Melanoma is the most lethal form of skin cancer. Melanoma is usually curable with surgery if detected early, however, treatment options for patients with metastatic melanoma are limited and the five-year survival rate for metastatic melanoma had been 15–20% before the advent of immunotherapy. Treatment with immune checkpoint inhibitors has increased long-term survival outcomes in patients with advanced melanoma to as high as 50% although individual response can vary greatly. A mutation within the MAPK pathway leads to uncontrollable growth and ultimately develops into cancer. The most common driver mutation that leads to this characteristic overactivation in the MAPK pathway is the B-RAF mutation. Current combinations of BRAF and MEK inhibitors that have demonstrated improved patient outcomes include dabrafenib with trametinib, vemurafenib with cobimetinib or encorafenib with binimetinib. Treatment with BRAF and MEK inhibitors has met challenges as patient responses began to drop due to the development of resistance to these inhibitors which paved the way for development of immunotherapies and other small molecule inhibitor approaches to address this. Resistance to these inhibitors continues to push the need to expand our understanding of novel mechanisms of resistance associated with treatment therapies.
- melanoma
- metastatic
- resistance
- BRAF
- immunotherapy
1. Introduction
2. Therapies
3. Anti-PD-1/PD-L1
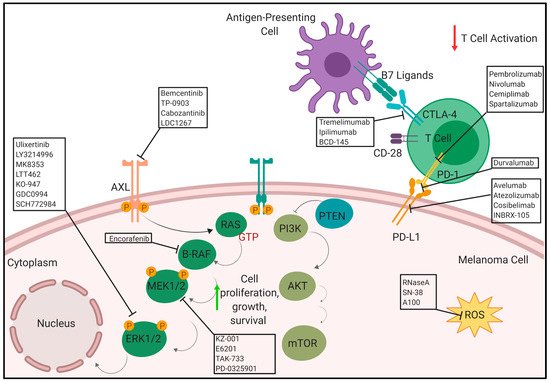
The immunogenic nature of melanoma was utilized to develop several immunotherapeutic treatment strategies especially with regards to the programmed cell death (PD-1) receptor and its ligand, PD-L1. Antibodies targeting the PD-1 axis has shown significant promise in the clinic for treatment of metastatic melanoma either as a monotherapy or in combination with Ipilimumab. There are several ongoing clinical trials using anti-PD1 and anti-PD-L1 antibodies. Programmed cell death protein 1 (PD-1) is an inhibitory receptor expressed on the surface of the cancer cells that inhibits the immune system via suppressing the T-cell activity. Anti-PD-1 monoclonal antibodies block the PD-1 receptor which maintains T-cells in activated state to suppress the tumor growth [23]. There are several anti-PD-1/PD-L1 monoclonal antibodies including pembrolizumab (Keytruda3.1. Pembrolizumab/Lambrolizumab/MK-3475/SCH 900475/Keytruda
This is a humanized monoclonal antibody targeting the PD1 receptor in the lymphocytes. It was developed by Merck and approved for treatment of metastatic melanoma in 2017 [24].3.2. Nivolumab/ONO-4538/BMS-936558/MDX1106/Opdivo
This is a human IgG4 anti-PD-1 monoclonal antibody. Nivolumab works as a checkpoint inhibitor that inhibits T-cell activation. [25]. It was developed by Medarex and Ono Pharmaceutical, and is marketed by Bristol-Myers Squibb (BMS) and Ono. Nivolumab was approved by the FDA for melanoma in 2014 [26][27].3.3. Avelumab/MSB0010718C/Bavencio
This is a humanized monoclonal antibody developed by Merck and Pfizer that targets the PD-L1. It has been approved by FDA for treatment Merkel-cell carcinoma, an aggressive type of skin cancer [28]. It blocks the PD-1/PD-L1 receptor/ligand complex formation leading to suppression of CD8+ T cells action [29]. There is a current clinical trial (NCT01772004) investigating the safety, tolerability, pharmacokinetics and clinical activity of avelumab in melanoma [30].3.4. Durvalumab/MEDI4736/Imfinzi
This is monoclonal antibody that blocks the interaction of PD-L1 with the PD-1 and CD80 (B7.1) molecules developed by Medimmune/AstraZeneca [31][32]. A phase I clinical trial (NCT02586987) is ongoing evaluating the safety and efficacy of selumetinib (AZD6244 Hyd-sulfate) in combination with durvalumab (MEDI4736) along with tremelimumab in patients with advanced solid tumours, including melanoma [33].3.5. Atezolizumab/MPDL3280A/Tecentriq
This is a fully humanized engineered monoclonal antibody of IgG1 isotype against PD-L1 developed by Genentech [34][35]. There is an active ongoing phase II trial (NCT02303951) which includes the combination of vemurafenib, cobimetinib and atezolizumab in stage III/IV advanced melanoma patients [36]. Another phase III study (NCT02908672) compares the efficacy of atezolizumab in combination with cobimetinib and vemurafenib versus placebo control plus cobimetinib and vemurafenib in unresectable and advanced melanoma patients with BRAFV600 mutation [37].3.6. Spartalizumab/PDR001
Spartalizumab (PDR001) is a humanized monoclonal antibody against the negative immuno-regulatory human cell surface receptor programmed death-1 (PD-1, PCD-1) was developed by Novartis. This suppresses T-cell activation as it binds to PD-1 on activated T-cells and inhibits the interaction with its ligands, (PD-L1, PD-1L1) and (PD-L2, PD-1L2) [38]. A phase I/Ib (NCT03891953) study evaluating the efficacy of spartalizumab in combination with DKY709 (immunomodulatory agent) in patients with advances solid tumors including melanoma is ongoing [39]. A phase II PLATforM (NCT03484923) study evaluating the efficacy and safety of spartalizumab in combinations with LAG525 (monoclonal antibody targeting LAG-3), capmatinib (MET inhibitor), canakinumab (monoclonal antibody targeting IL-1β) and ribociclib (CDK4/6 inhibitor) is ongoing in previously treated unresectable or metastatic melanoma [40]. A phase III COMBI-i study (NCT02967692) comparing the combination of spartalizumab, dabrafenib and trametinib versus dabrafenib and trametinib in previously untreated patients with unresectable or metastatic BRAFV600 mutant melanoma is initiated [41].3.1. Pembrolizumab/Lambrolizumab/MK-3475/SCH 900475/Keytruda
3.2. Nivolumab/ONO-4538/BMS-936558/MDX1106/Opdivo
3.3. Avelumab/MSB0010718C/Bavencio
3.4. Durvalumab/MEDI4736/Imfinzi
3.5. Atezolizumab/MPDL3280A/Tecentriq
3.6. Spartalizumab/PDR001
4. Anti-CTLA-4
In addition to PD-1, another immune checkpoint inhibitor, cytotoxic T-lymphocyte antigen 4 (CTLA-4), is important in melanoma. It is found on the surface of regulatory T cells (Treg) and activated T cells [42]. CTLA-4 competes with CD28, another receptor expressed on the surface of T cells, to interact with its two ligands CD80 and CD86, collectively known as the B7 ligands. When CTLA-4 binds with the B7 ligands, commonly found on antigen presenting cells (APC), it results in an immunosuppressive response, which is the inhibition of T cell activation via transendocytosis of CD80 and CD86 from their surfaces [43][44]. Typically, T cell activation requires co-stimulation from the CD28-B7 ligand interaction and the TCR-MHC interaction [45]. However, CTLA-4 has a stronger affinity for the B7 ligands, making it a good immune checkpoint inhibitor that keeps the immune response from turning into an autoimmune one [42]. CTLA-4 is expressed on tumor cells, infiltrating Tregs, and exhausted, activated T cells [46]. Tumor cells, therefore, take advantage of this natural immunosuppressive system in order to prevent an immune response against them. This provides a therapeutic approach which involves anti-CTLA-4 therapy. There are currently three main anti-CTLA-4 antibodies under preclinical and clinical trials for the treatment of melanoma: Tremelimumab, Ipilimumab (Yervoy), and BCD-145.4.1. Tremelimumab/Ticilimumab/CP 675.206
This human monoclonal antibody against CTLA-4 was developed by AstraZeneca [47]. A phase I active, clinical trial (NCT02141542) is -evaluating tremelimumab in combination with MEDI3617 (human anti-angiopoietin 2 monoclonal antibody) for unresectable Stage III/IV melanoma patients [48]. Another phase I active, clinical trial (NCT01103635) is examining tremelimumab in combination with CP-870,893 CD40 agonist monoclonal antibody) for metastatic melanoma [49].4.2. Ipilimumab/MDX010/BMS-734016
This human monoclonal antibody against CTLA-4 was developed by YERVOY Medarex/BMS. It was approved by the FDA in 2011 for the treatment of unresectable or metastatic melanoma [50]. There are current, active clinical trials devoted to assess the efficacy of ipilimumab in combination with other immunotherapies or targeted therapies for metastatic melanoma. A phase I clinical trial (NCT02115243) that assessed ipilimumab as a neoadjuvant followed by melphalan (chemotherapeutic) via isolated limb perfusion in patients with unresectable in-transit extremity melanoma is completed [51]. A phase Ib clinical trial (NCT02117362) evaluating ipilimumab in combination with GR-MD-02 (galnectin inhibitor) in metastatic melanoma patients has been completed [52]. A phase II clinical trial (NCT03153085) examining ipilimumab in combination with TBI-1401(HF10) in Japanese patients with Stage IIIb, IIIc, IV unresectable or metastatic malignant melanoma has been completed [53]. A phase II clinical trial (NCT01970527) looking at SBRT followed by Ipilimumab in patients with stage IV and recurrent melanoma has been completed [54].4.3. BCD-145
This human monoclonal antibody against CTLA-4 is developed by BIOCAD [55]. A completed phase I clinical trial (NCT03472027) studied the efficacy of BCD-145 in unresectable/metastatic melanoma [56]. The combination of anti-PD-1/PD-L1 and anti-CTLA-4 are also being tested in the clinic for stage III/IV melanoma patients. A phase I clinical trial (NCT02935790) evaluating ipilimumab and nivolumab in combination with ACY-241 (selective HDAC inhibitor) is completed [57]. Current clinical trials, outcomes and adverse events investigating the efficacy of anti-CTLA-4, anti-PD-1/PD-L1 therapies and their combinations used to treat metastatic melanoma patients are listed in Table 1 [58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105]. Table 1. Summary of clinical trials, outcomes and adverse events associated with anti-PD-1/PD-L1, anti-CTLA-4 and their combination in metastatic melanoma patients.| Treatment | Status | Sponsor | Phase and NCT | Clinical Outcomes | Adverse Events |
|---|---|---|---|---|---|
| Anti-PD-1/PD-L1 | |||||
| Pembrolizumab in Japenese patients with advanced melanoma (KEYNOTE-041) [58] | Completed | Merck Sharp & Dohme Corp | Ib, NCT02180061 | Pembrolizumab has promising anti-tumor activity and an acceptable safety profile in patients with cutaneous melanoma (n = 29). As per central review, the median overall survival (OS) and median duration of response was not reached. The 1 year OS was 82.7%. The median profession-free survival (PFS) and 6 months PFS was 4.2 months (95% CI: 2.8–7 months) and 41.4% respectively. The overall response, complete response (CR) and partial response (PR) were 24.1% (95% CI: 10.3–43.55), 6.9% (95% CI: 0.8–22.8%) and 17.2% (95% CI: 5.8–35.8%) respectively. |
Pruritus, anemia, maculopapular rash, malaise, and hypothyroidism |
| Study of pembrolizumab (MK-3475) versus chemotherapy in patients with advanced melanoma KEYNOTE-002) [59] | Completed | Merck Sharp & Dohme Corp. | II, NCT01704287 | The progression-free survival was improved in patients assigned to pembrolizumab 2 mg/kg (HR 0.57, 95% CI 0.45–0.73; p < 0.0001) and those assigned to pembrolizumab 10 mg/kg (0.50, 0.39–0.64; p < 0.0001) compared with those assigned to chemotherapy. 6-month progression-free survival was 34% (95% CI 27–41) in the pembrolizumab 2 mg/kg group, 38% (31–45) in the 10 mg/kg group, and 16% (10–22) in the chemotherapy group. | Fatigue, generalised oedema, myalgia, hypopituitarism, colitis, diarrhoea, anemia decreased appetite, hyponatremia, pneumonitis, neutropenia and leucopenia. |
| Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006) [60] | Completed | Merck Sharp & Dohme Corp. | III, NCT01866319 | Pemrolizumab showed superiority over ipilimumab at 5 year follow up time. 834 patients were stratified into three groups: (i) Pembrolizumab (10 mg/kg i.v. every 2 weeks), (ii) Pembrolizumab (10 mg/kg i.v. every 3 weeks) and Ipilimumab (3 mg/kg i.v. every 3 weeks). The median follow up was 57.5 months (IQR: 56.7–59.2 months). Combined Pembrolizumab groups: The median OS and PFS were 32.7 months (95% CI: 24.5–41.6 months) and 8.4 months (95% CI: 6.6–11.3 months) respectively. The ORR was 42% (95% CI: 38.1–46.5%). Ipilimumab groups: The median OS and PFS were 15.9 months (95% CI:13.3–22 months; HR 0.73; 95% CI for HR- 0.61–0.88; p = 0.00049) and 3.4 months (95% CI: 2.9–4.2 months; HR 0.57; 95% CI for HR: 0.48–0.67; p < 0.0001) respectively. The ORR was 17% (95% CI: 12.4–21.4%). |
Fatigue, colitis, diarrhea, asthenia, arthralgia, rash, pruritus, vitiligo. |
| Durvalumab in combination with Dabrafenib and Trametinib in patients with advanced melanoma [61] | Completed | MedImmune LLC | I. NCT02027961 | Durvalumab in combination with dabrafeib and trametinib had manageable safety profile. No maximum tolerated dose was identified (n = 50) and durvalumab 10 mg/kg was selected for further studies. | Pyrexia, fatigue, diarrhea, rash, vomiting and other drug related toxicities |
| Nivolumab in metastatic melanoma patients [62] | Active, not recruiting | BMS in collaboration with Ono Pharmaceutical Co. Ltd. | I, NCT00730639 | Treatment with nivolumab is associated with long-term survival in patients with melanoma (n = 72). The median duration to response and objective response rate (ORR) was 22.9 months (95% CI: 19.7–31.8 months) and 31.8% respectively. The median, estimated 3 years and 5 year overall survival rates were 20.3 months (95% CI: 12.5–37.9 months), 42.3% (95% CI: 32.7–51.6%) and 34.2% (95% CI: 25.2–43.4%) respectively. Patients who had an ORR had significantly higher mean baseline absolute lymphocytes count (1480 cells/uL) as compared to patients without response (1300 cells/uL; p = 0.4). | Anemia, hypothryoididm, gastrointestinal disorder, general disorder, muscular disorder, nasopharyngitis, decreased apatite, nervous and respiratory problems, vascular and skin disorder |
| Atezolizumab in combination with vemurafenib alone or in combination with cobimetinib [63] | Active, not recruiting | Genentech, Inc. | Ib, NCT01656642 | The triple combination was safe, tolerable and had a promising anti-tumor activity. Atezolimumab + Vemurafenib (n = 17): The best objective response rate and complete response rate was 76.5% (95% CI: 50.1–93.2%) and 17.6% respectively. All the patients demonstrated a reduction in the sum of the longest diameter of the target lesion. The median duration of response, PFS and OS was 10.6 months (95% CI: 9.1–37.6 months), 10.9 months (95% CI: 5.7–22 months) and 46.2 months (95% CI: 24.1-not reached) respectively. Estimated OS rates for 1 year were 82%. Atezolimumab + Vemurafenib + Cobimetinib (n = 39): The best objective response rate and complete response rate was 71.8% (95% CI: 55.1–85%) and 20.5% respectively. All the patients demonstrated a reduction in the sum of the longest diameter of the target lesion. The median duration of response and PFS was 17.4 months (95% CI: 10.6–25.3 months), 12.9 months (95% CI: 8.7–21.4 months) respectively. The median OS was not reached. Estimated OS rates for 1 year were 83%. Treatment with vemurafenib alone or in combination with cobimetinib exhibited an increase in the proliferating CD4+ T-helper cells and addition of atezolizumab led to further escalation in these cells. CD8+ cytotoxic T cells were augmented on addition of atezolizumab. | Increase in AST, ALT and blood bilirubin, hyponatremia, blood alkaline phosphatase, rash, diarrhea, vomiting. |
| Anti-CTLA-4 | |||||
| Tremelimumab in patients with advanced refractory and/or relapsed melanoma [64] | Completed | AstraZeneca | II, NCT00254579 | Tremelimumab showed a durable response in these patients (n = 241). The ORR was 6.6% and the duration of response was 8.9–29.8 months. The median OS and clinical benefit rate was 10.1 months (95% CI: 7.9–11.7 months) and 21% respectively. The survival rate at 1 and 2 years was 40% (95% CI: 34–46%) and 22% (95% CI: 17–27%) respectively. Median PFS and 6 months PFS was 2.8 months (95% CI: 2.7–2.8 months) and 15% respectively. As per Response Evaluation Criteria in Solid Tumors (RECIST) criteria, 3.3% of the patients achieved response at the target lesion. | Diarrhea, pruritus, rash, nausea, fatigue, vomiting and colitis. |
| Ipilimumab alone or in combination with dacarbazine, paclitaxel and carboplatin [65] | Completed | BMS in collaboration with Medarex | I, NCT00796991 | Ipilimumab could be combined safely with these chemotherapies with no major pharmacokinetic/pharmacodynamic interactions being observed in these patients. The combinations exhibited a good anti-tumor activity. Ipilumumab alone (n = 20): Estimated geometric mean for Area Under the Curve (AUC) (0-infinity) and maximum serum concentration (Cmax) for Ipilimumab metabolite (AIC) in presence of ipilimumab was changed by 0.970 (90% CI: 0.891–1.056) and 1.058 (90% CI: 0.974–1.150) fold respectively. Based on World Health Organization (WHO) and immune-related criteria ORR were 29.4% and 33.3% respectively and disease control rates b were 59.2% and 73.3% respectively. Ipilimumab + Decarbazine (n = 19): Estimated geometric mean for AUC (0-infinity) and Cmax for dacarbazine in presence of ipilimumab was changed by 0.912 (90% CI: 0.757–1.099) and 1.027 (90% CI: 0.848–1.243) folds respectively. Based on WHO and immune-related criteria ORR were 27.8% and 33.3% respectively, and disease control rates were 55.6% and 61.1% respectively. Ipilimumab + paclitaxel + carboplatin (n = 20): Estimated geometric mean for AUC (0-infinity) and Cmax for carboplatin/paclitaxel in presence of ipilimumab was changed by 0.970 (90% CI: 0.891–1.056) and 1.058(90% CI: 0.974–1.150) folds respectively. ORR based on WHO and immune related criteria were 11.1% and 27.8% respectively. Disease control rate based on WHO and immune related criteria were 44.4% and 55.6% respectively. There was a significant increase in the mean relative frequency and counts of HLA-DR+ CD4+ and CD8+ T cells after treatment initiation in all the three groups. | Rash, fatigue, diarrhea, pruritus, nausea, increase in ALT and AST, decreased neutrophil count. |
| Ipilimumab in combination with imatinib mesylate in patients with advanced malignancies [66] | Completed | M.D. Anderson Cancer Center in collaboration with NCI | I, NCT01738139 | The combination was well tolerated and safe and the MTD and recommended phase 2 dose for intravenous ipilimumab was 3 mg/kg every 3 weeks and imatinib mesylate at 400 mg orally twice daily. Twenty six patients were enrolled in dose escalation cohort Expression of ICOS and OX40 was increased on the CD4 + T cells upon ipilimumab treatment. | Fatigue, nausea, vomiting, edema, anemia, diarrhea, rash, fever. |
| Ipilimumab and high dose IFN-α2B as a neoadjuvant combination for locally/regionally advanced/recurrent melanoma [67] | Completed | Diwakar Davar, University of Pittsburgh | I, NCT01608594 | The combination was well tolerated and exhibited promising durable clinical response rates. 30 patients were enrolled. The median follow-up was 32 months and the pathologic complete response rate was 32% (95% CI: 18–51%). The radiologic response rate was 36% (95% CI: 21–54%). The median PFS was not reached and the probability of PFS at 12 and 6 months was 0.79 (95%CI: 0.65–0.95) and 0.86 (95% CI: 0.74–1) respectively. The probability of OS at 2 years and 1 year was 0.89 (95% CI: 0.79–1) and 0.93 (95% Ci: 0.84–1) respectively. The peripheral blood mononuclear cell was significantly lower at 12 weeks (p = 0.025). The tumor-infiltrating lymphocyte (TIL) was significantly higher in primary melanoma tumors for patients with pathologic complete response (p = 0.033). There was an increase in the number of tumor associated clones following the neoadjuvant treatment and it showed a strong correlation with TIL fraction (ρ = 0.7299; p = 0.0003) and TIL clone diversity (ρ= 0.882; p = 2.7–7). The increase in the tumor T-cell clonality in the primary tumor and a further increase in the clonality after neoadjuvant therapy was statistically significant with relapse-free survival. (p = 0.048 for tumor clonality and p = 0.018 for post treatment metastatic clonality). | Fever, fatigue, creatinine increase, skin, GI, hepatic, endocrine and hematologic disorders. |
| Ipilimumab in combination with PEG-Intrerferon (IFN) α2B on for Stage IIb/c/IV unresected melanoma [68] | Completed | H. Lee Moffitt Cancer Center and Research Institute in collaboration with Merck Sharp & Dohme Corp. | Ib, NCT01496807 | The maximum tolerated dose established was 3 mg/kg of Ipilimumab and 2 ug/kg/week of peginterferon alfa-2 b was efficacious and well tolerated. 30 patients were enrolled. Immune related response criteria: 3.33% and 36.67% of the subjects achieved a CR and PR respectively. The overall response rate was 40%. The median follow up time was 35.8 months (Range: 19.7–50.2 months). The median PFS was 5.9 months and median OS was not reached. At 40.3 months, 16.7% of the patients had a prolonged PFS without any need for further therapy. 85.6% of the subjects had an objective response. Here was a significant correlation between autoimmune vitiligo and objective response (p = 0.009). | Anemia, dry eye, GI disorders, chills, fatigue, fever, increase in blood enzymes, anorexia, muskoskeletal and connective tissue disorders, nervous system disorders. Cough, dyspnea, depression, skin and subcutaneous tissue disorders |
| Intratumoral injection of Ipilimumab and IL-2 for unresectable stage III/IV melanoma [69][70] | Completed | University of Utah | I, NCT01672450 | The combination was well tolerated and generated an enhanced systemic immune response at injected and non-injected lesions in these patients. No dose limiting toxicities were observed in the 12 enrolled patients. Immune-related response criteria: Clinical benefit rate was 50% (95% CI: 19–81%). The PR and overall ORR was 30% and 40% (95% CI: 10–70%) respectively. 67% of the subjects (95% CI: 40–94%) had local response on the injected lesion which was assessed by pathology and/or measurement. 88.9% of the patients (95% CI: 68–100%) had an abscopal effect observed at distant and locoregional sites. Based on imaging and/or pathology, 40% of the patients (95% CI: 10–70%) demonstrated an objective response. 60% of the subjects had an increase in the frequency of CD8+ T cells expressing Tbet (fold increase: 1.75; 95% CI: 1.14–2.36) and IFNgamma (fold increase: 2.02; 95% CI: 1.31–2.73). 40% (fold increase: 1.83; 95% CI: 1.61–2.05) and 50% (fold increase: 1.50; 95% CI: 1.32–1.68) of the patients had an increase in the CD8+ T cells expressing granzyme-B and perforins. | Chills, fatigue, flu like symptoms, pain and ulceration at site of injection, rash, soft tissue infection. No grade 4/5 toxicities were observed. |
| Ipilimumab in combination with Stereotactic Radiosurgery (SRS) or Whole Brain Radiation Therapy (WBRT) [71] | Completed | Sidney Kimmel Cancer Center at Thomas Jefferson University in collaboration with BMS | I, NCT01703507 | The combination of ipilimumab (3 mg/kg; n = 7 or 10 mg/kg; n = 9) and SRS was safe without any dose limiting toxicities. Arm A (WBTR; n = 5): The median follow-up time was 8 months (Range: 3.5–24.1 months). Median time to intracranial progression, PFS and OS were 2.53 months (Range 0.3–18 months), 2.5 months and 8 months respectively. Arm B (SRS; n = 11): the median follow-up time was 10.5 months (Range: 1.8–36.8 months). Median time to intracranial progression and PFS was 2.45 months (Range: 1–37 months) 2.1 months respectively. The median OS was not reached. Immune-related PR was 7%. | Neurotoxic effects, headache, GI toxicity, vomiting, subclinical intracranial hemorrhage, increase in ALT, dizziness, tinnitus. No grade 4/5 and radionecrosis were observed. |
| Ipilimumab in subjects with previously treated unresectable stage III/IV melanoma [72] | Completed | BMS in collaboration with Medarex | II NCT00289640 | The three fixed doses used in this study were ipilimumab 10 mg/kg, 3 mg/kg or 0.3 mg/kg administered every 3 weeks for four cycles (induction phase) followed by maintenance therapy administered every 3 months. Ipilimumab had a dose-dependent efficacy and safety in these subjects and 10 mg/kg dose was well tolerated with manageable safety. 10 mg/kg (n = 73): The median follow-up was 10.7 months (Interquartile range {IQR}: 3.6–20.4 months). Best overall response rate was 11.1% (95% CI: 4.9–20.7). This dose had greater increase in absolute lymphocyte count and serum ipilimumab concentrations compared to the other doses at 4 weeks. 3 mg/kg (n = 72): The median follow-up was 8.3 months (IQR: 4–20.4 months). Best overall response was 4.2% (95% CI: 0.9–11.7). 0.3 mg/kg (n = 32): The median follow-up was 8.3 months (IQR: 3.5–15.3 months). Best overall response was 0% (95% CI: 0–4.9). The best overall response was significant for 10 mg/kg group (p = 0.0015). Median OS for 10 mg/kg vs 3 mg/kg had an HR of 0.875 (95% CI: 0.593–1.291). Median OS for 10 mg/kg vs 0.3 mg/kg had a hazard ratio (HR) of 0.77 (95% CI: 0.525–1.130). | Immune-related grade 3–4 events were GI related, skin related, nausea, vomiting, pruritus, rash, endocrine. Some immune related grade 5 adverse events were observed. |
| Ipilimumab in combination with Fotemustine for unresectable locally advanced or metastatic malignant melanoma with or without brain metastasis (NIMIT-M1) [73] | Completed | Italian Network for Tumor Biotherapy in collaboration with BMS | II, NCT01654692 | The combination was efficacious and fotemustine did not impair the activity of Ipilimumab. The median follow up time was 39.9 months. The median duration of response, 3 and 2 year duration of response rates were 30.3 months (95% CI: 15.5–46.5 months), 49.2% (95% CI: 27.4–71%) and 55.4% (95% CI: 34.7–76.1%). Whole Study Population (n = 86): The median OS was 12.9 months (95% CI: 7.1–18.7 months). The 3 and 2 year survival rates were 28.5% and 33.4% respectively. The PFS and median brain PFS were 4.5 months (95% CI: 3.1–5.9 months) and 8.3 months (95% CI: 4.7–11.8 months) respectively. For patients with brain metastasis: The median OS, 3 and 2 years survival rates were 12.7 months (95% CI: 2.7–22.7 months), 27.8% and 38.9% respectively. The PFS and median brain PFS were 3.4 months (95% CI: 2.3–4.5 months) and 3 months (95% CI: 2.9–3.1 months). The median OS was not significant for patients with BRAF WT and V600E mutation. Subjects with improved OS had increased levels of circulating CD3 + CD4+ICOS+CD45RO+T cells as opposed to CD3+CD4+ICOS+CD45RA+ T cells. The expansion of memory T cells over naïve T-cells shows that the combination favors an increase in T-cell antigen-primed populations. | Rash, pruritus |
| Ipilimumab in advanced melanoma patients with preexisting humoral response to NY-ESO-1 [74] | Completed | National Center for Tumor Diseases, Heidelberg in collaboration with University Hospital Heidelberg | II, NCT01216696 | Ipilimumab demonstrated a higher clinical efficacy in patients with NY-ESO-1, maybe used as a surrogate for preexisting immune response to tumor antigens. 25 patients were enrolled in the study. The median duration of treatment was 64 days (Range: 1–352 days). Immune-related response criteria: the disease control rate was 52% (90% CI: 34.1–69.5%). 36% of the subjects had a PR. The PFS was 7.8 months (95%CI: 2.6-nr moths). No significant association was observed between best response and the amount of NY-ESO-1-specific T-cells. RECIST criteria: 24% of the subjects had a PR. The PFS was 2.9 months (95%CI: 2.5–8.1 months). The median OS was 22.7 months (95%CI: 9.5-nr months). The 1 year survival rate was 66.8% (95%CI: 0.44–0.82). The best overall response (BOR) had a statistically significant association with the OS (p = 0.0031). For a small subset of patients there was a statistically significant association between PFS and NY-ESO-1-specific CD3+ T-cells (HR: 1.039; p = 0.0478). |
Endocrine, GR, hepatobiliary, musculoskeletal and connective tissue disorders, headache, skin and subcutaneous tissue disorders |
| Vemurafenib followed by ipilimumab in V600 BRAF mutant, untreated metastatic melanoma patients [75] | Completed | BMS | II, NCT01673854 | The study was divided into two parts: one where patients received vemurafenib followed by ipilimumab and the other where subjects who progressed after ipilimumab received vemurafenib. The sequential treatment was efficacious and had a manageable safety profile. The use of targeted therapy followed by immune modulation therapy has helped to understand the optimum regimen of these therapies. VEM1-IPI: 46 patients were treated with vemurafenib followed by 46 patients on ipilimumab induction and eight patients on ipilimumab maintenance. The median duration of response and follow-up was 23.1 months (95%CI: 5.03-not reached). The BOR was 32.6%. The median PFS and OS was 4.5 months (95%CI: 4.17–6.57 months) and 18.5 months (95%CI: 11.96-not evaluated). VEM2: 19 patients progressed on pilimumab were treated with vemurafenib. The median follow-up and overall survival was 15.3 months and 18.5 months (95% CI: 11.96-not evaluated) respectively. The median PFS was 4.5 months (95% CI: 4.17–6.57 months). The BOR rate was 36.8%. 4.3% of patients had a CR and 28.3% had a PR. | Rash, erythema, pruritus, GI toxicities, hetaobiliary toxicities, nausea, vomiting. |
| Ipilimumab in combination with HF-10 (replication –competent HSV-1 oncolytic virus) in stage IIIb/c/IV unresectable or metastatic malignant melanoma [76] | Completed | Takara Bio Inc. in collaboration with Theradex | II, NCT02272855 | The combination was well tolerated, beneficial and elicited anti-tumor activity. The combination induced an immune-cell infiltration in the TME. 46 patienrs were enrolled. The best overall response rate was at 24 weeks. Immune-related response criteria: 18% and 23% of the patients had a CR and PR respectively. The median PFS and OS were 19 months and 26 months respectively. There was increase om the total tumor infiltrating CD8+ T-cells and lymphocytes along with a decrease in the CD4+ T-cells. | Treatment related grade 3/4 events. Majority of the AEs were due to Ipilimumab which are immune related events. |
| Ipilimumab in combination with standard melphalan and dactinomycin to isolate limb infusion (ILI) for advanced unresectable melanoma of the extremity [77] | Completed | Memorial Sloan Kettering Cancer Center in collaboration with BMS | II, NCT01323517 | The combination was safe and efficacious. 18 patients were enrolled. The median follow-up time was 18 months. At 3 months timepoint, 89% of the patients had a limb response from which 65% and 24% had a CR and PR, respectively. At 18 months, the median OS was 78%. The PFS at one year was 57%. The levels of eosinophils and ALC were elevated in all the subjects. | Limb toxicity, colitis, hypophysitis, rash. |
| Ipilimumab in Japanese patients with unresectable or metastatic melanoma [78] | Completed | BMS | II, NCT01990859 | Ipilimumab was well tolerated and demonstrated an anti-tumor response in these patients (n = 20). The median OS and PFS was 8.71 months (95% CI: 3.71-nr months) and 2.74 months (95% CI: 1.25–2.83 months) respectively. The disease control rate and best overall response rates were 20% (95% CI: 5.7–43.7) and 10% (95% CI: 1.2–31.7) respectively. 10% of the subjects had a CR. | Rash, pruritus, pyrexia, GI disorder, increase in AST and ALT, skin, liver and endocrine related immune events. No grade 4 drug related adverse events were observed. |
| Ipilimumab in combination with temozolamide in metastatic melanoma patients [79][80] | Completed | M.D. Anderson Cancer Center in collaboration with BMS | II, NCT01119508 | The combination exhibited an enhanced antitumor activity. 64 patients were enrolled. The median duration of response and median follow-up was 35 months (Range: 2–57 months) and 20 months (Range: 2–60 months). 15.6% of the subjects had a CR and PR. The median PFS and OS was 5 months and 24.5 months respectively. 6 months PFS was 45%. The PFS for patients with bone metastasis was significantly decreased (p = 0.014) but not for subjects with liver metastasis. 21% and 7% of the subjects with liver metastasis had a CR and PR respectively while no ORR was observed in subjects with bone metastasis | Pruritus, skin rash, nausea, constipation, diarrhea, colitis, increase in ALT and AST, hematologic toxicities. No drug related grade 5 toxicities were observed. |
| Ipilimumab as monotherapy for previously treated unresectable stage III/IV melanoma [81][82] | Completed | BMS in collaboration with Medarex | II, NCT00289627 | Ipilimumab demonstrated good clinical activity in these patients which consists of subjects who did not respond to prior therapy. 155 patients were enrolled in the study. The median follow-up and OS was 10 months (Range 0.32–33.1 months) and 10.2 months (95% CI: 7.6–16.3%). The best overall response rate and disease control rate was 5.8% (95% CI: 2.7–10.7%) and 27% (95% CI: 20–35%) respectively. 5.8% of the subjects had a PR and no CR was observed. The 1 year, 18 months and 2 year survival rates were 47.2% (95% CI: 39.5–55.1%), 39.4% (95% CI: 31.7–47.2%) and 32.8% (95% CI: 25.4–40.5%), respectively. | Immune related grade 3 and 4 events - skin and GI tract, liver and endocrine. No grade 5 immune related adverse events were observed. |
| Ipilimumab in combination with autologous TriMix –DC therapeutic vaccine for previously treated unresectable stage III/IV melanoma [83][84] | Completed | Bart Neyns, Universitair Ziekenhuis Brussel in collaboration with Vrije Universiteit Brussel | II, NCT01302496 | The combination was well tolerated and demonstrated a durable anti-tumor response. 39 patients were enrolled in the trial. The median follow-up time, estimated median PFS and OS were 36 months (Range: 22–43 months), 27 weeks (95% CI: 9–44 weeks) and 59 weeks (Range: 72–185 weeks) respectively. PFS and OS rates at year one- 33% (95% CI: 18–48%), 59% (95% CI: 43–74%); year two- 22%(95% CI: 9–36%), 38%(95% CI: 23–53%) and year three- 18% (95% CI: 5–31%), 34% (95% CI: 19–50%). Immune-related response criteria: 6 months disease control rate was 51% (95% CI: 36–67%). The ORR was 38%. 20% and 18% of the subjects had a CR and PR respectively. The outcome was poor in patients with brain metastasis. There was a significant increase in the eosinophils, peripheral blood lymphocytes and monocytes after from the baseline treatment with the combination. A significant increase in the CD8+ (p < 0.001), CD4+ (p < 0.001), HLA-DR+ activation marker on CD4+ cells (p < 0.001), CD3+ (p < 0.001), ratio of CD4+ to CD8+ (p = 0.003) and Tregs (p = 0.016) was observed after the combination treatment. | Dermal injection site reactions, post-infusion chills, flu-like symptoms. Immune related adverse events- dermatitis, colitis, diarrhea, hypophysitis, neumonitis, lymphadenopathy. Grade 3/4 immune related adverse events were observed with no grade 5 events. |
| Ipilimumab in combination with WBRT for melanoma patients and brain metastases (GEM STUDY, GRAY-B) [85][86] | Completed | Grupo Español Multidisciplinar de Melanoma in collaboration with BMS | II, NCT02115139 | The combination was well tolerated and safe. Fifty eight patients were enrolled in the study. The overall survival at 1 year was 31.8% (95% CI: 18.8–44.8%). The median OS and PFS was 5.8 months (95% CI: 3.6–5.9 months) and 4.8 months (95% CI: 2.2–3.4 months). | WBRT related- vomiting and headache. Immune therapy related- diarrhea, intestinal perforation, increase in AST and ALT, headache. |
| Ipilimumab in combination with SRS for subjects with brain metastasis [87] | Completed | University Hospital, Lille in collaboration with BMS | II, NCT02662725 | High dose of ipilimumab and SRS was effective with a manageable safety profile. 57 patients were enrolled in the study. Median survival time for reference population vs study population was 5.6 months vs 13.2 months (HR: 0.29, 95% CI: 0.19–0.39; p < 0.0001) with 49% disease control rate. | Colitis, hepatitis, hypophiisitis, headache. One subject showed radionecrosis. |
| Ipilumumab alone or in combination with decarbazine in previously untreated metastatic melanoma patients [88] | Completed | BMS | II, NCT00050102 | The combination was well tolerated and a durable, clinically meaningful responses were observed. Decarbazine did not affect the PK of ipilimumab. Ipilimumab alone (n = 37): The median follow up time and median OS were 16.4 months and 11.4 months (95% CI: 6.1–15.6 months) respectively. The objective response rate and disease control rate were 5.4% (95% CI: 0.7–18.2%) and 21.6% (95% CI: 9.8–38.2%), respectively. 5.4% of the subjects had a PR. The survival rates for 1 year, 2 year and 36 months were 45%, 21% and 9%, respectively. Combination (n = 35): The median follow up time and the OS were 20.9 months and 14.3 months (95% CI: 10.2–18.8 months) respectively. The objective response rate and disease control rate was 14.3% (95% CI: 4.8–30.3%) and 37.1% (95% CI: 21.5–55.1%) respectively. 5.7% and 8.6% of the patients had a CR and PR for more than 24 weeks respectively. The survival rates for 1 year, 2 year and 36 months were 62%, 24% and 20% respectively. CD4+ and CD8+ expressing HLA-DR T cells were increased in both the groups. |
Colitis, muscle weakness, anemia, tachycardia, abdominal pain. Fatigue, dehydration, increased ALT/AST, rash, pruritus, vasculitis. |
| Ipilimumab in combination with HF10 for unresectable Stage IIIb/c/IV or metastatic malignant melanoma [89] | Completed | Takara Bio Inc. in collaboration with Theradex | II, NCT02272855 | The combination was well tolerated with positive antitumor activity and there were no dose limiting toxicities. 46 patients were enrolled. The median PFS and OS was 19 months and 21.8 months respectively. Immune-related response criteria: The best overall response rate and disease stability rate was 41% and 68%, respectively. 16% and 25% of the patients had CR and PR, respectively. | Embolism, lymphedema, diarrhea, hypoglycemia, groin pain, immune related events. |
| Decarbazine alone or in combination with ipilimumab for unresectable stage III/IV melanoma [90] | Completed | BMS in collaboration with Medarex | II, NCT00324155 | The combination of ipilimumab and decarbazine was well tolerated and had a long-term durable overall survival. Ipilimumab and decarbazine group (n = 250): The median survival follow-up time and OS was 11 months (Range: 0.4–71.9 months) and 11.2 months (95% CI: 9.5–13.8 months) respectively. At 5 years, 18.2% of the patients were alive which was significantly higher than that in the other group (p = 0.002). 7.5% and 42.5% of the patients had a CR and PR respectively. No median OS was reached for the responders while that for the non-responders was was 14.3 months (95% CI: 11.4–16.9 months; HR: 0.28, 95% CI: 0.16–0.47). Decarbazine and placebo group (n = 252): The median survival follow-up time and OS was 8.9 months (Range: 0.1–73.2 months) and 9.1 months (95% CI: 7.8–10.5 months; HR: 0.69; 95% CI: 0.57–0.84) respectively. At 5 years, 8.8% of the patients were alive. 35% of the patients had a PR and no CR was achieved. The median OS for non responders and responders was 12.3 months (95% CI: 10.9–15.4 months) and 20.2 months (95% CI: 14.6–45.3 months; HR: 0.51, 95% CI: 0.32–0.84), respectively. | Rash, pruritus, vitiligo, GI, liver and endocrine related events. Grade 3 to 4 immune related adverse events were observed in skin with no grade 5 events. |
| Ipilimumab antibody (MDX-010) alone or in combination with melanoma peptide vaccine (MDX-1379; gp100) for previously untreated unresectble stage III/IV melanoma [91] | Completed | BMS | III, NCT00094653 | Overall ipilimumab resulted in survival of 20% of the patients for more than 2 years. 45% of the patients who survived for more than 2 years survived for more than 3 years. Ipilimumab + placebo (n = 137): 25% of the patients survived for more than 2 years and 3 years. The disease control rate for on-study response and for patients surviving more than 2 years was 28.5% (1.5% CR and 9.5% PR) and 83.3% (8.3% CR and 41.7% PR) respectively. Gp100 vaccine alone (n = 136): 17% and 10% of the patients survived for more than 2 year and 3 years respectively. The disease control rate for on-study response and for patients surviving more than 2 years was 11% (1.5% PR) and 43.8%, respectively. Combination (n = 403): 19% and 15% of the patients survived for more than 2 years and 3 years respectively. The disease control rate for on-study response and for patients surviving more than 2 years was 20.1% (0.2% CR and 5.5% PR) and 66.7% (1.9% CR and 22.2% PR), respectively. | Immune related adverse events- colitis, vitiligo, diarrhea, hypogonadism, proctitis, dermatologic, GI, endocrine related events, increase ALT. No grade 4/5 immune related adverse effects were observed. |
| Ipilimumab doses- 3 mg/kg vs 10 mg/kg for previously treated or untreated unresectable or metastatic melanoma [92] | Completed | BMS | III, NCT01515189 | Both doses were well tolerated, with 10 mg/kg dose having more treatment-related events. 10 mg/kg group (n = 364): The median follow-up time, OS and PFS were 14.5 months (IQR: 4.6–42.3 months), 15.7 months (95% CI: 11.6–17.8 months) and 2.8 months (95% CI: 2.8–3 months) respectively. 2% and 13% of the patients had a CR and PR respectively. The 1 year, 2 year and 3 year overall survival was 54.3% (95% CI: 49–59.3%), 38.5% (95% CI: 33.4–43.5%) and 31.2% (95% CI: 26.4–36%), respectively. 3 mg/kg group (n = 362): The median follow-up time, OS and PFS was 11.2 months (IQR: 4.9–29.4 months), 11.5 months (95% CI: 9.9–13.3 months) and 2.8 months (95% CI: 2.8–2.8 months), respectively. 2% and 10% of the patients had a CR and PR respectively. The 1 year, 2 year and 3 year overall survival was 47.6% (95% CI: 42.4–52.7%), 31% (95% CI: 26.2–35.8%) and 23.2% (95% CI: 18.9–27.7%), respectively. HR between both the groups for median overall survival was 0.84 (95% CI: 0.7–0.99; p = 0.04). EORTC QLQ-C30 global health status were significantly declined in both the groups form the baseline. |
Headache, diarrhea, colitis, increase in ALT, hypophysitis. No grade 5 toxicities were observed. |
| Ipilimumab alone or in combination with talimogene laherparepvec (T-VEC) in patients with previously untreated unresected, Stage IIIb-IV melanoma [93] | Active, not recruiting | Amgen | Ib/II, NCT01740297 | The combination was well tolerated and had a greater systemic antitumor response (in uninjected and visceral lesions) as compared to single agent. Ipilimumab + Laherparepvec (n = 98): The median duration of treatment with laherparepved and ipilimumab was 21.1 and 9.1 weeks, respectively. The median followup time and time to response was 68 weeks (Range: 0–156 weeks) and 5.8 months (95% CI: 5.4–10.9 months) respectively. 39% of the patients had an ORR (13% CR and 26% PR) and visceral lesions decrease was observed in 52% of the patient population. The median PFS was 8.2 months (95% CI: 4.2–21.5 months). Ipilimumab alone (n = 100): The median duration of treatment of ipilumumab was 9.1 weeks. The median followup time was 58 weeks (Range: 0–152 weeks). The median time to response was not estimated (HR = 1.41; 95% CI: 0.8–2.5). 18% of the patients had an ORR (7% CR and 11% PR; OR: 2.9, 95% CI: 1.5–5.5, p = 0.002) and visceral lesion decrease was observed in 23% of the patient population. The median PFS was 6.4 months (95% CI: 4.2–21.5 months). | Fatigue, chills, GI disorders, pruritus, rash and nausea. |
| Ipilimumab in Stage IV melanoma patients receiving palliative radiation therapy [94] | Active, not recruiting | Stanford University | II, NCT01449279 | The combination was safe and efficacious. 22 patients were enrolled and treated in the study. 50% of the patients had benefited from the therapy including CR and PR at 55 week follow-up. 27.3% of the patients had an ongoing systemic complete response to the combination (95% CI- 9.7–56.9%) with no evidence of disease at 55 week. 27.3% (95% CI- 9.7–56.96%) of the patients has an initial PR without progression for median of 40 weeks (Range: 29–53). The median PFS was 26 weeks (Range: 2–65; 95% CI: 16.3–35.7) and the median overall survival was 55 weeks (Range: 8–141; 95% CI: 39.2–70.8) with the patients receiving the combination. The median time for response for patients who had CR or PR was 19 weeks (Range: 12–52). The strong antitumor response in patients with CR or PR can be attributed to increased levels of IL-2 producing CD8+ T cells and central memory CD8+ T cells in comparison to patients with melanoma could be used as biomarkers further. | Colitis, hypophysitis, rash, anemia, nausea and radiation dermatitis. |
| Ipilimumab alone or in combination with sargramostim (GM-CSF) in Stage III/IV melanoma that cannot be removed surgically [95] | Active, not recruiting | NCI | II, NCT01134614 | The combination was advantageous and had a lower toxicity profile. The median follow-up was 13.3 months (Range: 0.03–19.9 months). Ipilimumab + Sargarmostim (n = 132): The median OS was 17.5 months (95% CI: 14.9-not reached). The one year survival rate was 68.9% (95% CI: 60.6–85.5%). Ipilimumab alone (n = 122): The median OS was 12.7 months (95% CI: 10-not reached). The one year survival rate was 52.9% (95% CI: 43.6–62.2%). There was no difference in PFS across both the groups. |
GI toxicity, pulmonary toxicities, |
| Ipilimumab as adjuvant therapy after complete resection of high risk stage III melanoma [96] | Active, not recruiting | BMS | III, NCT00636168 | Addition of Ipilimumab as adjuvant therapy benefited patients with microscopic involvement only (sentinel node-positive) and for patient with macroscopic or palpable nodes. The median follow up was 5.3 years. Ipilimumab group (n= 475): The 5 years recurrence-free survival and OS was 40.8% and 65.4% respectively. The rate of distant metastasis free survival at 5 years was 48.3%. Placebo group (n = 476): The 5 years recurrence free survival and OS was 30.3% (HR: 0.76; 95% CI: 0.64–0.89; p < 0.001) and 54.4% (HR: 0.72; 95% CI: 0.58–0.88; p = 0.001) respectively. The rate of distant metastasis free survival at 5 years was 38.9% (HR: 0.76; 95% CI: 0.64–0.92; p = 0.002). |
Immune related adverse events- GI, hepatic, endocrine, skin and neurologic. |
| Combination of Anti-PD-1/PD-L1 and anti-CTLA-4 | |||||
| Ipilimumab in low dose as an adjuvant in combination with nivolumab after resection of melanoma macrometastases [97] | Completed | Universitair Ziekenhuis Brussel | Ib, NCT02941744 | Ipilumumab at low doses in combination with nivolumab had an acceptable safety profile. Ipilumumab (50 mg) + Nivolumab (10 mg) (n = 34): the median follow up was 86 weeks. One year relapse-free survival, overall survival and distant metastasis-free survival was 55% (95% CI: 39–72%); 97% (95% CI: 94–100%) and 79% (95% CI: 65–92%) respectively. Median relapse free survival was 84 weeks (95% CI: 28–139 weeks). Nivolumab (10 mg) (n = 22): The median follow up was 36 weeks. One year relapse-free survival and overall survival was 78% (95% CI: 73–82%) and 100% respectively. Distant metastasis was not observed. The median relapse free survival was not reached. | 4–8% grade 3 immune related adverse events were across both the cohorts. |
| LTX-315 alone or in combination with Ipilimumab or Pembrolizumab in patients with transdermally accessible tumors [98][99] | Completed | Lytix Biopharma AS in collaboration with Theradex and ICON plcI | I, NCT01986326 | Combination of immune checkpoint inhibitors with LTX-315 was safe and tolerable and demonstrated a potent anti-tumor activity. Of 6 melanoma patients received LTX-315 in combination with Ipilimumab, stable disease was observed in 33% of the patients. LTX-315 when administered to patients with solid tumors resulted in increase in number of CD8+ T cells at the site of treated lesions along with tumor infiltrating lymphocyte population. Clonal expansion of T-cells in blood was observed after treatment with LTX-315 as revealed by T-cell receptor sequencing. | LTX-315-related grade 3 and 4 adverse events (allergic/anaphylaxis) were observed along with tingling post injection, rash, fatigue, diarrhea, hypo and hyper tension, weakness. |
| Nivolumab and Ipilimumab alone or in combination in patients with previously untreated unresectable or metastatic melanoma (CheckMate067) [100] | Active, not recruiting | BMS | III, NCT01844505 | Combination of ipilimumab and nivolumab or nivolumab alone was superior over monotherapy with ipilimumab. No new toxic effects associated with chronic use of these therapies were observed. Nivolumab plus Ipilimumab group (n = 314): Median overall survival was more than 60 months. Hazard ratio for death versus Ipilimumab group was 0.52. 5 year overall survival rate was 52%. Nivolumab group (n = 316): Median overall survival was 36.9 months. Hazard ratio for death versus Ipilimumab group was 0.63. 5 year overall survival rate was 44%. Ipilimumab group (n = 315): Median overall survival was 19.9 months. 5 year overall survival rate was 26%. |
|
| Ipilimumab in combination with Nivolumab in patients with unresectable Stage III/IV malignant melanoma [101] | Active, not recruiting | BMS in collaboration with Medarex and Ono Pharma USA Inc | Ib, NCT01024231 | The combination of Ipilimumab and nivolumab had durable clinical activity in patients with advanced melanoma. 94 patients were enrolled in the study. At the target lesions, the mean reduction in tumor burden was around 64.7%. The median follow-up was 30.3 to 55 months while the median OS was not reached at 3 years. The median PFS was 6.2 months (95% CI: 3.2–11 months). The median duration of response was 22.3 months (95% CI: 13.8–25.8 months). The best overall response rate and objective response rate by modified WHO criteria were 19.1% and 41.5% (95% CI: 31.4–52.1%). The CR and PR rates were 22.3% and 16% respectively. The OS and median PFS rates at 1 year were 81% (95% CI: 71–87%) and 37% (95%CI: 27–47%) respectively. The OS and median PFS rates at 2 years were 72% (95% CI: 62–80%) and 28% (95% CI: 19–38%) respectively. The OS and median PFS at year 3 were 63% (95% CI: 52–72%) and 17% (95% CI: 8–29%) respectively. | Grade 3 and 4 immune related toxicities such as rash, diarrhea, increase in lipase, AST, ALT and amylase, rthralgia, colitis, were observed. |
| Nivolumab alone or in combination with ipilimumab in melanoma patients with brain metastases [102] | Active, not recruiting | Melanoma Institute Australia in collaboration with Melanoma and Skin Cancer Trials Limited and BMS | II, NCT02374242 | The combination of Nivolumab and ipilimumab was active in melanoma brain metastases with a durable intracranial and extra cranial response. Cohort A (n = 36): Nivolumab (3 mg/kg) + Ipilumumab (3 mg/kg) Cohort B (n = 27): Nivolumab (3 mg/kg) Cohort C (n = 16): patients with brain metastases for whom local therapy failed/showing neurological symptoms/leptomeningeal disease were administered nivolumab (3 mg/kg). As per RECIST criteria, the median follow up was 17 months (IQR: 8–25 months). The overall survival at 6 months in cohorts A.B and c were 78% (95% CI: 65–94%), 68% (95% CI: 52–89%) and 44% (95% CI: 25–76% respectively. Intracranial response: The overall response in cohorts A, B and C was 46% (95% CI: 29–63), 20% (95% CI: 7–41%) and 6% (95% CI: 0–30%) respectively. The PFS at 6 months in cohorts A, B and C was 53% (95% CI: 38–73%), 25% (95% CI: 9–44%) and 13% (95% CI: 5–65%) respectively. Extra cranial response: the overall response in cohorts A, B and C was 57% (95% CI: 37–75%), 29% (95% CI: 11–52%) and 25% respectively. The PFS at 6 months in cohorts A, B and C were 51% (35–76%), 35% (95% CI: 19–64%) and 19% (95% CI: 5–65%) respectively. | Grade 1 and 2 treatment related adverse events were commonly observed such as skin, GI, endocrine, musculoskeletal, respiratory related and fatigue. Grade 3/4 related adverse events were infrequent. |
| Pembrolizumab in combination with reduced dose Ipilimumab or Pegylated Interferon Alfa-2b in patients with advanced melanoma (KEYNOTE-29) [103] | Active, not recruiting | Merck Sharp & Dohme Corp. | I/II, NCT02089685 | While the combination of Pembrolizumab and ipilimumab had good antitumor activity and manageable safety profile, the combination of pembrolizumab and PEF-INF did not. Pembrolizumab and Ipilimumab (n = 12): The median follow-up was 25.1 months (Range: 0.8–38.7 months). The median duration of response was not reached. The objective response rate as per independent central review was found to be 42% (95% CI: 15–72%). The CR and PR rates were 8.33% and 33.33% respectively. As per investigator review, the objective response rate and PR were 33% (95% CI: 10–655) and 33.33% respectively. Pembrolizumab and PEG-IFN (n = 17): The median follow up was 22.2 months (Range- 25–377 months). As per central and investigator review, the objective response rate was 20% and the partial response rate was 20%. |
Pembrolizumab and Ipilimumab: Grade 1/2 treatment related adverse events- fatigue, diarrhea, rash, nausea, colitis, increased lipase, ALT and AST. Immune-related adverse events- colitis, hyper and hypo thyroidism. Pembrolizumab and PEG-IFN: Treatment related AEs- elevation is AST, ALT nerve disorder, fatigue, chills, pyrexia, diarrhea, rash, pruritus, nausea, anemia. Immune-related AEs- hyperthyroidism, pneumonitis, hepatitis. |
| Nivolumab combined with Ipilimumab or Ipilimumab alone in patients with untreated, unresectableor metastatic melanoma [104] | Active, not recruiting | BMS | II, NCT01927419 | The median follow-up was 24.5 months (IQR: 9.1–25.7 months). Ipilimumab alone (n = 47): The 2 years overall survival was 53.6% (95% CI: 38.1–66.8%). The objective response rate and PR were 11% (95% CI: 3–23%) and 11% respectively. The median PFS was 3 months (95% CI: 27–5.1 months; HR: 0.36; 95% CI for HR: 0.22–0.56, p < 0.0001). The PFS and 1 and 2 year was 16% (95% CI: 6.6–28.9) and 152% (95% CI: 3.8–25.2%) respectively. Nivolumab plus Ipilimumab (n = 95): The 2 years overall survival was 63.8% (95% CI: 53.3–72.6%). The objective response rate, CR and PR were 59% (95% CI: 48–69%), 22% and 37% respectively. The median PFS was not reached. The PFS at 1 and 2 years was 52.5% (95% CI: 41.6–62.3%) and 51.3% (95% CI: 40.4–61.2%) respectively. The median overall survival was not reached in either group (HR: 0.74, 95% CI 0.43–1.26; p = 0.26). | Colitis, Diarrhea, Hypophysitis, Pneumonitis, Anaemia, Hypothyrodism, increased ALT. |
| Nivolumab administered sequentially with Ipilimumab in subjects with advanced or metastatic melanoma (CheckMate064) [105] | Active, not recruiting | BMS | II, NCT01783938 | Nivolumab followed by ipilimumab was more clinically beneficial. Nivolumab followed by Ipilimumab (n = 70): The median overall survival was not reached. The 1 year overall survial was 76% (95% CI: 64–85%). The overall response rate, CR and PR was 56% (95% CI: 43.3–67%), 12% and 44% respectively. Ipilimumab followed by Nivolumab (n = 70): The median overall survival was 16.9 months (95% CI: 9.2–26.5 months; HR: 0.48; 95% CI for HR: 0.29–0.8). The 1 year overall survival was 54% (95% CI: 42–65%). The overall response rate, CR and PR was 31% (95% CI: 20.9–43.6%), 6% and 26% respectively. | Pruiritus, rash, fatigue, chills, pyrexia, vitiligo, diarrhoea, nausea, increased ALT, AST and lipase. |
