find new molecules (e.g., Asciminib, Flumatinib;Figure 1) active on resistant CML (in particular on T315I mutation), for the treatment of different diseases (e.g., Ruxolitinib;Figure 1) currently without an effective therapy. InTable 1, selected TKIs (the most important for CML treatment and more recent and innovative than other ones) and their applications are reported. Issues related to solubility and resistance onset could be solved using safe and efficient delivery vehicles, that could improve the therapeutic efficacy, minimize toxicity, ameliorate tumor targetability and decrease drug resistance [2][3].
| Compound | Target | Number of Clinical Trials | Diseases | FDA Approval |
|---|---|---|---|---|
| Imatinib | Abl, PDGFR, Kit | 754 | CML, GIST, GVHD, many hematological and solid tumors | 2001 |
| Dasatinib | Abl, PDGFR, Kit, Src | 320 | CML, ALL, lymphoma, NSCLC and others solid tumors | 2006 |
| Nilotinib | Abl, PDGFR, c-Kit, LCK, EPHA3, EPHA8, DDR1, DDR2, MAPK11, ZAK | 219 | CML, ALL, GIST | 2007 |
| Ponatinib | Abl, Src, FGFR, PDGFR, VEGFR, | 67 | CML, ALL | 2012 |
| Asciminib | Abl | 13 | CML | // |
| Flumatinib | Abl, PDGFR, c-Kit, CSFR | 5 | CML | // |
| Sunitinib | PDGFR, Kit, FLT3, VEGFR, CSF1R | 610 | RCC, GIST | 2006 |
| Sorafenib | PDGFR, c-Kit, FLT3, VEGFR, B-Raf | 870 | RCC, liver and thyroid cancers | 2007 |
| Axitinib | Abl, PDGFR, VEGRF, c-Kit | 161 | RCC | 2012 |
| Ruxolitinib | JAK1, JAK2 | 258 | Myelofibrosis, polycythemia vera, GVHD, many other different diseases | 2011 |
Imatinib (IM, Gleevec®, Glivec®) was approved for CML in 2001 and today represents the first-line therapy for this type of hematological tumor, being able to block phosphorylation of Bcr-Abl, a fusion protein kinase which plays a fundamental role in CML development [4]. As IM inhibits PDGFR and c-Kit, two other transmembrane TKs, it has been approved as frontline therapy for: (i) gastrointestinal stromal tumors (GIST), characterized by mutated and over-expressed c-Kit or PDGFR-b [5]; (ii) other myeloid malignancies and hypereosinophilic syndromes and (iii) systemic mastocytosis [6][7] Most of them are obviously focused on CML, but also on solid tumors, such as acute lymphoblastic lymphoma (ALL), GIST, melanoma, sarcoma, glioblastoma and papillary thyroid cancer; interestingly, some trials concern asthma (NCT01097694), chronic graft-versus-host disease (GVHD) (NCT01862965), steroid-refractory sclerotic/fibrotic type GVHD (NCT01898377), multiple sclerosis (MS) (NCT03674099) and COVID-19 (NCT04422678), this compound having good immunosuppressive properties.
Currently, 350 clinical trials (63 in recruitment) regarding Dasatinib are focused on CML, ALL, Hodgkin and non-Hodgkin lymphoma, neck, head, breast, NSCLC, melanoma, mesothelioma, ovarian, colorectal, glioblastoma and CNS tumors (Table 1). In addition, Dasatinib, also acting on PDGFR, Kit, Src, Tek and Btk [8], could be useful as an immunosuppressive agent for immunological disorders [9]. Nilotinib is currently being studied in 219 clinical trials (41 in recruitment,Table 1) to evaluate its efficacy in CML, ALL, GIST and sarcoma (soft tissue sarcoma) patients, but also Huntington’s (NCT03764215), Parkinson’s (NCT02954978) and different forms of dementia pathologies (NCT02947893, NCT04002674).
Sorafenib (Figure 1) is an approved pankinase inhibitor able to target the Ras/Raf/Mek/Erk cascade pathway, PDGFR, VEGFR1/2 and the c-Kit receptor, and to block cell proliferation of different solid tumors; in particular, hepatocellular carcinoma (HCC) [10]. However, its long-term application in clinical practice was hampered by serious dermal toxicity and drug resistance, low water solubility and the first-pass effect [11] and consequent low drug concentration in tumor tissue. In addition, it can induce paradoxical activation of the MAPK pathway in both malignant and normal stromal cells [12] and this fact in hepatic stellate cells (HSCs) leads to their activation with consequent liver damage. Other pankinase inhibitors (e.g., Sunitinib and Axitinib;Figure 1) have been more recently approved for advanced RCC, unresectable HCC, thyroid cancer and GIST, and many other clinical trials are ongoing also on different solid tumors and leukemia types [13][14].
Seventy-six clinical trials (31 in recruitment,Table 1) focused on CML, ALL and different solid tumors (as NSCLC, GIST, glioblastoma, breast and many others) are reported for Ponatinib, (Iclusig,Figure 1) [15], approved in 2012 for CML treatment. In 2013, the FDA temporarily suspended Ponatinib sales because of the risk of life-threatening blood clots and severe narrowing of blood vessels, but at the end of the same year, this suspension was partially lifted.
Very recently, Novartis announced the results of a phase III ASCEMBL study (multicenter, open-label, randomized study) regarding Asciminib (ABL001,Figure 1), a new Abl allosteric inhibitor; the study evaluated Asciminib administration in adult patients with Philadelphia chromosome-positive CML in chronic phase, previously treated with two or more TKIs for 24 weeks [16][17][18]. On the basis of these interesting results, the FDA has granted Fast Track designation for Asciminib. Now, 14 clinical trials (two of them completed,Table 1) are focused on this compound (alone or in association with IM or Nilotinib) for CML and other leukemic patients; only one clinical trial is focused on asthma treatment (NCT03549897).
Flumatinib (HHGV678,Figure 1) is an orally bioavailable TKI, recently approved in China [19]; it inhibits the wild-type and mutated Bcr-Abl, PDGFR and mast/stem cell growth factor receptor (SCFR and c-Kit). Up to date, five clinical trials (one completed,Table 1) regarding only CML are in progress.
Ruxolitinib (Jakafi,Figure 1) is a selective JAK1 and JAK2 inhibitor approved for myelofibrosis (2011), polycythemia vera (2014) and GVHD in adult and pediatric patients (2019), but now it is also under study for COVID-19 (NCT04414098, NCT04359290, NCT04348071), atopic dermatitis (NCT039208529) and vitiligo (NCT04530344) (Table 1).
Although new compounds are continuously placed on the market and many are effective against different mutations, problems regarding poor solubility, resistance and severe side effects are not completely overcome. In part, the evolutionary probability of resistance can also be overcome with the association of two or more compounds, but this approach does not seem to be conclusive; consequently, the advent of nanotechnologies seems to be of great importance. In addition, it is also possible that the administration of one single nanoparticle containing several drugs may be more effective than the administration of several nanoparticles each containing one compound [20].
2. Nanoparticles of Tyrosine Kinase Inhibitors
A major part of these new nanoformulations have been patented in the last ten years [21][22]; in general, IM and Dasatinib represent the most studied compounds, whereas new molecules, such as Asciminib, Axitinib and others, are less investigated. Interesting results have been obtained for Sorafenib, Ponatinib and Nilotinib, as reported below. Regarding the routes of administration, these nanocarriers are usually injected intravenously; recent reports describe alternative administration routes thorough intratecal [23] and subcutaneous injection [24].
2.1. Imatinib
2.2. Dasatinib
Many patents focus on Dasatinib nanoformulations and most of them are innovative and very recent [25][26][27]. As previously reported, a major problem for oral administration of Dasatinib is its poor bioavailability caused by a low solubility, inappropriate partition coefficient, low drug permeation through lipid membrane, first-pass metabolism, P-glycoprotein-mediated efflux and drug degradation in the gastrointestinal tract due to the pH of the stomach or enzymatic degradation [28]. Animal data suggest that, due to an extensive first-pass effect, the bioavailability of Dasatinib is about 14–34%. Thus, limited aqueous solubility is the bottleneck for the therapeutic outcome of Dasatinib. The majority of Dasatinib nanoformulations have been developed to treat CML cell lines, but as reported below, also for solid tumor treatment. In addition, in the last years Dasatinib-loaded NPs have been developed for different diseases, in particular ocular diseases (proliferative vitreoretinopathy, PVR) [29][30].2.3. Nilotinib
2.4. Ponatinib
2.5. Sunitinib
2.6. Sorafenib
References
- Robertson, S.C.; Tynan, J.; Donoghue, D.J. RTK mutations and human syndromes: When good receptors turn bad. Trends Genet. 2000, 16, 265–271.
- Moradpour, Z.; Barghi, L. Novel Approaches for Efficient Delivery of Tyrosine Kinase Inhibitors. J. Pharm. Pharm. Sci. 2019, 22, 37–48.
- Yin, Y.; Yuan, X.; Gao, H.; Yang, Q. Nanoformulations of small molecule protein tyrosine kinases inhibitors potentiate targeted cancer therapy. Int. J. Pharm. 2020, 573, 118785.
- Druker, B.J.; Lydon, N.B. Lessons learned from the development of an Abl tyrosine kinase inhibitor for chronic mye-logenous leukemia. J. Clin. Investig. 2000, 15, 3–7.
- Pisters, P.W.; Patel, S.R. Gastrointestinal stromal tumors: Current management. J. Surg. Oncol. 2010, 102, 530–538.
- Cheah, C.Y.; Burbury, K.; Apperley, J.F.; Huguet, F.; Pitini, V.; Gardembas, M.; Ross, D.M.; Forrest, D.; Genet, P.; Rousselot, P.; et al. Patients with myeloid malignancies bearing PDGFRB fusion genes achieve durable long-term remissions with imatinib. Blood 2014, 123, 3574–3577.
- Eilers, G.; Czaplinski, J.T.; Mayeda, M.; Bahri, N.; Tao, D.; Zhu, M.; Hornick, J.; Lindeman, N.I.; Sicinska, E.; Wagner, A.J.; et al. CDKN2A/p16 Loss Implicates CDK4 as a Therapeutic Target in Imatinib-Resistant Dermatofibrosarcoma Protuberans. Mol. Cancer Ther. 2015, 14, 1346–1353.
- Schenone, S.; Brullo, C.; Musumeci, F.; Botta, M. Novel dual Src/Abl inhibitors for hematologic and solid malignancies. Expert Opin. Investig. Drugs 2010, 19, 931–945.
- Hantschel, O.; Rix, U.; Schmidt, U.; Bürckstümmer, T.; Kneidinger, M.; Schütze, G.; Colinge, J.; Bennett, K.L.; Ellmeier, W.; Valent, P.; et al. The Btk tyrosine kinase is a major target of the Bcr-Abl inhibitor dasatinib. Proc. Natl. Acad. Sci. USA 2007, 104, 13283–13288.
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angio-genesis. Cancer Res. 2004, 64, 7099–7109.
- Jain, L.; Woo, S.; Gardner, E.R.; Dahut, W.L.; Kohn, E.C.; Kummar, S.; Mould, D.R.; Giaccone, G.; Yarchoan, R.; Venitz, J.; et al. Population pharmacokinetic analysis of sorafenib in patients with solid tumours. Br. J. Clin. Pharmacol. 2011, 72, 294–305.
- Duncan, J.S.; Whittle, M.C.; Nakamura, K.; Abell, A.N.; Midland, A.A.; Zawistowski, J.S.; Johnson, N.L.; Granger, D.A.; Jordan, N.V.; Darr, D.B.; et al. Dynamic Reprogramming of the Kinome in Response to Targeted MEK Inhibition in Triple-Negative Breast Cancer. Cell 2012, 149, 307–321.
- Abdelgalil, A.A.; Alkahtani, H.M.; Al-Jenoobi, F.I. Sorafenib. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: New York, NY, USA, 2019; Volume 44, pp. 239–266.
- Boland, P.; Wu, J. Systemic therapy for hepatocellular carcinoma: Beyond sorafenib. Chin. Clin. Oncol. 2018, 7, 50.
- Tan, F.H.; Putoczki, T.L.; Stylli, S.S.; Luwor, R.B. Ponatinib: A novel multi-tyrosine kinase inhibitor against human ma-lignancies. OncoTargets Ther. 2019, 12, 635–645.
- Wylie, A.A.; Schoepfer, J.; Jahnke, W.; Cowan-Jacob, S.W.; Loo, A.; Furet, P.; Marzinzik, A.L.; Pelle, X.; Donovan, J.; Zhu, W.; et al. The allosteric inhibitor ABL001 enables dual targeting of BCR–ABL1. Nature 2017, 543, 733–737.
- Hughes, T.P.; Mauro, M.J.; Cortes, J.E.; Minami, H.; Rea, D.; DeAngelo, D.J.; Breccia, M.; Goh, Y.T.; Talpaz, M.; Hoch-haus, A.; et al. Asciminib in Chronic Myeloid Leukemia after ABL Kinase Inhibi-tor Failure. N. Engl. J. Med. 2019, 381, 2315–2326.
- Schoepfer, J.; Jahnke, W.; Berellini, G.; Buonamici, S.; Cotesta, S.; Cowan-Jacob, S.W.; Dodd, S.; Drueckes, P.; Fabbro, D.; Gabriel, T.; et al. Discovery of Asciminib (ABL001), an Allosteric Inhibitor of the Tyrosine Kinase Activity of BCR-ABL1. J. Med. Chem. 2018, 61, 8120–8135.
- Zhang, L.; Li, M.; Yanli, Z.; Huanling, Z.; Jiuwei, C.; Aining, S.; Yu, H.; Jie, J.; Hao, J.; Xi, Z.; et al. Frontline flumatinib versus imatinib in patients with chronic myeloid leukemia in chronic phase: Results from the China randomized phase III study. J. Clin. Oncol. 2019, 37, 7004.
- Goldman, A.; Kulkarni, A.; Kohandel, M.; Pandey, P.; Rao, P.; Natarajan, S.K.; Sabbisetti, V.; Sengupta, S. Rationally Designed 2-in-1 Nanoparticles Can Overcome Adaptive Resistance in Cancer. ACS Nano 2016, 10, 5823–5834.
- Onda, T.; Masuda, A.; Yamakawa, K.; Tomiyama, C.; Yoneta, Y.; Akatsu, Y.; Yamamoto, K.; Mochizuki, A.; Inventors; Nippon Kayaku Co Ltd. Block Copolymer Conjugate of Physiologically Active Substance. United States Patent US 10,357,573, 23 July 2019.
- Dravid, V.P.; Nandwana, V.; Inventors; Northwestern University Assignee. Nanoparticle-Lipid Composite Carriers and Uses Thereof. United States Patent Application US 16/615,260, 4 June 2020.
- Xu, H.; Ji, H.; Li, Z.; Qiao, W.; Wang, C.; Tang, J. In vivo Pharmacokinetics and in vitro Release of Imatinib Mesylate-Loaded Liposomes for Pulmonary Delivery. Int. J. Nanomed. 2021, 16, 1221–1229.
- Soundararajan, R.; Wang, G.; Petkova, A.; Uchegbu, I.F.; Schätzlein, A.G. Hyaluronidase Coated Molecular Envelope Technology Nanoparticles Enhance Drug Absorption via the Subcutaneous Route. Mol. Pharm. 2020, 17, 2599–2611.
- Gao, J.; Qiao, Z.; Liu, S.; Xu, J.; Wang, S.; Yang, X.; Wang, X.; Tang, R. Preparation of small molecule prodrug composed of pH-sensitive orthoester and dasatinib conjugate. Eur. J. Pharm. Biopharm. 2021, 163, 188–197.
- Horner, G.; Rai, P.; Agrawal, S.; Parker, B. Remotely Triggered Therapy. United States Patent Application US 17/000,205, 17 December 2020.
- Tran, D.; Le, S.B. Methods for Targeted Treatment and Prediction of Patient Survival in Cancer. PCT Int. Appl. WO 2020163639 A1 20200813, 13 August 2020.
- Zhang, L.; Wang, S.; Zhang, M.; Sun, J. Nanocarriers for oral drug delivery. J. Drug Target. 2013, 21, 515–527.
- Chauhan, R.; Balgemann, R.; Greb, C.; Nunn, B.M.; Ueda, S.; Noma, H.; McDonald, K.; Kaplan, H.J.; Tamiya, S.; O’Toole, M.G. Production of dasatinib encapsulated spray-dried poly (lactic-co-glycolic acid) particles. J. Drug Deliv. Sci. Technol. 2019, 53, 101204.
- Li, Q.; Lai, K.L.; Chan, P.S.; Leung, S.C.; Li, H.Y.; Fang, Y.; To, K.; Choi, C.H.J.; Gao, Q.Y.; Lee, T.W. Micellar delivery of dasatinib for the inhibition of pathologic cellular processes of the retinal pigment epithelium. Colloids Surf. B Biointerfaces 2016, 140, 278–286.
- Cai, L.; Zhou, H.; Liu, L.; He, H.; Liang, R. Preparation Method and Application of Nanoparticle Drug-Carrying System. Faming Zhuanli Shenqing CN 110859817, 6 March 2020.
- McKeon, F.; Duleba, M.; Zhang, Y.; Xie, J.; Xian, W.; Vincent, M. Screening Methods for Identifying Therapeutic Agents for Treating Chronic Inflammatory Injury, Metaplasia, Dysplasia and Cancers of Epithelial Tissues. PCT Int. Appl. WO 2020219963, 29 November 2020.
- Koehl, N.J.; Griffin, B.T.; Holm, R.; Holm, R.; Kuentz, M. Chase Dosing of Lipid Formulations to Enhance Oral Bioavailability of Nilotinib in Rats. Pharm. Res. 2020, 37, 124.
- Jesson, G.; Brisander, M.; Andersson, P.; Demirbueker, M.; Derand, H.; Lennernaes, H.; Malmsten, M. Carbon Dioxide-Mediated Generation of Hybrid Nanoparticles for Improved Bioavailability of Protein Kinase Inhibitors. Pharm. Res. 2014, 31, 694–705.
- Fan, Q.-Q.; Zhang, C.-L.; Qiao, J.-B.; Cui, P.-F.; Xing, L.; Oh, Y.-K.; Jiang, H.-L. Extracellular matrix-penetrating nanodrill micelles for liver fibrosis therapy. Biomaterials 2020, 230, 119616.
- Cortese, B.; D’Amone, S.; Palamà, I.E. Wool-Like Hollow Polymeric Nanoparticles for CML Chemo-Combinatorial Therapy. Pharmaceutics 2018, 10, 52.
- Robinson, W.H.; Postolova, A.; Raghu, H. Tyrosine Kinase Inhibitor Formulations for the Treatment of Mast Cell-Mediated Inflammatory Diseases and Methods of Use Thereof. United States Patent Application US 20170312282, 2 November 2017.
- Zhang, Y.; Zhang, H.; Peng, R. Preparation and in vitro release of ponatinib nanosuspensions. Guangdong Yaoxueyuan Xuebao 2014, 30, 544–548.
- Zinger, A.; Baudo, G.; Naoi, T.; Giordano, F.; Lenna, S.; Massaro, M.; Ewing, A.; Kim, H.R.; Tasciotti, E.; Yustein, J.T.; et al. Reproducible and Characterized Method for Ponatinib Encapsulation into Biomimetic Lipid Nanoparticles as a Platform for Multi-Tyrosine Kinase-Targeted Therapy. ACS Appl. Bio Mater. 2020, 3, 6737–6745.
- Kallus, S.; Englinger, B.; Senkiv, J.; Laemmerer, A.; Heffeter, P.; Berger, W.; Kowol, C.R.; Keppler, B.K. Nanoformulations of anticancer FGFR inhibitors with improved therapeutic index. Nanomedicine 2018, 14, 2632–2643.
- Zhang, J.; Tao, C.; Song, X.; Li, W. Preparation Method of Injectable Sunitinib Nanoparticle. Faming Zhuanli Shenqing CN 108030927, 15 May 2018.
- Otroj, M.; Taymouri, S.; Varshosaz, J.; Mirian, M. Preparation and characterization of dry powder containing sunitinib loaded PHBV nanoparticles for enhanced pulmonary deliver. J. Drug Deliv. Sci. Technol. 2020, 56, 101570.
- Joseph, J.J.; Sangeetha, D.; Gomathi, T. Sunitinib loaded chitosan nanoparticles formulation and its evaluation. Int. J. Biol. Macromol. 2016, 82, 952–958.
- Shi, J.; Ju, R.; Sun, M.; Li, X.; Zhao, Y.; Zeng, F.; Lu, W. Development of targeted sunitinib plus vinorelbine liposomes modified with DSPE-PEG2000-pemetrexed conjugate and the inhibitory effect to resistant breast cancer in vitro. J. Chin. Pharm. Sci. 2014, 23, 287–294.
- Shi, J.; Sun, M.; Li, X.; Zhao, Y.; Ju, R.; Mu, L.; Yan, Y.; Li, X.; Zeng, F.; Lu, W. A combination of targeted sunitinib lipo-somes and targeted vinorelbine liposomes for treating invasive breast cancer. J. Biomed. Nanotechnol. 2015, 11, 1568–1582.
- Bhatt, P.; Narvekar, P.; Lalani, R.; Chougule, M.B.; Pathak, Y.; Sutariya, V. An in vitro Assessment of Thermo-Reversible Gel Formulation Containing Sunitinib Nanoparticles for Neovascular Age-Related Macular Degeneration. AAPS PharmSciTech 2019, 20, 1–14.
- Zhang, H.; Zhang, F.; Yan, S. Preparation, in vitro release, and pharmacokinetics in rabbits of lyophilized injection of sorafenib solid lipid nanoparticles. Int. J. Nanomed. 2012, 7, 2901–2910.
- Xu, X.; Tang, X.; Wu, X.; Feng, X. Biosynthesis of sorafenib coated graphene nanosheets for the treatment of gastric cancer in patients in nursing care. J. Photochem. Photobiol. B Biol. 2019, 191, 1–5.
- Almeida, P.V.; Shahbazi, M.; Correia, A.; Maekilae, E.; Kemell, M.; Salonen, J.; Hirvonen, J.; Santos, H. A multifunctional nanocomplex for enhanced cell uptake, endosomal escape and improved cancer therapeutic effect. Nanomedicine 2017, 12, 1401–1420.
- Sheng, X.; Huang, T.; Qin, J.; Li, Q.; Wang, W.; Deng, L.; Dong, A. Preparation, pharmacokinetics, tissue distribution and antitumor effect of sorafenib-incorporating nanoparticles in vivo. Oncol. Lett. 2017, 14, 6163–6169.
- Park, S.Y.; Kang, Z.; Thapa, P.; Jin, Y.S.; Park, J.W.; Lim, H.J.; Lee, J.Y.; Lee, S.; Seo, M.; Kim, M.; et al. Development of sorafenib loaded nanoparticles to improve oral bioavailability using a quality by design approach. Intern. J. Pharm. 2019, 566, 229–238.
- Kim, D.H.; Kim, M.-D.; Choi, C.-W.; Chung, C.-W.; Ha, S.H.; Kim, C.H.; Shim, Y.-H.; Jeong, Y.-I.; Kang, D.H. Antitumor activity of sorafenib-incorporated nanoparticles of dextran/poly(dl-lactide-co-glycolide) block copolymer. Nanoscale Res. Lett. 2012, 7, 91.
- Wang, C.-F.; Mäkilä, E.M.; Kaasalainen, M.H.; Liu, D.; Sarparanta, M.; Airaksinen, A.J.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Copper-free azide–alkyne cycloaddition of targeting peptides to porous silicon nanoparticles for intracellular drug uptake. Biomaterials 2014, 35, 1257–1266.
- Hong, F.; Tuyama, A.; Lee, T.F.; Loke, J.; Agarwal, R.; Cheng, X.; Garg, A.; Fiel, M.I.; Schwartz, M.; Walewski, J.; et al. Hepatic stellate cells express functional CXCR4: Role in stromal cell-derived factor-1α-mediated stellate cell activation. Hepatology 2009, 49, 2055–2067.
- Sung, Y.-C.; Liu, Y.-C.; Chao, P.-H.; Chang, C.-C.; Jin, P.-R.; Lin, T.-T.; Lin, J.-A.; Cheng, H.-T.; Wang, J.; Lai, C.P.; et al. Combined delivery of sorafenib and a MEK inhibitor using CXCR4-targeted nanoparticles reduces hepatic fibrosis and prevents tumor development. Theranostics 2018, 8, 894–905.
- Chen, Y.; Liu, Y.C.; Sung, Y.C.; Ramjiawan, R.R.; Lin, T.T.; Chang, C.C.; Jeng, K.S.; Chang, C.F.; Liu, C.H.; Gao, D.Y.; et al. Overcoming sorafenib evasion in hepatocellular carcinoma using CXCR4-targeted nanoparticles to co-deliver MEK-inhibitors. Sci. Rep. 2017, 7, 44123.
- Yu, X.-N.; Deng, Y.; Zhang, G.-C.; Liu, J.; Liu, T.-T.; Dong, L.; Zhu, C.-F.; Shen, X.-Z.; Li, Y.-H.; Zhu, J.-M. Sorafenib-Conjugated Zinc Phthalocyanine Based Nanocapsule for Trimodal Therapy in an Orthotopic Hepatocellular Carcinoma Xenograft Mouse Model. ACS Appl. Mater. Interfaces 2020, 12, 17193–17206.
- Li, Z.; Ye, L.; Liu, J.; Lian, D.; Li, X. Sorafenib-loaded nanoparticles based on biodegradable dendritic polymers for enhanced therapy of hepatocellular carcinoma. Intern. J. Nanomed. 2020, 15, 1469–1480.
- Tang, X.; Chen, L.; Li, A.; Cai, S.; Zhang, Y.; Liu, X.; Jiang, Z.; Liu, X.; Liang, Y.; Ma, D. Anti-GPC3 antibody-modified sorafenib-loaded nanoparticles significantly inhibited HepG2 hepatocellular carcinoma. Drug Deliv. 2018, 25, 1484–1494.
- Cao, H.; Wang, Y.; He, X.; Zhang, Z.; Yin, Q.; Chen, Y.; Yu, H.; Huang, Y.; Chen, L.; Xu, M.; et al. Codelivery of Sorafenib and Curcumin by Directed Self-Assembled Nanoparticles Enhances Therapeutic Effect on Hepatocellular Carcinoma. Mol. Pharm. 2015, 12, 922–931.
- Zhang, J.; He, B.; Qu, W.; Cui, Z.; Wang, Y.; Zhang, H.; Wang, J.; Zhang, Q. Preparation of the albumin nanoparticle system loaded with both paclitaxel and sorafenib and its evaluation in vitro and in vivo. J. Microencapsul. 2011, 28, 528–536.
- Zan, Y.; Dai, Z.; Liang, L.; Deng, Y.; Dong, L. Co-delivery of plantamajoside and sorafenib by a multi-functional nano-particle to combat the drug resistance of hepatocellular carcinoma through reprograming the tumor hypoxic microenvironment. Drug Deliv. 2019, 26, 1080–1091.
- Li, Z.; Rana, T.M. Therapeutic targeting of microRNAs: Current status and future challenges. Nat. Rev. Drug Discov. 2014, 13, 622–638.
- Li, M.; Su, Y.; Zhang, F.; Chen, K.; Xu, X.; Xu, L.; Zhou, J.; Wang, W. A dual-targeting reconstituted high density lipoprotein leveraging the synergy of sorafenib and antimiRNA21 for enhanced hepatocellular carcinoma therapy. Acta Biomater. 2018, 75, 413–426.
- Liu, J.; Boonkaew, B.; Arora, J.; Mandava, S.H.; Maddox, M.M.; Chava, S.; Callaghan, C.; He, J.; Dash, S.; John, V.T.; et al. Comparison of Sorafenib-Loaded Poly (Lactic/Glycolic) Acid and DPPC Liposome Nanoparticles in the in Vitro Treatment of Renal Cell Carcinoma. J. Pharm. Sci. 2015, 104, 1187–1196.
- Poojari, R.; Kini, S.; Srivastava, R.; Panda, D. Intracellular interactions of electrostatically mediated layer-by-layer assembled polyelectrolytes based sorafenib nanoparticles in oral cancer cells. Colloids Surf. B Biointerfaces 2016, 143, 131–138.
