Src family kinases (SFKs) are key regulators of cell proliferation, differentiation, and survival. The expression of these non-receptor tyrosine kinases is strongly correlated with cancer development and tumor progression. Thus, this family of proteins serves as an attractive drug target.
- G protein-coupled receptors
- GPCR
- SFK
- Src kinases
- G proteins
- arrestin
- allosteric regulation
- biased signaling
- non-receptor tyrosine kinases
- SH3 domains
- polyproline motifs
- kinase activation
- signaling
1. Introduction
Src family kinases (SFKs) are non-receptor protein-tyrosine kinases that regulate essential processes such as cell proliferation, differentiation, survival, migration, and metabolism [1].
SFKs are upregulated in malignancies, and their expression levels as well as specific activity are elevated in brain, breast, colon, lung, and pancreatic carcinomas [2][3][4][5][6][7][8][9][2,3,4,5,6,7,8,9]. For acute myeloid leukemia and colorectal cancer, a direct correlation between expression level of some SFK family members and patient survival was observed [10][11][10,11].
The human SFKs consist of eight typical family members (Src, Fyn, Yes, Fgr, Hck, Blk, Lck, and Lyn) and three atypical family members (Brk, Frk, Srms) based on sequence similarity. In some nomenclatures, atypical family members are considered a separate family also called the Frk family [12][13][12,13]. Most of the SFKs, such as Yes, Fgr, Blk, Hck, Lck, and Lyn have an important regulatory role in signaling pathways of hematopoietic cells. A majority of SFKs are essential in immune response; whereas Fyn and Lck are activated immediately after T-cell receptor stimulation, expression of Fgr, Hck, Lyn is induced in stimulated mature monocytes and macrophages [14][15].
SFKs represent drug targets with great therapeutic potential, especially in cancer treatment, since SFKs are involved in cancer progression in various stages (reviewed in [15][16][16,17]). Approved drugs for cancer treatment targeting Src family kinases, such as Dasatinib, show a high level of toxicity [17][18] due to their unselective inhibition of SFKs in cancer and healthy cells. In-depth structural, functional, and mechanistic knowledge of each single SFK in combination with a detailed understanding of their expression regulation can be the basis for a more specific therapeutic approach with limited side effects.
2. Activation mechanism of SFKs
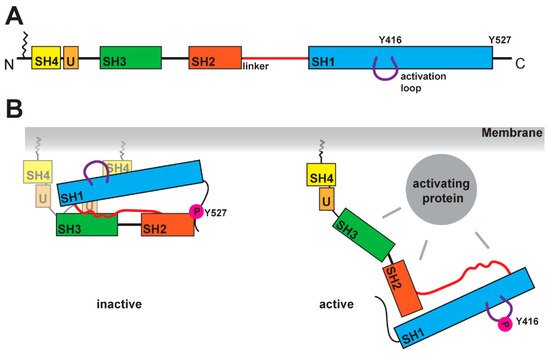
SFKs are composed of distinct domains (Figure 1A). The N-terminal region, also called the SH4 domain, contains a myristoylation or palmitoylation site, which acts as a membrane anchor and is a key element for the localization of SFKs [19,20]. The unique domain, which is located after the SH4 domain, has a regulatory function for membrane localization and can form a fuzzy intramolecular complex with the neighboring SH3 domain [21,22,2321–23]. SH3 domains serve as binding elements and are known to interact with a variety of polyproline motifs (reviewed in [24]). AftFollowerd by a linker, SFKs contain t the SH2 -domain, is encoded, which is known to interact with phosphorylated tyrosine residues, and following. After a longer linker region, the kinase domain follows, containing an N-lobe and a C-lobe. This domain It entails two regulatory phosphorylation sites (Y-416 and Y-527 for Src as a representative example, Figure 1B) [25,26]. The first regulatory site is the activating autophosphorylation site, and the second one the negative -regulatory site. The phosphorylation and dephosphorylation of these tyrosine residues cause dramatic structural changes and affect the activity of the kinase. In the inactive structure, the Y-527 is phosphorylated by CSK (C-terminal Src kinase) or CHK (CSK homologous kinase). [27,28], which results in an interaction of the kinase domain with the SH2 -domain [25,29,30]. This inactive conformation is further stabilized by the binding of the SH3 -domain with the polyproline motif in the linker region between SH2 -domain and kinase domain [30,31]. A recent finding showed a possible involvement of the SH4 domain, which binds in the inactive conformation to the kinase domain [32]. This compact stconformateion results in a closed conformation of the N- and C-lobes in the kinase domain, which results in a shielding of the Y-416 in the active site. In this closed conformation, thea binding of ATP and substrates is blocked. In the active conformation, the interactions of SH2 and SH3 domains are displaced bywith other binding partners, which results in an open conformation (Figure 1B). This grants accessibility of the active site and allows for an autophosphorylation of Y-416.
In general, SFKs are activated by several different growth factor receptor tyrosine kinases. For example, the SH2 domains interact with SHP-1 protein tyrosine phosphatase, CRK-associated substrate, or protein tyrosine phosphatase-1B [18][19][20][33,34,35]. Proteins with typical polyproline motifs such as cyclin-dependent kinase-5, KCNB1,
Additionally, for a number of G protein-coupled receptors (GPCRs), SFK activation was shown. However, the exact activation mechanism of this interaction is poorly understood. It has been postulated that there are three ways of GPCR-mediated SFK activation: through arrestins, G proteins, or direct binding. A detailed understanding of the mechanism is highly desirable due to the potential druggability of GPCRs and the crucial role of SFKs in cancer development and progression.
3. Src Activation through a GPCR–Arrestin Complex
Until now, the best understood GPCR-mediated activation of SFK is arrestin-based. As early as 1999, arrestin-2-mediated Src activation by beta-2 adrenergic receptor (β2AR) stimulation was detected [21][63]. Later on, this was observed for multiple other receptors. Until recently, there was no evidence of how this interaction could take place.
Nevertheless, in the ‘tail’ conformation, arrestin internalization and signaling are still possible. The most dominant conformational change during the activation of arrestin is the rotation of the N- and C-domains towards each other. With this domain rotation, multiple small conformational changes appear (also called switch regions for arrestin-3) The interaction of SFK SH3 domains with polyproline motifs in arrestin are substantial for the activation of SFKs [22][76].
Yang et al showed that the receptor phospho-tail allosterically regulates the different conformations within the polyproline motifs in arrestin-2, which subsequently allows for the binding of the SFK SH3 domain. leading to the adoption of an open active conformation of the kinase [22][76]. There are only a few activation studies for arrestin-3-mediated Src activation. For dopamine D1 receptor, activation of Src in the presence of arrestin-3 was shown [23][45].
4. Src Activation by G Proteins
G proteins (heterotrimeric guanine nucleotide-binding regulatory proteins) contain α, ß, and γ subunits. Agonist-bound GPCRs activate G proteins by facilitating the exchange of GDP to GTP at the α subunit. Activation of SFKs by G proteins can be achieved through either the α subunit or the ßγ subunit. The described two switch regions in G proteins are defined regions crucial for binding of effectors such as Ras protein or adenylyl cyclase [24][25][26][87,88,89].
The two sites of phosphorylation are Y37 and Y377 [27][28][93,94], and both promote GTP hydrolysis. The different regulatory mechanisms by G protein phosphorylation are reviewed elsewhere [29][95]. In transducin, an additional phosphorylation site, Y142, was found [30][96]. Overall, an arrestin-independent G protein-mediated activation of Src is still not fully understood and requires further investigation.
