Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Nasrul Wathoni.
Natural polymer is a frequently used polymer in various food applications and pharmaceutical formulations due to its benefits and its biocompatibility compared to synthetic polymers. One of the natural polymer groups (i.e., polysaccharide) does not only function as an additive in pharmaceutical preparations, but also as an active ingredient with pharmacological effects.
- polysaccharide
- sacran
- Aphanothece sacrum
1. Introduction
Polysaccharides are the most used natural polymers in pharmacy, in which they are safe, biocompatible, and biodegradable [1]. Polysaccharides such as chitosan, alginate, carrageenan, and cellulose are the most commonly used in pharmaceutical formulations. However, they suffer from several drawbacks, such as poor elasticity [2] and low water retention [3]. Sacran is a polysaccharide polymer that has a high water retention, high potential to develop, and a better elasticity than other polymers.
Sacran is a polysaccharide derived from cyanobacteria. Cyanobacteria are one of the algae that are also popular with blue-green algae [4] and are diverse in terms of genetics. They are not only spread in freshwater, ocean, and land, but can also be found in extreme ecosystems such as hot springs, hypersaline areas, cold environments, and barren deserts [5].
Cyanobacteria are the only prokaryotes that perform oxygenic photosynthesis as a potential biocatalyst, to directly convert CO2 into chemicals and other valuable products [6,7,8][6][7][8]. Cyanobacteria can produce polysaccharides with different functional groups, such as carboxylic acid, sulfate, phosphate, and amino acids that are responsible for adsorption. The texture of the polysaccharide produced by the cyanobacteria looks like a gel. Therefore, it has been a good candidate for the food industry and medicine materials [9]. Reports about the function of polysaccharides produced by freshwater cyanobacteria are still limited. As a result, research about the function of polysaccharides produced by freshwater cyanobacteria has widely been developed [10]. One of the cyanobacteria that live in freshwater is Aphanothece sacrum (A. sacrum).
A. sacrum is a green plant that has long been consumed by the Japanese and grown in Kogane river [9]. A. sacrum can produce a polysaccharide called sacran. Sacran has a negative charge in water. Due to its high viscoelasticity and water retention, it has been applied in many cosmetics and medical products [11].
2. Aphanothece sacrum
2.1. Aphanothece sacrum
Cyanobacteria are well known as toxin formers in various water systems all over the world, and they have contributed to the toxicity level of communities as they can release toxic compounds [12,13,14,15,16][12][13][14][15][16]. However, much research dealing with the metabolites of cyanobacteria has shown that cyanobacterial elements have biological activities related to human health [16]. It has been identified that Cyanobacteria can produce and secrete polysaccharides with different functional groups, such as carboxylic acid, sulfate, phosphate, and amino acids that are responsible for adsorption [17].
Aphanothece sacrum (A. sacrum) is a unicellular cyanobacterium found in freshwater. It belongs to seaweed types that can produce a polysaccharide known as sacran [9]. A. sacrum is a microalga grown in the Kyushu river, Japan [18], and it has a jelly matrix and a long cluster size [10]. It was classified biologically in the 19th century by Suringar and has been recognized for more than 100 years. However, research dealing with this issue is rarely found as its material availability is low and its cultivating method is less efficient. A. sacrum has a higher water content than other popular jellies such as Nostoc commune. From this information, it can be inferred that A. sacrum has a greater capacity for water retention [10]. It also contains high contents of Ca, Fe, Cu, and Mn that has made it different from other seaweed types [19]. A. sacrum is the only microalgae possibly grown as food materials for more than 300 years [9].
2.2. Characteristic of Aphanothece sacrum
A. sacrum is a prokaryotic alga that does not have chloroplasts to perform photosynthesis, and it has been recognized as a very primitive alga. It is evolutionarily and ecologically close to photosynthetic bacteria. Aphanothece, one of the algae, only has chlorophyll a, not chlorophyll a and b like other higher plants. It is a mediator between photosynthetic bacteria and green plants that have chlorophyll a and ferredoxin chloroplast types, but it resembles photosynthetic bacteria in other particular features [20,21,22][20][21][22].
In the research of Fujishiro et al. [19], the DNA analysis of an isolated A. sacrum chain showed that this chain carries two genes of ferredoxin (I and II), an amino acid sequence. In line with that, research undertaken by Wada et al. (1974) has shown that the ferredoxin molecule of A. sacrum contains an amino acid that is similar to the ferredoxin of other chloroplast types. The acidic amino acid is more prominent than the basic one. This ferredoxin lacks methionine and tryptophan. It retains one residue of histidine, arginine, and phenylalanine. There are only four cysteine residues found in this ferredoxin, which are also possessed by other plants such as Bumilleriopsis, Equisetum, Zea mays, Cypenis, and Gossypium. Moreover, the results of this research also explained that one molecule of ferredoxin of A. sacrum contains two atoms of iron and common volatile sulfur. It also provided the composition of the ferredoxin amino acid of A. sacrum.
2.3. Sacran
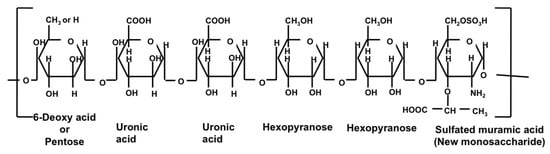
Nowadays, natural polymers are frequently used in several pharmaceutical preparations. One of the natural polymers is sacran, which is derived from Aphanothece sacrum [23]. This polysaccharide contains anionic groups such as carboxylate and sulfonate in high concentrations (32% mol of the total sugar) [9,24,25][9][24][25]. Therefore, sacran has a negative charge in water [11]. Sacran is a sulfated natural polymer extracted from the extracellular jelly matrix of A. sacrum, a cyanobacterium in freshwater [26]. Sacran contains 11 types of monosaccharides (Glc, Gal, Pria, Xyl, Rha, Fuc, GalA, GlcA, and traces of Ara, GalN, and Mur), with a molecular weight of 1–2.2 × 107 g/mol and a chain length of more than 30 μm [27,28][27][28].
Previous research has reported that the physical characters of sacran are unique. It builds gel-like layers that contain water with polyol; 1.3-butanedyol and 1.2-pentanedyol. Its film prevents the penetration by water and chemicals [27]. In solution, sacran is a polyelectrolyte, and because of its electric charge, the conformation of the sacran chain changes depending on its concentration. For instance, a helix transition concentration occurs when a chain of sacran changes from a random coil into a double helix at 0.09%, and gelation concentration occurs when sacran transitions from liquid to gel at 0.25% [28,29][28][29]. With increasing concentration, the sacran chain changes into a rigid rod form, showing liquid crystalline (LC) properties [30,31][30][31]. Sacran can form a gel-like film that is difficult to re-dissolve in water. It is formed due to polyol 1,3-butilen glycol, which is more superior to skin protection without polyol [32].
Sacran is a heteropolysaccharide composed of various sugar residues. From 11% of the monosaccharides, it contains a sulfate and carboxyl group [28,33][28][33]. Sacran is considered a safe biomaterial as A. sacrum is a cyanobacterium that has long been used by the people of Kyushu, Japan as a food to treat allergies and gastroenteritis. Sacran can also retain more water than hyaluronic acid or xanthan gum, and it can form hydrogel through the interaction between electrostatic and heavy metal cations [34]. Sacran can orientate itself depending on its concentration. Its structure looks like jelly that can adsorb cationic metals. Moreover, it generates an extracellular jelly matrix that can protect cells [25,35][25][35]. Macroscopically, the geometric structure of sacran consists of membrane partitions of three-dimensional cuboid cells to evaporate a uniaxially oriented water solution; thus, it can be developed for medical and pharmaceutical industries [36,37][36][37].
2.4. Extraction and Isolation of Sacran
A. sacrum is a plant that has been cultivated massively in the freshwater of Japan. With metal ions at its extracellular matrix, it forms a jelly-like material, which provides protection to the cellular structure of the algae. The composition of sacran within A. sacrum is very high, with 70% of dry A. sacrum [38]. Sacran is extracted from A. sacrum by evaporating the solvent [39], and this polysaccharide is extracted using a basic solvent generated from the biomaterial that has been washed with acid so that the minerals can be removed [38]
A sample of A. sacrum is frozen and thawed to damage the cell membranes, and it is then washed by water to remove components easily dissolved in water such as pigments (phycobiliprotein). Subsequently, the sample is washed using isopropanol. By stirring a large portion of sample three times, green decolorization emerges, which is gathered through filtration by applying gauze. An isopropanol-washed sample is then added into 0.1 M of NaOH solution at a temperature of 100 °C, and it is agitated at room temperature for 4 h to generate a transparent solution. This solution is then neutralized by HCl until the pH decreases to 8.0–9.0, and then it is filtered. The filtrate is concentrated using a rotating evaporator so that the solution becomes thick. To achieve deposition of the white fibrous materials, the thick solution is poured slowly into isopropanol. Afterward, to remove the added salt or generated salt from the extraction process, the fibers are re-dissolved in hot water and the solution is dialyzed against pure water for a month by changing the external water solution using regenerated cellulose membrane every day (MWCO: 14,000). The internal solution with sacran is re-thickened. The isopropanol fibrous deposit is gathered and dried in a vacuum oven (Figure 2Figure 1). The sacran water solution produced does not show a specific absorption in the 220–600 nm wavelength range by using ultraviolet spectroscopy (UV-Vis). This indicates that the solution is not contaminated by proteins, nucleic acids, chromophores, and/or other chemicals that have UV-Vis absorption. The yield of sacran extraction is very high (i.e., 70% of dry A. sacrum) [40,41,42][40][41][42].
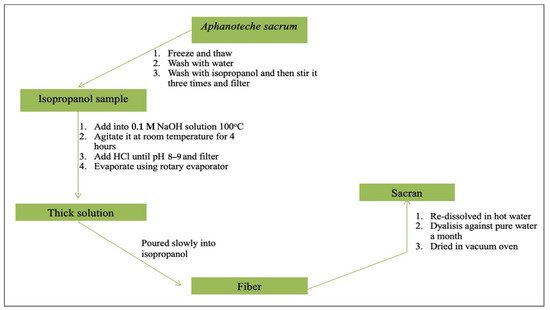

Figure 21. Scheme of sacran extraction process.
2.5. Characteristics of Sacran
Sacran is a heteropolysaccharide composed of sugar residue (galactose, glucose, mannose, xylose, rhamnose, fucose, and galacturonic acid, and glucuronic acid). It also contains traces of alanine, galactosamine, uronic acid, and muramic acid; 11% of the monosaccharide contains sulfate groups, while 22% of the monosaccharide contains carboxyl groups (Figure 3Figure 2) [43]. The extraction of sacran from A. sacrum collected from numerous locations in Japan has generated a product that is physically similar to high-purity cotton [40,43][40][43].
Sacran characterization has been conducted on solutions containing sacran. The results of GC-MS confirmed that there are Glc, Gal, Man, Xyl, Rha, Fuc, GalA, and GlcA with composition ratios of 25.9, 11.0, 10.0, 16.2, 10.2, 6.9, 4.0, and 4.2, respectively. The T-ICR-MS of sacran with methanolysis confirmed that there is a sulfated muramic acid, a unique sugar that has only be found in sacran so far [44][45]. The results of FTIR confirmed that several groups consist of R-COOH, R-SO4−, and R-OH [45][46]. A GC-MS study showed that there are uronic acid–Glc or Gal (m/z 355.0869), uronic acid–uronic acid (m/z 369.0667), uronic acid–uronic acid–Gal/Glc (m/z 545.162), uronic acid–Gal–Glc–hexose (m/z 693.2089), sulfated dimethyl uronic acid (m/z 301.0239), sulfated dimethyl muramic acid (m/z 358.081), dimethylated (uronic acid–uronic acid) (m/z 435.1127), sulfated dimethylated (uronic acid–Gal–Glc) (m/z 463.0773), and Glc–Gal–N-acetyl muramic acid (m/z 630.21). FT-ICR-MS also revealed the sequence of muramic acid connectors for hexose, Glc, and Gal. These results confirmed that muramic acid is not the constituent of the cell wall but of the capsule polysaccharide of A. sacrum [44][45].
According to the analysis of Budpud et al. [47], when 0.5 wt.% of fluorescein isothiocyanate-stained sacran is observed under a super-resolution confocal microscope, it clearly shows that sacran exists in solution with a microfiber of 0.5–1 μm in diameter. Besides, this microfiber could break into particles at a submicrometer scale in NaCl solution. As Na+ can replace the proton of carboxylate groups, it is added to the sacran solution.
To evaluate the ability of metal ions to absorb sacran, Okajima et al. [48] conducted a test by pouring 0.5% of sacran into a metal ion solution. The result showed that sacran is able to absorb metal ions such as magnesium, calcium, manganese, iron, zinc, nickel, copper, strontium, and barium. Next, the photoreaction result of anionic gel connected with the metal cation was visualized. As a result, the gel of sacran with trivalent metal ions gradually contracts depending on the photoirradiation energy, while alginate gel as a comparison degrades [49].
A hydrogel film that has been generated by sacran at a 0.5% w/v concentration produced a film with a thickness of 0.05 mm and a 20 Wt/Wo swollen ratio, which is considered a large value [23]. Yusof et al. [28] in their research also showed that the observed flow properties of sacran solution had a low rate of shear in which its shear viscosity increased with time [50], and it was constant in an increased viscosity of 6 Pa.s. for 900 s. The increased viscosity improved with the decrease in the rate of shear. Research has found that the shear viscosity does depend on a rate of shear period of 0.8/s. Meanwhile, the shear viscosity vastly decreased in the first 10 s at a higher rate of shear of more than 1.0 s−1, also known as thixotropy (positive). The sacran chain was aligned with the direction of flow at low and high rates of shear. Therefore, the viscosity change completely depended on the binding time constant of inter-chain sacran. In other words, the viscosity will only increase if the rate of shear of the binding time constant is short. However, the viscosity of sacran solution has been shown to not completely depend on concentration. A sacran concentration above 0.20 wt.% was considered a neutral electric chain in a solution. Afterward, when the sacran concentration was 0.20 wt.% with the addition of NaCl (100 mL), its viscosity did not change. When the concentration increased above 0.20 wt.% with NaCl addition, the sacran chain was clearly shown to behave as a noncharged chain in a solution [41]. It has been empirically confirmed that the degree of hydrogel swelling significantly increases with the degree of dissociation. A significant increase in molar conductivity is caused by the increase in the dissociation degree of a carboxyl group. The results confirm that the free counterion of sacran contributed to the upcoming electrical conductivity upon sacran chains in a particular concentration. Moreover, sacran chains can presumably change conformation into a more compact structure by breaking down the electric charge in the sacran chain [41]. The result of XPS (X-ray photoelectron spectroscopy) at this charge suggested that with the increase in the sulfate group charge in sacran, sacran will form aggregates around the negative bias electrode [51].
References
- Qi, X.; Wu, L.; Su, T.; Zhang, J.; Dong, W. Polysaccharide-based cationic hydrogels for dye adsorption. Colloids Surf. B Biointerfaces 2018, 170, 364–372.
- Jayakumar, R.; Kumar, P.S.; Mohandas, A.; Lakshmanan, V.-K.; Biswas, R. Exploration of alginate hydrogel/nano zinc oxide composite bandages for infected wounds. Int. J. Nanomed. 2015, 10, 53–66.
- Rasib, S.Z.M.; Akil, H.M.; Khan, A.; Hamid, Z.A.A. Controlled release studies through chitosan-based hydrogel synthesized at different polymerization stages. Int. J. Biol. Macromol. 2019, 128, 531–536.
- Miller, T.R.; Bartlett, S.L.; Weirich, C.A.; Hernandez, J. Automated subdaily sampling of cyanobacterial toxins on a buoy reveals new temporal patterns in toxin dynamics. Environ. Sci. Technol. 2019, 53, 5661–5670.
- Miller, T.R.; Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L. Cyanobacterial toxins of the laurentian great lakes, their toxicological effects, and numerical limits in drinking water. Mar. Drugs 2017, 15, 160.
- Hagemann, M.; Hess, W.R. Systems and synthetic biology for the biotechnological application of cyanobacteria. Curr. Opin. Biotechnol. 2018, 49, 94–99.
- Nielsen, A.Z.; Mellor, S.B.; Vavitsas, K.; Wlodarczyk, A.; Gnanasekaran, T.; de Jesus, M.P.R.H.; King, B.; Bakowski, K.; Jensen, P.E. Extending the biosynthetic repertoires of cyanobacteria and chloroplasts. Plant J. 2016, 87, 87–102.
- Ko, S.C.; Lee, H.J.; Choi, S.Y.; Choi, J.-I.; Woo, H.M. Bio-solar cell factories for photosynthetic isoprenoids production. Planta 2018, 249, 181–193.
- Ohki, K.; Kanesaki, Y.; Suzuki, N.; Okajima, M.; Kaneko, T.; Yoshikawa, S. Physiological properties and genetic analysis related to exopolysaccharide (EPS) production in the fresh-water unicellular cyanobacterium Aphanothece sacrum (Suizenji Nori). J. Gen. Appl. Microbiol. 2019, 65, 39–46.
- Okajima-Kaneko, M.; Ono, M.; Kabata, K.; Kaneko, T. Extraction of novel sulfated polysaccharides from Aphanothece sacrum (Sur.) Okada, and its spectroscopic characterization. Pure Appl. Chem. 2007, 79, 2039–2046.
- Igata, K.; Sakamaki, T.; Inutsuka, Y.; Higaki, Y.; Okajima, M.K.; Yamada, N.L.; Kaneko, T.; Takahara, A. Cationic polymer brush/giant polysaccharide sacran assembly: Structure and lubricity. Langmuir 2020, 36, 6494–6501.
- Mantzouki, E.; Lürling, M.; Fastner, J.; Domis, L.D.S.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156.
- Jaša, L.; Sadílek, J.; Kohoutek, J.; Straková, L.; Maršálek, B.; Babica, P. Application of passive sampling for sensitive time-integrative monitoring of cyanobacterial toxins microcystins in drinking water treatment plants. Water Res. 2019, 153, 108–120.
- Basu, A.; Dydowiczova, A.; Ctverackova, L.; Jasa, L.; Trosko, J.E.; Blaha, L.; Babica, P. Assessment of hepatotoxic potential of cyanobacterial toxins using 3D in vitro model of adult human liver stem cells. Environ. Sci. Technol. 2018, 52, 10078–10088.
- Dao, T.-S.; Vo, T.-M.-C.; Wiegand, C.; Bui, B.-T.; Dinh, K.V. Transgenerational effects of cyanobacterial toxins on a tropical micro-crustacean Daphnia lumholtzi across three generations. Environ. Pollut. 2018, 243, 791–799.
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural product biosynthetic diversity and comparative genomics of the cyanobacteria. Trends Microbiol. 2015, 23, 642–652.
- Kaneko, T.; Okajima, M.; Tateyama, S. Structure and properties of sacran, one of supergiant polysaccharides, and its biomimetic functionalization. Nippon. GOMU KYOKAISHI 2014, 87, 146–152.
- Gomes Ferreira, M. Study of Anti-Inflammatory Bioactivity of Cyanobacterial Strains Using Murine Macrophage RAW 264.7 Cells. Master’s Thesis, University of Porto, Porto, Portugal, 2016; pp. 265–267.
- Fujishiro, T.; Ogawa, T.; Matsuoka, M.; Nagahama, K.; Takeshima, Y.; Hagiwara, H. Establishment of a pure culture of the hitherto uncultured unicellular cyanobacterium aphanothece sacrum, and phylogenetic position of the organism. Appl. Environ. Microbiol. 2004, 70, 3338–3345.
- Wada, K.; Kagamiyama, H.; Shin, M.; Matsubara, H. Ferredoxin from a blue-green alga, aphanothece sacrum (suringar) OKADA1. J. Biochem. 1974, 76, 1217–1225.
- Hase, T.; Wakabayashi, S.; Wada, K.; Matsubara, H. Amino acid sequence of aphanothece sacrum ferredoxin II (minor component). J. Biochem. 1978, 83, 761–770.
- Matsuda, S.; Sugawa, H.; Shirakawa, J.-I.; Ohno, R.-I.; Kinoshita, S.; Ichimaru, K.; Arakawa, S.; Nagai, M.; Kabata, K.; Nagai, R. Aphanothece sacrum (Sur.) okada prevents cataractogenesis in type 1 diabetic mice. J. Nutr. Sci. Vitaminol. 2017, 63, 263–268.
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically crosslinked-sacran hydrogel films for wound dressing application. Int. J. Biol. Macromol. 2016, 89, 465–470.
- Okajima, M.K.; Miyazato, S.; Kaneko, T. Cyanobacterial megamolecule sacran efficiently forms LC gels with very heavy metal ions. Langmuir 2009, 25, 8526–8531.
- Alcântara, A.C.S.; Darder, M.; Aranda, P.; Tateyama, S.; Okajima, M.K.; Kaneko, T.; Ogawa, M.; Ruiz-Hitzky, E. Clay-bionanocomposites with sacran megamolecules for the selective uptake of neodymium. J. Mater. Chem. A 2014, 2, 1391–1399.
- Shikinaka, K.; Okeyoshi, K.; Masunaga, H.; Okajima, M.K.; Kaneko, T. Solution structure of cyanobacterial polysaccharide, sacran. Polymer 2016, 99, 767–770.
- Doi, M.; Sagawa, Y.; Momose, S.; Tanaka, T.; Mizutani, T.; Okano, Y.; Masaki, H. Topical treatment with sacran, a sulfated polysaccharide from Aphanothece sacrum, improves corneocyte-derived parameters. J. Dermatol. 2017, 44, 1360–1367.
- Yusof, F.A.A.; Yamaki, M.; Kawai, M.; Okajima, M.K.; Kaneko, T.; Mitsumata, T. Rheopectic behavior for aqueous solutions of megamolecular polysaccharide sacran. Biomolecules 2020, 10, 155.
- Li, L.; Takada, A.; Ma, W.; Fujikawa, S.; Ariyoshi, M.; Igata, K.; Okajima, M.K.; Kaneko, T.; Takahara, A. Structure and properties of hybrid film fabricated by spin-assisted layer-by-layer assembly of sacran and imogolite nanotubes. Langmuir 2020, 36, 1718–1726.
- Amornwachirabodee, K.; Okajima, M.K.; Kaneko, T. Uniaxial swelling in LC hydrogels formed by two-step cross-linking. Macromolecules 2015, 48, 8615–8621.
- Okeyoshi, K.; Okajima, M.K.; Kaneko, T. Milliscale self-integration of megamolecule biopolymers on a drying gas–aqueous liquid crystalline interface. Biomacromolecules 2016, 17, 2096–2103.
- Doi, M.; Sagawa, Y.; Mizutani, T.; Okano, Y.; Momose, S.; Tanaka, T.; Masaki, H. Possibilities of sacran-polyol complexes in skin care. J. Soc. Cosmet. Chem. Jpn. 2017, 51, 117–125.
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Abu Hashim, I.I.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; et al. Anti-inflammatory effects of novel polysaccharide sacran extracted from cyanobacterium aphanothece sacrum in various inflammatory animal models. Biol. Pharm. Bull. 2016, 39, 1172–1178.
- Motoyama, K.; Tanida, Y.; Hata, K.; Hayashi, T.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Kaneko, S.; Arima, H. Potential use of a megamolecular polysaccharide sacran as a hydrogel-based sustained release system. Chem. Pharm. Bull. 2014, 62, 636–641.
- Okajima, M.K.; Le Nguyen, Q.T.; Nakamura, M.; Ogawa, T.; Kurata, H.; Kaneko, T. Double-metal complexation of heterogels containing cyanobacterial polysaccharides. J. Appl. Polym. Sci. 2012, 128, 676–683.
- Okeyoshi, K.; Okajima, M.K.; Kaneko, T. Unidirectionally-oriented Membrane formation of supra-polysaccharides sacran and application to drug delivery system. Yakugaku Zasshi 2018, 138, 503–507.
- Sornkamnerd, S.; Okajima, M.K.; Matsumura, K.; Kaneko, T. Micropatterned cell orientation of cyanobacterial liquid-crystalline hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 44834–44843.
- Okajima, M.K.; Kumar, A.; Fujiwara, A.; Mitsumata, T.; Kaneko, D.; Ogawa, T.; Kurata, H.; Isoda, S.; Kaneko, T. Anionic complexes of MWCNT with supergiant cyanobacterial polyanions. Biopolymers 2013, 99, 1–9.
- Wathoni, N.; Hasanah, A.N.; Mohammed, A.F.A.; Pratiwi, E.D.; Mahmudah, R. Accelerated wound healing ability of sacran hydrogel film by keratinocyte growth factor in alloxan-induced diabetic mice. Int. J. Appl. Pharm. 2018, 10, 57–61.
- Okajima, M.K.; Sornkamnerd, S.; Kaneko, T. Development of functional bionanocomposites using cyanobacterial polysaccharides. Chem. Rec. 2018, 18, 1167–1177.
- Mitsumata, T.; Miura, T.; Takahashi, N.; Kawai, M.; Okajima, M.K.; Kaneko, T. Ionic state and chain conformation for aqueous solutions of supergiant cyanobacterial polysaccharide. Phys. Rev. E 2013, 87, 042607.
- Okajima, M.K.; Nakamura, M.; Ogawa, T.; Kurata, H.; Mitsumata, T.; Kaneko, T. Spongy hydrogels of cyanobacterial polyanions mediate energy-saving electrolytic metal-refinement. Ind. Eng. Chem. Res. 2012, 51, 8704–8707.
- Zhao, Y.; Hien, K.T.T.; Mizutani, G.; Rutt, H.N.; Amornwachirabodee, K.; Okajima, M.; Kaneko, T. Optical second-harmonic images of sacran megamolecule aggregates. J. Opt. Soc. Am. A 2017, 34, 146–152.
- Wathoni, N. Design and Evaluation of Sacran/Cyclodextrin Hydrogel Films for Wound Dressing Materials Graduate School of Pharmaceutical Sciences Department of Physical Pharmaceutics Nasrul Wathoni. Ph.D. Thesis, Kumamoto University, Kumamoto, Japan, 2017.
- Ngatu, N.R.; Okajima, M.K.; Yokogawa, M.; Hirota, R.; Eitoku, M.; Muzembo, B.A.; Dumavibhat, N.; Takaishi, M.; Sano, S.; Kaneko, T.; et al. Anti-inflammatory effects of sacran, a novel polysaccharide from Aphanothece sacrum, on 2,4,6-trinitrochlorobenzene–induced allergic dermatitis in vivo. Ann. Allergy Asthma Immunol. 2012, 108, 117–122.e2.
- Kaneko, T.; Okajima, M.K. Super liquid crystalline polysaccharides produced by ultimately-ecological microreactors. Yakugaku Zasshi 2018, 138, 489–496.
- Budpud, K.; Okeyoshi, K.; Okajima, M.K.; Kaneko, T. Vapor-sensitive materials from polysaccharide fibers with self-assembling twisted microstructures. Small 2020, 16, e2001993.
- Okajima, M.K.; Kaneko, D.; Mitsumata, T.; Kaneko, T.; Watanabe, J. Cyanobacteria that produce megamolecules with efficient self-orientations. Macromolecules 2009, 42, 3057–3062.
- Okajima, M.K.; Le Nguyen, Q.T.; Tateyama, S.; Masuyama, H.; Tanaka, T.; Mitsumata, T.; Kaneko, T. Photoshrinkage in polysaccharide gels with trivalent metal ions. Biomacromolecules 2012, 13, 4158–4163.
- Mitsumata, T. Negative thixotropic behavior for sacran aqueous solutions. Yakugaku Zasshi 2018, 138, 497–501.
- Zhao, Y.; Li, Y.; Hien, K.T.T.; Mizutani, G.; Ito, N.; Rutt, H.N.; Okajima, M.; Kaneko, T. Electric field effect on optical second-harmonic generation of amphoteric megamolecule aggregates. J. Phys. Soc. Jpn. 2017, 86.
More