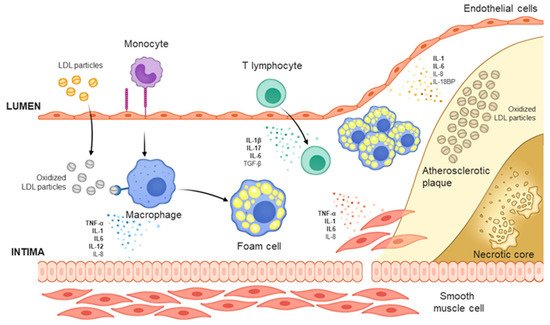
Cardiovascular disease is the leading cause of death worldwide, and its prevalence is increasing due to the aging of societies. Atherosclerosis, a type of chronic inflammatory disease that occurs in arteries, is considered to be the main cause of cardiovascular diseases such as ischemic heart disease or stroke. In addition, the inflammatory response caused by atherosclerosis confers a significant effect on chronic inflammatory diseases such as psoriasis and rheumatic arthritis.
- atherosclerosis
- inflammation
- antibody therapy
1. Overview
Cardiovascular disease is the leading cause of death worldwide, and its prevalence is increasing due to the aging of societies. Atherosclerosis, a type of chronic inflammatory disease that occurs in arteries, is considered to be the main cause of cardiovascular diseases such as ischemic heart disease or stroke. In addition, the inflammatory response caused by atherosclerosis confers a significant effect on chronic inflammatory diseases such as psoriasis and rheumatic arthritis. Here, we review the mechanism of action of the main causes of atherosclerosis such as plasma LDL level and inflammation; furthermore, we review the recent findings on the preclinical and clinical effects of antibodies that reduce the LDL level and those that neutralize the cytokines involved in inflammation. The apolipoprotein B autoantibody and anti-PCSK9 antibody reduced the level of LDL and plaques in animal studies, but failed to significantly reduce carotid inflammation plaques in clinical trials. The monoclonal antibodies against PCSK9 (alirocumab, evolocumab), which are used as a treatment for hyperlipidemia, lowered cholesterol levels and the incidence of cardiovascular diseases. Antibodies that neutralize inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-17, and IL-12/23) have shown promising but contradictory results and thus warrant further research.
2. Cardiovascular disease
| Therapeutic/Study Name | Antibody Name | Target | Patients | Result |
|---|---|---|---|---|
| GLACIER | MLDL1278A | oxLDL (MDA-modified human ApoB-100) | CVD patients | Non significantly reduce carotid plaque |
| FOURIER | Evolocumab | PCSK9 | patients with clinically evident CVD(prior MI, stroke or PAD) | LDL-C level and primary outcomes (MI, stroke, cardiovascular death, coronary revascularization, unstable angina) reduction |
| ODYSSEY | Alirocumab | PCSK9 | patients diagnosed with ACS | LDL-C level and primary outcomes (non-fatal MI, ischemic stroke, unstable angina) reduction |
| SPIRE | Bococizumab | PCSK9 | CV or high risk patients | LDL-C level and primary ennpoint reduction in LDL-C >100 mg/dL group |
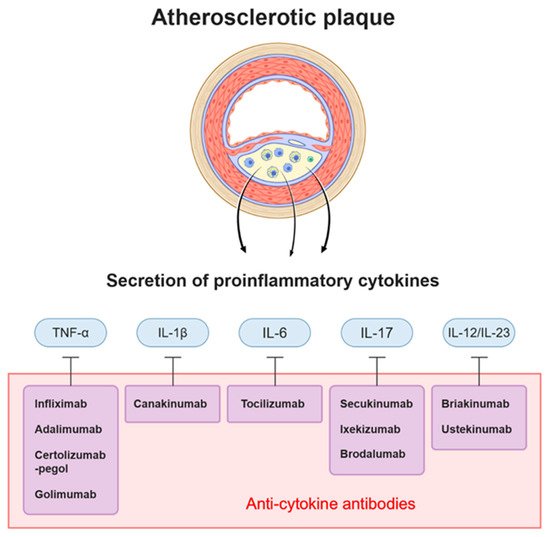
| ATTACH | Infliximab | TNF-α | Heart failure | Deteriorated heart failure |
| STROBE (follow up study) | Infliximab | TNF-α | Psoriasis | Significantly reduce the cardiovascular risk |
| Di Minno et al. [21] | Adalimumab, Infliximab | TNF-A | Psoriatic arthritis | Decreased atherosclerosis of carotid artery |
| CANTOS | Canakinumab | IL-1B | CAD after MI + hsCRP | Decreased hsCRP level and incidence of the primary endpoint (nonfatal myocardial infarction, stroke, cardiovascular death) |
| ASSIL-MI | Tocilizumab | IL-6 | ACS | Increased myocardial salvage |
| Mease et al. [22] | Secukinumab | IL-17 | Psoriatic arthritis | Non significant increased MACE |
| Uncover | Ixekizumab | IL-17 | Moderate to severe psoriasis | Reduced Psoriasis Area and Severity Index (PASI) score |
| Langley et al. [23] | Briakinumab | IL-12/23 | Psoriasis | Increased MACE |
| Uniti | Ustekinumab | IL-12/23 | Moderate to severe Crohn’s disease | Significantly higher rate of response |
3. Discussion
References
- Otreba, M.; Kosmider, L.; Rzepecka-Stojko, A. Polyphenols’ Cardioprotective Potential: Review of Rat Fibroblasts as Well as Rat and Human Cardiomyocyte Cell Lines Research. Molecules 2021, 26, 774.
- Chen, J.; Zhang, X.; Millican, R.; Sherwood, J.; Martin, S.; Jo, H.; Yoon, Y.S.; Brott, B.C.; Jun, H.W. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021, 170, 142–199.
- Palasubramaniam, J.; Wang, X.W.; Peter, K. Myocardial Infarction-From Atherosclerosis to Thrombosis Uncovering New Diagnostic and Therapeutic Approaches. Arter. Throm. Vas. 2019, 39, E176–E185.
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769.
- Cholesterol Treatment Trialists Collaborators; Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590.
- Wu, M.-Y.; Li, C.-J.; Hou, M.-F.; Chu, P.-Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034.
- Shah, P.K.; Lecis, D. Inflammation in atherosclerotic cardiovascular disease. F1000Research 2019, 8, 1402.
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56.
- Valanti, E.-K.; Dalakoura-Karagkouni, K.; Siasos, G.; Kardassis, D.; Eliopoulos, A.G.; Sanoudou, D. Advances in biological therapies for dyslipidemias and atherosclerosis. Metabolism 2021, 116, 154461.
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380.
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337.
- Park, Y.M.; Febbraio, M.; Silverstein, R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Investig. 2009, 119, 136–145.
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.-L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.S.; Smyth, D.; et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166–1172.
- Libby, P. Targeting Inflammatory Pathways in Cardiovascular Disease: The Inflammasome, Interleukin-1, Interleukin-6 and Beyond. Cells 2021, 10, 951.
- Cybulsky, M.I.; Gimbrone, M.A., Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 1991, 251, 788–791.
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arter. Thromb. Vasc. Biol. 2011, 31, 969–979.
- Niu, N.; Xu, S.; Xu, Y.; Little, P.J.; Jin, Z.G. Targeting Mechanosensitive Transcription Factors in Atherosclerosis. Trends Pharm. Sci. 2019, 40, 253–266.
- Tiller, K.E.; Tessier, P.M. Advances in Antibody Design. Annu. Rev. Biomed. Eng. 2015, 17, 191–216.
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233.
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1–30.
- Di Minno, M.N.D.; Iervolino, S.; Peluso, R.; Scarpa, R.; Di Minno, G. Carotid Intima-Media Thickness in Psoriatic Arthritis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 705–712.
- Mease, P.J.; McInnes, I.B.; Kirkham, B.; Kavanaugh, A.; Rahman, P.; Van Der Heijde, D.; Landewé, R.; Nash, P.; Pricop, L.; Yuan, J.; et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N. Engl. J. Med. 2015, 373, 1329–1339.
- Langley, R.G.; Papp, K.; Gottlieb, A.B.; Krueger, G.G.; Gordon, K.B.; Williams, D.; Valdes, J.; Setze, C.; Strober, B. Safety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasis. J. Eur. Acad. Derm. Venereol. 2012.
- Morelli, M.B.; Chavez, C.; Santulli, G. Angiopoietin-like proteins as therapeutic targets for cardiovascular disease: Focus on lipid disorders. Expert Opin. Ther. Targets 2020, 24, 79–88.
- Geladari, E.; Tsamadia, P.; Vallianou, N.G. ANGPTL3 Inhibitors- Their Role in Cardiovascular Disease Through Regulation of Lipid Metabolism. Circ. J. 2019, 83, 267–273.
- Jarr, K.-U.; Nakamoto, R.; Doan, B.H.; Kojima, Y.; Weissman, I.L.; Advani, R.H.; Iagaru, A.; Leeper, N.J. Effect of CD47 Blockade on Vascular Inflammation. N. Engl. J. Med. 2021, 384, 382–383.
- Caligiuri, G. CD31 as a Therapeutic Target in Atherosclerosis. Circ. Res. 2020, 126, 1178–1189.
