Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Francisco A. Macías.
Annona cherimola Mill., or the custard apple, is one of the species belonging to the Annonaceae family, is widely used in traditional medicine, and has been reported to be a valuable source of bioactive compounds.
- Annona cherimola
- acetogenins
- bioactivity
1. Introduction
Plants are one of the most important and diverse sources of chemical structures and bioactive compounds. One of the species that has been used in traditional medicine for a long time and with a valuable source of bioactive molecules is Annona cherimola Mill. (ACM) or the custard apple [1]. It belongs to the Annonaceae family, which comprises more than 140 genera and approximately 2500 species [2]. ACM is an extensively known deciduous tree whose edible fruit is known as the cherimoya. Cherimoyas are considered an exotic fruit native to inter-Andean valleys from Peru and Ecuador [3]. They can be found and are commercially cultivated in several mild-temperature regions around the world, such as Portugal, Italy, Taiwan or Spain [4]. Around 2406 hectares where mainly two varieties of this plant are cultivated, ‘Fino de Jete’ and ‘Campa’ [5] can be found in the coast of Granada-Malaga region (southern of Spain) also known as ‘Costa Tropical’ [6], which was granted by the European Union a Protected Designation of Origin (PDO) [7] in 2002. In fact, the specific soil and climate conditions as well as the particular requirements regarding the handling of a crop with a rather brief postharvest life [8] have made of Spain its world leading producer [2,9][2][9]. The commercialization of this cultivar from Coast of Granada-Malaga around the rest of the European regions has grown by 20% in the last year [10].
ACM is mainly cultivated for the food industry and although it is often consumed as fresh fruit in many countries, a varied range of food products and beverages are made up from cherimoya pulp [11]. In addition to its organoleptic properties and nutritional value, ACM has a certain potential use in folk medicine, particularly for the treatment of skin disorders [12]. Additionally, some hepatoprotective, anti-inflammatory and antitumoral properties have been described [1,2,9][1][2][9]. Over the last few decades, a growing number of studies have focused on the phytochemical composition of ACM extracts in order to explain their traditional applications and to determine the compounds in ACM that are responsible of its biological activity. Some phytochemicals, such as flavonoids, tocopherols, tannins, acetogenins, saponins, polyphenols among others, have been isolated from ACM roots, seeds, pulp and leaves [5,9,13][5][9][13]. Since the first acetogenin, uvaricin was isolated by Jolad et al. in 1982—a product of interest for its cytotoxic activity—and other investigations have focused on this class of compounds, which are exclusively found in the Annonaceae family [14]. Since then, over 500 acetogenins from the Annonaceae family have been described [15].
2. Classification of ACGs from ACM
The most generally used classification for annonaceous ACGs is based on the structure of its systems of central rings. Hence, linear, mono-THF, adjacent or non-adjacent bis-THF, tri-THF, epoxy and THP ACGs, could be further divided attending to the number and location of the hydroxyl groups along the chain, and also according to the type of terminal lactone [15,18][15][16].
Most of the ACGs found in ACM contain one or two THF rings. In addition, a number of variations have usually been registered with regard to the number and position of the hydroxyl groups. Between one and four hydroxyl groups can be found along the aliphatic chain or at the terminal lactone group. Moreover, some of the differences in the number and position of the hydroxyl groups, besides their stereochemistry, are crucial for their biological activity. The most common THF and γ-lactone ring structures can be seen in Figure 21. This classification has been made following the same criteria as Neske and cols [15].
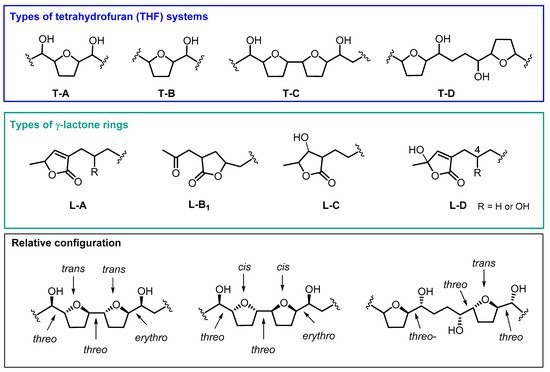

Figure 21.
Most common structures of annonaceous acetogenins isolated from ACM.
A total of 41 ACGs have been identified and isolated from roots, seeds, stems and, recently, from the deciduous leaves of ACM (Table 1). Of all these compounds, a total of 20 ACGs can be found in the ACMs from the Spanish ‘Tropical Coast’ region (Figure 32).
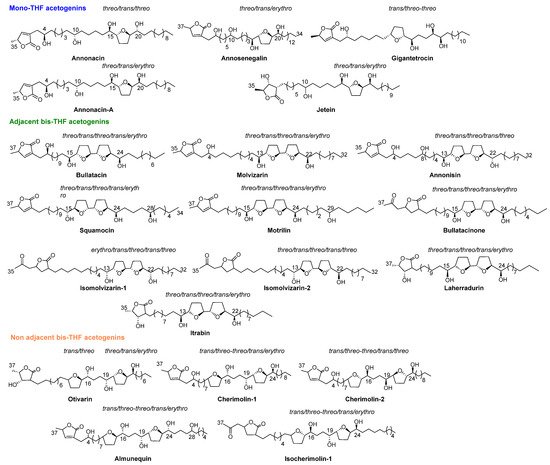

Figure 32.
Annonaceous acetogenins isolated from ACMs harvested at ‘Tropical Coast’.
Table 1.
Characteristics and structural features of the 41 ACGs described in ACM.
| CAS Number | Name | Other Names | OH Positions | THF System | Relative THF Configuration | Type of γ-Lactone Ring | Molecular Formula | Organ | Number of Biological Studies (Scifinder Database) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 133352-34-8 | Corossolin | Corossoline | 10,15,20 | T-A | th/t/th | L-A | C35H64O6 | Seeds | 15 | [23,24][17][18] |
| 246165-35-5 | Annocherin | 4,15,20 | T-A | th/t/th | L-A | C35H62O7 | Seeds | 2 | [25][19] | |
| 111035-65-5 | Annonacin † | 4,10,15,20 | T-A | th/t/th | L-A | C35H64O7 | Seeds, deciduous leaves | 133 | [20,26][20][21] | |
| 130853-76-8 | Annonacin-A † | 4,10,15,20 | T-A | th/t/er | L-A | C35H64O7 | Seeds | 17 | [27][22] | |
| 137550-92-6 | Annomontacin | 4,10,17,22 | T-A | th/t/th | L-A | C37H68O7 | Seeds | 18 | [26][21] | |
| 155969-86-1 | Xylomaticin | 4,10,15,20 | T-A | th/t/th | L-A | C37H68O7 | Seeds | 7 | [23][17] | |
| 155969-65-6 | Gonionenin | (2,4-cis)-Gonioneninone | 4,10,13,18 | T-A | th/t/th | L-A | C37H66O7 | Seeds | 8 | [23][17] |
| 176200-77-4 | Annosenegalin † | 4,10,15,20 | T-A | th/t/er | L-A | C37H68O7 | Seeds | 1 | [27][22] | |
| 172586-13-9 | Cis annonacin | 4,10,15,20 | T-A | th/c/th | L-A | C35H64O7 | Seeds | 10 | [28][23] | |
| 344940-10-9 | Annocherimolin | 4,9,13,18 | T-A | th/t/th | L-A | C37H66O7 | Seeds | 1 | [29][24] | |
| 373362-55-1 | Annomocherin | 4,10,15,20 | T-A | th/t/th | L-A | C35H62O7 | Seeds | 1 | [26][21] | |
| 134955-48-9 | Gigantetrocin † | 4,14,17,18 | T-B | t/th-th | L-A | C35H64O7 | Seeds | 13 | [27][22] | |
| 344940-09-6 | Annomolin | 4,7,8,18 | T-B | th-t/th | L-A | C35H64O7 | Seeds | 1 | [29][24] | |
| 152784-18-4 and 152784-19-5 | cis/trans isoannonacins | cis-Annonacin-A-one and trans-Annonacin-A-one | 10,15,20 | T-A | th/t/th | L-B1 | C35H64O7 | Seeds | 7 | [28][23] |
| 246165-37-7 and 246165-38-8 | (2,4)-cis- and trans annocherinones | 15,20 | T-A | th/t/th | L-B1 | C35H62O7 | Seeds | 2 | [25][19] | |
| 627518-99-4 and 627519-01-1 | Annomolon-A + 34-epi | 15,20,34 | T-A | th/t/th | L-D | C35H62O7 | Seeds | 1 | [30][25] | |
| 627519-00-0 and 627519-02-2 | Annomolon-B + 34-epi | 4,15,20,34 | T-A | th/t/th | L-D | C35H62O8 | Seeds | 1 | [30][25] | |
| 139294-55-6 | Jetein † | 10,15,20 | T-A | th/t/er | L-C | C35H66O7 | Seeds | 2 | [31][26] | |
| 102989-24-2 | Asimicin | Squamocin H | 4,15,24 | T-C | th/t/th/t/th | L-A | C37H66O7 | Seeds | 57 | [32][27] |
| 123123-32-0 | Bullatacin † | Annonareticin; LI 12105; Rolliniastatin 2; Squamocin G | 4,15,24 | T-C | th/t/th/t/er | L-A | C37H66O7 | Seeds | 229 | [31][26] |
| 138551-26-5 | Molvizarin † | 4,13,22 | T-C | th/t/th/t/er | L-A | C35H62O7 | Seeds, deciduous leaves | 17 | [20,33][20][28] | |
| 194413-43-9 | Annonisin † | 4,8,13,22 | T-C | th/t/th/t/th | L-A | C35H62O8 | Deciduous leaves | 3 | [20] | |
| 120298-30-8 | Squamocin † | Annonin I; Squamocin A | 15,24,28 | T-C | th/t/th/t/er | L-A | C37H66O7 | Seeds, roots | 162 | [31,34][26][29] |
| 138551-27-6 | Motrilin † | 15,24,29 | T-C | th/t/th/t/er | L-A | C37H66O7 | Seeds, deciduous leaves | 28 | [20,33][20][28] | |
| 159934-23-3 | Squamocin B | 13,22,26 | T-C | th/t/th/t/er | L-A | C35H62O7 | Seeds | 10 | [32][27] | |
| 123012-00-0 | Bullatacinone † | Isorolliniastatin-2 | 15,24 | T-C | th/t/th/t/er | L-B1 | C37H66O7 | Roots | 69 | [34][29] |
| 161169-72-8 | Isomolvizarin-1 † | 13,22 | T-C | th/t/th/t/er | L-B1 | C35H62O7 | Roots | 0 | [34][29] | |
| 158252-75-6 | Isomolvizarin-2 † | 13,22 | T-C | th/t/th/t/th | L-B1 | C35H62O7 | Roots | 1 | [34][29] | |
| 125276-75-7 | Laherradurin † | 15,24,35 | T-C | th/t/th/t/er | L-C | C37H68O7 | Seeds | 13 | [35][30] | |
| 139294-54-5 | Itrabin † | 13,22,33 | T-C | th/t/th/t/er | L-C | C35H64O7 | Seeds | 8 | [33][28] | |
| 832683-48-4 | Tucumanin | 15,24,35 | T-C | th/t/th/t/th | L-C | C37H68O7 | Seeds | 3 | [32][27] | |
| 92280-15-4 | Otivarin † | 16,19,24,35 | T-D | t/th-th/t/er | L-C | C37H68O8 | Seeds | 6 | [31][26] | |
| 92280-14-3 | Cherimolin-1 † | Bullatalicin | 4,16,19,24 | T-D | t/th-th/t/er | L-A | C37H66O8 | Seeds, deciduous leaves | 16 | [20,31][20][26] |
| 151637-38-6 | Cherimolin-2 † | Bullatanocin | 4,16,19,24 | T-D | t/th-th/t/th | L-A | C37H66O8 | Seeds | 10 | [31][26] |
| 125620-82-8 | Almunequin † | Squamostatin-A | 16,19,24,28 | T-D | t/th-th/t/er | L-A | C37H66O8 | Seeds, roots | 12 | [31,34][26][29] |
| 241822-07-1 | Aromin-A | 15,20 | T-D | t-th/t/er | L-A | C35H60O7 | Stems | 1 | [36][31] | |
| 157966-80-8 | Isocherimolin-1 † | 16,19,24 | T-D | th-th/t/er | L-B1 | C37H66O8 | Roots | 2 | [34][29] |
† ACM from the Spanish ‘Tropical Coast’; th = threo; er = erythro; t = trans; c = cis.
ACM from the Spanish ‘Tropical Coast’; th = threo; er = erythro; t = trans; c = cis.
2.1. Mono THF Acetogenins
From 1990 to date, twenty-two ACGs from ACM have been described. This represents the main structural group of ACGs isolated from ACM and they have been mostly found as mono-THF α,α’-dihydroxylated ACGs (T-A). The most frequent configuration of the diastereoisomers is threo/trans/threo. Most of the mono THF acetogenins from ACM possess a α,β-unsaturated methyl γ-lactone ring (L-A).
2.2. Adjacent Bis-THF Acetogenins
This second structural classification constitutes the next most frequent group. Thirteen ACGs from ACM have been described as adjacent bis-THF acetogenins. This bis-THF system is found flanked at the α and α’ positions by two hydroxyl groups (T-C). Moreover, three types of γ-lactone moiety have been reported, including α,β-unsaturated methyl γ-lactone (L-A); acetonyl γ-lactone (L-B1) and β-hydroxyl methyl γ-lactone (L-C) (Table 1 and Figure 32). Nearly all of the ACGs in this class present a threo/trans/threo/trans/erythro configuration.
2.3. Non-Adjacent Bis-THF Acetogenins
This constitutes the minor group, with only six ACGs from ACM described until present; two of them display a hydroxyl group at C4, namely cherimolin-1 and cherimolin-2. Two types of γ-lactone systems, L-A and L-B1, have been described in this group.
3. How to Apply ACGs from Annona Cherimola Mill.
The acetogenins that can be found in Annona cherimola have been the center of attention of many researchers because of their cytotoxic effect in different cancer lines [53][32], as well as for their tumoral and mitochondrial complex I inhibitory capacities [32,54][27][33]. In addition, these natural products have also exhibited antiparasitic [48][34], insecticidal [55,56][35][36] and bactericide [57][37] activity with really promising results.
Despite this wide array of possibilities, the isolation and identification of acetogenins still represent an obstacle in reaching their practical application. Nevertheless, certain counter current chromatographic techniques, together with a more recent method based on absorbent biopolymers, seem to provide more efficient ways to collect acetogenins [58,59,60][38][39][40]. Thanks to these new isolation methods, a growing number of studies on cell lines, isolated enzymes and complexes have been completed. Larger scale procedures, however, are still to overcome amount, solubility and bioavailability limitations.
The formulations that are usually employed to test the effect of acetogenin from Annona cherimola on cell lines are mainly obtained with the use of organic solvents. Dimethyl sulphoxide is the most employed solvent in these tests, being applied either as co-solvent (1%) with water or as single solvent. Annonacin and molvizarin, obtained through this single solvent formulation, have been tested for larvicidal [56][36], anti-protist (leishmanicidal and trypanocidal) [61][41] and different insecticidal activities, such as oviposition capacity [13]. Apart from that, kidney studies with VERO cells have also been carried out on acetogenins combined with rollisniastatin-2 and on squamocin, where pure a DMSO solution was mixed with cell cultures [61,62][41][42]. Methanol and ethanol solutions have also been employed to test acetogenin obtained from Annona cherimola. Its insecticidal activity against mosquito and different lepidoptera larvae were assayed by He and co-workers [48][34]. Tolosa et al. [55][35] employed methanol to solve motrilin, rolliniastatin-2, bullatacin and annonacin. Mclaughlin’s research team [63,64][43][44] used ethanol solutions and the same acetogenins plus aromin-A to evaluate their effects on lung, breast and colon tumor cell lines. Hidalgo et al. [65][45] conducted a number of bioassays against lepidoptera larvae, where other lesser known Annona cherimola acetogenins, such as almunequin, cherimolin-1, cherimolin-2 and squamocin were tested, and acetone was used as an alternative solvent.
Notwithstanding the good results achieved by solving the acetogenins in organic solvents, this method is not feasible for further objectives, such as their application to human assays, since their inoculation while mixed in pure ethanol or DMSO is not an acceptable approach. This is one of the reasons why some authors have focused on alternative formulae that intend to compensate poor water solubility. In short, two methods mainly based on encapsulation techniques have been devised to improve the performance of Annona cherimola acetogenins. The first one uses polymeric compounds to produce core/shell structures that mask the lower physicochemical properties of the lactones. Polyethylene glycol (PEG) with certain modifications is the most commonly used polymeric compound, but also a monomethoxy derivative (mPEG), ε-caprolactone (PCL), poly (lactide-co-glycolide) (PLGA) or cholesterol (Chol) have also been tested. These polymeric nanoparticles (NPs) have been successfully tested for the treatment of different types of breast cancer. For instance, Wang’s group tested the effect of bullatacin encapsulated in PCL-PEG nanoparticles against a 4T1 cancer cell line as well as in vivo breast tumor-bearing mice. They obtained a high percentage of 20–60 nm-size encapsulated NPs (>70%) which demonstrated twice the ability to reduce tumor volume than free bullatacin [66][46]. In a patented expansion of their study, the same authors applied their encapsulation method to squamocin and applied the formula to HeLa cell lines and ex vivo HeLa tumor-bearing mice. Tumor volume was thereby reduced by 64.11%, in comparison to the 43.1% reduction achieved by free squamocin. This novel method exhibited up to 144 h of controlled release of the drug [67][47]. A similar method has been patented by Ao et al. to apply the use of polymeric nanoparticles to cherimolin and motrilin for oral administration instead of being injected through the mice tail vein [68][48].
The same authors have also patented a method to modify the different polymers that had been previously assayed so that they would include different stabilizers, such as albumin serum or soybean lecithin, in the nanoparticle suspension. This new method was employed to encapsulate bullatacin, but they exhibited a shorter delivery time (controlled release for 96 h) compared to when just the polymer nanoparticles were administered. Nevertheless, this method seems to improve the antitumor efficacy of bullatacin against liver tumor, with a maximum inhibition rate of 74.8% in the tumor volume [69][49]. The size of the nanoparticles used in this method was similar to that of nanoparticles which had not been added a stabilizer, which means that these co-polymers did not affect the chemical structure layout [70][50].
In a different line of investigation, micelles based on pseudorotaxanes with cyclodextrins (CDs) are becoming a prominent approach for the encapsulation of Annona cherimola acetogenins. Wang’s working group has used supramolecular polymer micelles (SMPMs) with β-cyclodextrin and 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) to encapsulate bullatacin (Figure 43). One of the methods used employed folic acid as the guest molecule to be hosted in the cyclodextrin toroid, which would act as a building-block for the micelle, while another method employed soybean lecithin, a really standardized guest molecule in SMPMs science [71,72][51][52]. The nanostructures generated with the cyclodextrin molecules seem to be bigger than the particles synthesized using polymers, with sizes between 140 and 200 nm. In addition, the encapsulation percentages are also lower, with values between 45% and 60%. Despite these apparently worse results, this method allows for the incorporation of oligoelements, thanks to which it presents the advantage of serving a double purpose: anti-tumoral agent and nutraceutical supplier. In the case of the β-CD/folic acid micelles, the structure includes vitamin B9, which facilitates bio-recognition and targeting towards the liver. Besides from everything mentioned above, natural CDs are chemical compounds approved as a food additive by the EU since 2018, which supports their use as an excipient instead of other substances [73][53]. With respect to the inhibition of 4T1 tumors volume, there are no significant differences between the two methods, when compared against polymeric nanoparticles, with inhibition values at around 70%. It should be noted that drug release times change drastically as the micelles’ composition changes. In the case of the β-CD/folic acid, the drug release times are quite similar to those of polymeric nanoparticles, with 142 h required for the total delivery of the drug [74][54]. On the other hand, HP-β-CD/Lecithin presents an 80 h release profile [75][55]. The currently published papers do not cast any light over this behavior, but it could be attributed to the higher solubility of HP-β-CD in water when compared to that of β-CD. This improved solubility of the micelles in the media would facilitate their degradation. A similar method has been patented by Hong et al., where SMPMs are synthesized by means of three natural cyclodextrins (α, β and γ) that act as guests for different molecular weight polyethylene-glycol units ranging from 600 to 2000. No specific values have been reported on encapsulation percentages, although over 50% has been claimed. It should also be noted that bullatacin release time is limited to 72 h. When the different methods are applied to the same acetogenin and their data (Table 3) are compared, as in case of bullatacin, it seems that the guest molecule in the SMPMs building-block is crucial with respect to core release times, and their increment in molecular weight/molecular volume reduce the drug delivery time.
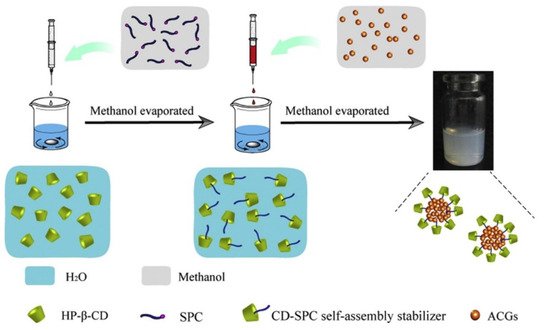

Table 3.
SMPMs: Reported values of acetogenins.
Gutiérrez et al. employed SMPMs based on α-CD and urea to encapsulate annonacin. In line with other studies, cytotoxicity to liver cells was the main objective, and it was proven that annonacin is selective to tumor cells, with over 87% of cell survival when the acetogenin is applied to non-tumoral liver cells (HEK-293) [20]. In this case, although both the encapsulation percentage and the drug delivery time provided the lowest values, it should be highlighted that annonacin is completely different from other previously encapsulated and tested acetogenins, since it presents a mono-THF structure instead of the bis-THF structure of bullatacin. No studies on the subject have been found to allow the comparison between these two methods.
Most of the formulation cases have focused on bullatacin, the most abundant acetogenin, but it should be emphasized that the authors used an acetogenin enrichment fraction and based their studies on determining bullatacin content, since it is the main compound in the fraction. However, an efficient encapsulation procedure for single acetogenins should be developed, similarly to the one previously described for anonacin, so that any antitumoral efficacy can be attributed to the only compound in the media and not to the possible synergistic effect that the different mixtures of acetogenins may exhibit.
References
- Jamkhande, P.G.; Ajgunde, B.R.; Jadge, D.R. Annona cherimola Mill. (Custard apple): A review on its plant profile, nutritional values, traditional claims and ethnomedicinal properties. Orient. Pharm. Exp. Med. 2017, 17, 189–201.
- Quílez, A.M.; Fernández-Arche, M.A.; García-Giménez, M.D.; De la Puerta, R. Potential therapeutic applications of the genus Annona: Local and traditional uses and pharmacology. J. Ethnopharmacol. 2018, 225, 244–270.
- Popenoe, W. The native home of the Cherimoya. J. Hered. 1921, 12, 228–239.
- Anaya-Esparza, L.M.; Ramírez-Marez, M.V.; Montalvo-González, E.; Sánchez-Burgos, J.A. Cherimoya (Annona cherimola Mill.). In Fruit and Vegetable Phytochemicals; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 993–1002.
- García-Salas, P.; Verardo, V.; Gori, A.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of lipid composition of the two principal cherimoya cultivars grown in Andalusian Region. LWT Food Sci. Technol. 2016, 65, 390–397.
- INEbase INe Instituto Nacional de Estadística. Available online: (accessed on 24 January 2019).
- Boletín Oficial de la Junta de Andalucía (BOJA); Consejería de Agricultura y Pesca: Sevilla, Spain, 2002; núm. 124, 20553–20562.
- Palma, T.; Aguilera, J.; Stanley, D. A review of postharvest events in cherimoya. Postharvest Biol. Technol. 1993, 2, 187–208.
- Díaz de Cerio, E.; Aguilera Saez, L.M.; Gómez Caravaca, A.M.; Verardo, V.; Fernández Gutiérrez, A.; Fernández, I.; Arráez Román, D. Characterization of bioactive compounds of Annona cherimola L. leaves using a combined approach based on HPLC-ESI-TOF-MS and NMR. Anal. Bioanal. Chem. 2018, 410, 3607–3619.
- HORTO INFO. Available online: (accessed on 24 January 2019).
- Le Ven, J.; Schmitz-Afonso, I.; Lewin, G.; Brunelle, A.; Touboul, D.; Champy, P. Identification of the environmental neurotoxins annonaceous acetogenins in an Annona cherimolia Mill. alcoholic beverage using HPLC-ESI-LTQ-Orbitrap. J. Agric. Food Chem. 2014, 62, 8696–8704.
- Barreca, D.; Laganà, G.; Ficarra, S.; Tellone, E.; Leuzzi, U.; Galtieri, A.; Bellocco, E. Evaluation of the antioxidant and cytoprotective properties of the exotic fruit Annona cherimola Mill. (Annonaceae). Food Res. Int. 2011, 44, 2302–2310.
- Colom, O.A.; Salvatore, A.; Willink, E.; Ordonez, R.; Isla, M.I.; Neske, A.; Bardon, A. Insecticidal, mutagenic and genotoxic evaluation of annonaceous acetogenins. Nat. Prod. Commun. 2010, 5, 391–394.
- Liaw, C.C.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Historic perspectives on Annonaceous acetogenins from the chemical bench to preclinical trials. Planta Med. 2010, 76, 1390–1404.
- Neske, A.; Hidalgo, J.R.; Cabedo, N.; Cortes, D. Acetogenins from Annonaceae family. Their potential biological applications. Phytochemistry 2020, 174, 112332.
- Bermejo, A.; Figadère, B.; Zafra-Polo, M.C.; Barrachina, I.; Estornell, E.; Cortes, D. Acetogenins from Annonaceae: Recent progress in isolation, synthesis and mechanisms of action. Nat. Prod. Rep. 2005, 22, 269–303.
- Dal, H.K.; Fang, Z.; Young, E.L.; Mi, H.W. Xylomaticin and gonionenin, cytotoxic annonaceous acetogenins from the seeds of Annona cherimolia. Nat. Prod. Sci. 2007, 13, 355–358.
- Kim, D.-H.; Woo, M.-H. Corrosolin and compound-2: Cytotoxic annonaceous acetogenins from the seeds of Annona cherimolia. Yakhak Hoeji 1999, 43, 584–590.
- Woo, M.H.; Kim, D.H.; Fotopoulos, S.S.; McLaughlin, J.L. Annocherin and (2,4)-cis- and trans-annocherinones, monotetrahydrofuran annonaceous acetogenins with a C-7 carbonyl group from Annona cherimolia seeds. J. Nat. Prod. 1999, 62, 1250–1255.
- Gutiérrez, M.T.; Durán, A.G.; Mejías, F.J.R.; Molinillo, J.M.G.; Megias, D.; Valdivia, M.M.; Macías, F.A. Bio-guided isolation of acetogenins from Annona cherimola deciduous leaves: Production of nanocarriers to boost the bioavailability properties. Molecules 2020, 25, 4861.
- Kim, D.H.; Son, J.K.; Woo, M.H. Annomocherin, annonacin and annomontacin: A novel and two known Bioactive mono-tetrahydrofuran annonaceous acetogenins from Annona cherimolia seeds. Arch. Pharm. Res. 2001, 24, 300–306.
- Sahpaz, S.; González, M.C.; Hocquemiller, R.; Zafra-Polo, M.C.; Cortes, D. Annosenegalin and annogalene: Two cytotoxic mono-tetrahydrofuran acetogenins from Annona senegalensis and Annona cherimolia. Phytochemistry 1996, 42, 103–107.
- Woo, M.-H.; Chung, S.-O.; Kim, D.-H. cis-Annonacin and (2,4)-cis-and trans-isoannonacins: Cytotoxic monotetrahydrofuran annonaceous acetogenins from the seeds of Annona cherimolia. Arch. Pharm. Res. 1999, 22, 524–528.
- Kim, D.H.; Ma, E.S.; Suk, K.D.; Son, J.K.; Lee, J.S.; Woo, M.H. Annomolin and annocherimolin, new cytotoxic annonaceous acetogenins from Annona cherimolia seeds. J. Nat. Prod. 2001, 64, 502–506.
- Son, J.K.; Kim, D.H.; Woo, M.H. Two New Epimeric pairs of acetogenins bearing a carbonyl group from Annona cherimolia Seeds. J. Nat. Prod. 2003, 66, 1369–1372.
- Cortes, D.; Myint, S.H.; Dupont, B.; Davoust, D. Bioactive acetogenins from seeds of Annona cherimolia. Phytochemistry 1993, 32, 1475–1482.
- Barrachina, I.; Neske, A.; Granell, S.; Bermejo, A.; Chahboune, N.; El Aouad, N.; Alvarez, O.; Bardon, A.; Zafra-Polo, M.C. Tucumanin, a β-Hydroxy-γ-lactone Bistetrahydrofuranic Acetogenin from Annona cherimolia, is a Potent Inhibitor of Mitochondrial Complex I. Planta Med. 2004, 70, 866–868.
- Cortes, D.; Myint, S.H.; Hocquemiller, R. Molvizarin and motrilin: Two novel cytotoxic bis-tetrahydro-furanic γ-lactone acetogenins from Annona cherimolia. Tetrahedron 1991, 47, 8195–8202.
- Duret, P.; Gromek, D.; Hocquemiller, R.; Cavé, A.; Cortes, D. Isolation and Structure of Three New Bis-Tetrahydrofuran Acetogenins from the Roots of Annona cherimolia. J. Nat. Prod. 1994, 57, 911–916.
- Ríos, J.; Cortes, D.; Valverde, S. Acetogenins, Aporphinoids, and Azaanthraquinone from Annona cherimolia Seeds. Planta Med. 1989, 55, 321–323.
- Chen, C.Y.; Chang, F.R.; Chiu, H.F.; Wu, M.J.; Wu, Y.C. Aromin-A, an annonaceous acetogenin from Annona cherimola. Phytochemistry 1999, 51, 429–433.
- Nakanishi, Y.; Chang, F.R.; Liaw, C.C.; Wu, Y.C.; Bastow, K.F.; Lee, K.H. Acetogenins as selective inhibitors of the human ovarian 1A9 tumor cell line. J. Med. Chem. 2003, 46, 3185–3188.
- Duval, R.A.; Lewin, G.; Peris, E.; Chahboune, N.; Garofano, A.; Dröse, S.; Cortes, D.; Brandt, U.; Hocquemiller, R. Heterocyclic analogues of squamocin as inhibitors of mitochondrial complex I. On the role of the terminal lactone of annonaceous acetogenins. Biochemistry 2006, 45, 2721–2728.
- He, K.; Zeng, L.; Ye, Q.; Shi, G.; Oberlies, N.H.; Zhao, G.-X.; Njoku, C.J.; McLaughlin, J.L. Comparative SAR Evaluations of Annonaceous Acetogenins for Pesticidal Activity. Pestic. Sci. 1997, 49, 372–378.
- Tolosa, D.; Colom, O.Á.; Bardón, A.; Neske, A. Insecticidal effects of acetogenins from rollinia occidentalis seed extract. Nat. Prod. Commun. 2012, 7, 1645–1646.
- Arriaga, Â.M.C.; Feitosa, E.M.A.; Lemos, T.L.G.; Santiago, G.M.P.; Lima, J.Q.; De Oliveira, M.C.F.; e Vasconcelos, J.N.; Rodrigues, F.E.A.; Gomes, T.B.M.; Braz-Filho, R. Chemical constituents and insecticidal activity of Rollinia leptopetala (Annonaceae). Nat. Prod. Commun. 2008, 3, 1934578X0800301021.
- Luna-Cazares, L.M.; Gonzalez-Esquinca, A.R. Susceptibility of complete bacteria and spheroplasts of Escherichia coli, Pseudomonas aeruginosa and Salmonella typhi to rolliniastatin-2. Nat. Prod. Res. 2010, 24, 1139–1145.
- Hopp, D.C.; Conway, W.D.; McLaughlin, J.L. Using countercurrent chromatography to assist in the purification of new Annonaceous acetogenins from Annona squamosa. Phytochem. Anal. 1999, 10, 339–347.
- Duret, P.; Waechter, A.I.; Margraff, R.; Foucault, A.; Hocquemiller, R.; Cave, A. High-speed countercurrent chromatography: A promising method for the separation of the Annonaceous acetogenins. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 627–635.
- Gopal, J.; Muthu, M.; Dhakshanamurthy, T.; Kim, K.J.; Hasan, N.; Kwon, S.J.; Chun, S. Sustainable ecofriendly phytoextract mediated one pot green recovery of chitosan. Sci. Rep. 2019, 9, 1–12.
- Waechter, A.I.; Yaluff, G.; Inchausti, A.; De Arias, A.R.; Hocquemiller, R.; Cavé, A.; Fournet, A. Leishmanicidal and trypanocidal activities of acetogenins isolated from Annona glauca. Phyther. Res. 1998, 12, 541–544.
- Sahpaz, S.; Laurens, A.; Hocquemiller, R.; Cavé, A.; Cortes, D. Senegalene, une nouvelle acCtogCnine olkfinique mono-tktrahydrofuranique des graines d’Annona senegalensisl. Can. J. Chem. 1994, 72, 3–6.
- Alfonso, D.; Johnson, H.A.; Colman-Saizarbitoria, T.; Presley, C.P.; McCabe, G.P.; McLaughlin, J.L. SARs of annonaceous acetogenins in rat liver mitochondria. Nat. Toxins 1996, 4, 181–188.
- Ratnayake, S.; Gu, Z.M.; Miesbauer, L.R.; Smith, D.L.; Wood, K.V.; Evert, D.R.; McLaughlin, J.L. Parvifloracin and parviflorin: Cytotoxic bistetrahydrofuran acetogenins with 35 carbons from Asimina parviflora (Annonaceae). Can. J. Chem. 1994, 72, 287–293.
- Hidalgo, J.R.; Parellada, E.A.; Blessing, L.D.T.; Bardón, A.; Ameta, K.L.; Vera, N.; Neske, A. Natural and derivatized acetogenins promising for the control of Spodoptera frugiperda smith. J. Agric. Chem. Environ. 2016, 05, 200–210.
- Hong, J.; Li, Y.; Li, Y.; Xiao, Y.; Kuang, H.; Wang, X. Annonaceous acetogenins nanosuspensions stabilized by PCL–PEG block polymer: Significantly improved antitumor efficacy. Int. J. Nanomed. 2016, 11, 3239–3253.
- Ao, H.; Gao, Y.; Guo, Y.; Han, M.; Li, H.; Wang, X.; Zhou, X. Nanoparticle with Effect-Enhancing and Toxicity-Reducing Effect on Synergistic and Attenuating Effects on Annonaceous Acetogenins Drugs, and Preparation Method and Application Thereof. CN109223769A, 18 January 2019.
- Ao, H.; Fu, J.; Guo, Y.; Han, M.; Li, H.; Wang, X.; Wang, Y. Pharmaceutical Composition for Selective Killing or Efficient killing at nm Level of Drug-Resistant Tumors and Use Thereof. WO2020156329, 6 August 2020.
- Hong, J.; Li, X.; Li, Y.; Wang, Y. Nanosuspension of Annonaceous Acetogenin Drugs and Preparation Method of Nanosuspension. CN106420604A, 22 February 2017.
- Li, H.; Li, Y.; Ao, H.; Bi, D.; Han, M.; Guo, Y.; Wang, X. Folate-targeting annonaceous acetogenins nanosuspensions: Significantly enhanced antitumor efficacy in HeLa tumor-bearing mice. Drug Deliv. 2018, 25, 880–887.
- Mejías, F.J.R.; Gutiérrez, M.T.; Durán, A.G.; Molinillo, J.M.G.; Valdivia, M.M.; Macías, F.A. Provitamin supramolecular polymer micelle with pH responsiveness to control release, bioavailability enhancement and potentiation of cytotoxic efficacy. Colloids Surfaces B Biointerfaces 2019, 173, 85–93.
- Dong, H.; Li, Y.; Cai, S.; Zhuo, R.; Zhang, X.; Liu, L. A facile one-pot construction of supramolecular polymer micelles from α-cyclodextrin and poly (ε-caprolactone ). Angew. Chem. 2008, 47, 5573–5576.
- European Commission. Commission Implementing Regulation (EU) 2018/1023 of 23 July 2018; EU: Brussels, Belgium, 2018.
- Hong, J.; Sun, Z.; Li, Y.; Guo, Y.; Liao, Y.; Liu, M.; Wang, X. Folate-modified Annonaceous acetogenins nanosuspensions and their improved antitumor efficacy. Int. J. Nanomed. 2017, 12, 5053–5067.
- Hong, J.; Li, Y.; Xiao, Y.; Li, Y.; Guo, Y.; Kuang, H.; Wang, X. Annonaceous acetogenins (ACGs) nanosuspensions based on a self-assembly stabilizer and the significantly improved anti-tumor efficacy. Colloids Surfaces B Biointerfaces 2016, 145, 319–327.
- Hong, J.; Liu, Y.; Wang, X. Annonaceous acetogenins Nanoparticles Taking Cyclodextrin and Lecithin as Vectors as well as Preparation Method and Application of Annonaceous acetogenins Nanoparticles. CN Patent CN106389385A, 15 February 2017.
More
