Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Atefeh Rabiee.
The concerning worldwide increase of obesity and chronic metabolic diseases, such as T2D, dyslipidemia, and cardiovascular disease, motivates further investigations into preventive and alternative therapeutic approaches. Over the past decade, there has been growing evidence that the formation and activation of thermogenic adipocytes (brown and beige) may serve as therapy to treat obesity and its associated diseases owing to its capacity to increase energy expenditure and to modulate circulating lipids and glucose levels. Thus, understanding the molecular mechanism of brown and beige adipocytes formation and activation will facilitate the development of strategies to combat metabolic disorders.
- adipose tissue
- development
- molecular circuits
- secretome
- thermogenesis
- metabolism
- obesity
- therapy
1. Introduction
Obesity is the main driver of insulin resistance (IR), type two diabetes (T2D), and metabolic syndrome. Obese subjects, especially the ones with a high percentage of intra-abdominal fat, have a greater risk of developing cardiovascular disease (CVD), the leading cause of death in industrial countries [1]. The prevalence of obesity is multifactorial and includes socioeconomic, educational status, issues concerning mental health, genetics, sedentarism, and diet [2]. It is now appreciated that obesity develops when energy consumption (food intake) overcomes energy expenditure. This induces white adipose tissue (WAT) expansion followed by reduced mass and activity of brown/beige adipocytes (fat cells), thereby contributing to the development of metabolic disorders during obesity [3].
WAT is the principal site for energy storage, while brown and beige adipocytes are the sites for energy expenditure (EE) due to their thermogenic capacity [4]. Adipose tissue (AT) is also an important endocrine organ responsible for the secretion of many molecules, including lipids [5[5][6],6], proteins [7,8[7][8][9],9], and miRNAs [10]. These factors serve as paracrine-endocrine signals, critical for the function of AT itself, as well as non-adipose tissues, regulation of whole-body metabolism, and insulin sensitivity [11]. Therefore, interventions that can induce the formation and activation of brown and beige adipocytes such as cold exposure [12], pharmacological activation of the adrenergic pathways [13], or even genetic manipulation of adipocytes [14] are attractive therapies to improve metabolic health in obese humans.
2. Brown and Beige Adipose Tissue in Obesity, Aging and Metabolic Disease
Obesity is the major contributor to the development of metabolic diseases such as IR, T2D, dyslipidemia, and CVD. These metabolic disorders are also observed during aging [342][15] raising the hypothesis that unhealthy excess of body fat may accelerate the aging processes. In this regard, diet-induced obese mice are shorter-lived compared to their controls [343][16]. Similarly, in obese humans, the risk of premature death is increased by 1.45 to 2.76 folds [344][17]. The pathophysiology of obesity and aging-associated diseases are complex and share dysregulations at the cellular level [342,345][15][18]. Consistent with this, robust evidence suggests that changes in AT distribution and metabolic dysfunction are implicated in the development and disease progression during obesity and aging [346,347,348][19][20][21]. Here we discuss how obesity changes AT biology and its implication for the development of the metabolic syndrome. Some factors altering the AT and contributing to obesity and aging are summarized in Figure 1.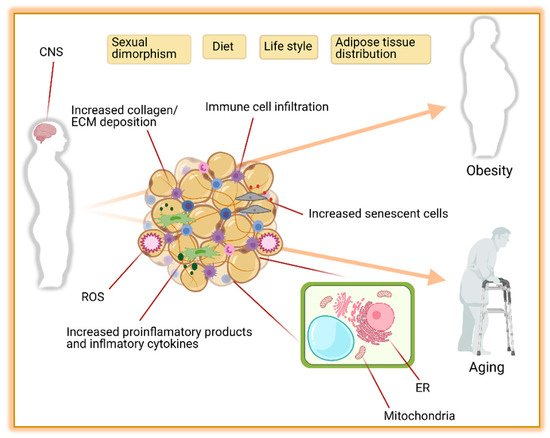
Figure 1. The leading causes of obesity and aging are driven by adipose tissue distribution, function, and environment. Contributions of the central nervous system (CNS), sexual dimorphism, diet, life style, and adipose tissue distribution to obesity and aging are well known. In addition, the composition of adipose tissue itself with increased collagen, extra cellular matrix (ECM), reactive oxygen species (ROS), immune cells, macrophages, and senescent cells is another major contributor to obesity and aging. Furthermore, the functionality of mitochondria and endoplasmic reticulum (ER) in adipocytes plays an important role in preventing obesity and aging complications. Figure created with ©BioRender.io.
2.1. Adipose Tissue Distribution
In humans, AT distribution can be influenced by sexual hormones, diet, and aging. In general, females exhibit higher scWAT (gynoid fat deposition) and BAT mass, while vWAT is more preeminent in men (android fat deposition) [349][22]. During obesity, even though AT expansion is observed in all types of fat depots, female subjects very often present lower visceral and larger subcutaneous AT compared with males [350][23]. This sexual dimorphism is also observed in BAT, where BAT mass [30][24], and Ucp1 mRNA expression are still higher in women [351][25]. Genetics and hormones are the major players in sexual dimorphism [352][26], however, some evidence suggests that these differences persist even after menopause [30][24]. Interestingly, this dimorphism is associated with a lower risk to develop metabolic diseases in women and may contribute to a longer lifespan compared to men [353][27].
2.2. Metabolic Function
It is now appreciated that AT function is also regulated in a sex-dependent manner that is widely reviewed elsewhere [349,354,355][22][28][29]. Here we will give an overall view of some biological processes that are impaired in the AT of obese mice and humans. These processes are interconnected and mediate the development of obesity-associated diseases.
2.2.1. Sympathetic Nervous System (SNS)
Overactivation of the sympathetic nervous system is often observed in obese subjects which contributes to the development of high blood pressure and cardiovascular diseases [356,357,358,359,360][30][31][32][33][34]. In AT, hyperactivation of the SNS pathway induces negative feedback, and downregulates the abundance of adrenergic receptors, decreasing the lipolytic [357][31], and thermogenic capacity [361][35]. This contributes to an increased WAT expansion, whitening of beige adipocytes [362][36], and decreased basal EE. Additionally, whitening of beige fat induces macrophage infiltration, brown adipocyte death and increased senescent cells, crown-like structure (CLS) formation, fibrosis, and local inflammation [362][36].
2.2.2. Endoplasmic Reticulum Stress (ER)
This organelle is composed of a membranous network responsible for the synthesis, maturation, and trafficking of proteins. It is also highly sensitive to nutrient availability. Upon nutrient overload, the increased protein synthesis followed by their misfolding and accumulation in the ER lumen induces ER stress. As a result, proteins from the unfolded protein response (UPR) Atf6, Perk, and Ire1 are recruited to reestablish the ER homeostasis [363][37]. In obesity, this process is hyperactivated in multiples tissues including adipose. This contributes to AT inflammation and insulin resistance [364,365][38][39]. Mechanistically, Atf6 and Perk acts through activation of NF-kB which translocate to the nucleus and induces the expression of pro-inflammatory cytokines such as IL-1 and TNFα, while Ire1a interacts with the tumor necrosis factor-a (TNFα)-receptor-associated factor 2 (Traf2), activates Jnk and IkB kinase (IKK) and downstream mediators of inflammation [363,366][37][40]. Adipocyte ER stress also leads to increased basal lipolysis through downregulation of perilipin and insulin receptor, decrease adiponectin assembling and secretion, as well as decrease in leptin release [366,367][40][41].
2.2.3. Mitochondrial Dysfunction
As the central contributors to energy metabolism, mitochondria play key roles in the production of ATP, oxidative phosphorylation, production of reactive oxygen species (ROS), and Ca2+ homeostasis. Mitochondria also play an important role in AT homeostasis and remodeling [368,369][42][43]. The rate-limiting steps of oxidative reaction that regulate the thermogenesis in the beige adipocytes take place in mitochondria. Brown and beige fat depots are packed with mitochondria (the cells’ tiny power plants) with high expression of Ucp1 across the mitochondria inner membrane which uncouples the respiratory chain from ATP (energy) and thereby, it increases thermogenesis by heat production. The browning of the WAT is accompanied by an increase in the number of mitochondria caused by de novo biogenesis of mitochondria as well as mitochondrial fission (fission separates one into two) [370][44]. Contrarily, a reduced number of mitochondria resulted from mitochondrial fusion (fusion joins two mitochondria together), and mitochondrial disappearance (mitophagy) is reported during beige to white fat transition [371,372][45][46]. Mitochondrial dysfunction is present in many organs including WAT and BAT. It is characterized by increased mitochondrial DNA (mtDNA) mutations and damage, decreased oxidative phosphorylation (OXPHOS), reduced activity of metabolic enzymes, as well as changes in mitochondrial morphology, dynamics, and biogenesis [373,374,375][47][48][49]. In line with this, multiple symmetric lipomatosis (MSL), an adipose disorder (AD) characterized by upper body lipomatous masses, is frequently linked to multiple mutations in mitochondrial genes such as Mttk (gene encoding mitochondrial tRNA lysine involved in the assembly of proteins that carry out oxidative phosphorylation), and Mfn2 (gene encoding mitofusin 2 that helps to regulate the morphology of mitochondria by controlling the fusion process) [376,377][50][51].
2.2.4. Inflammation and Endocrine Dysfunction
During obesity, adipocytes increase in size and number to accommodate the excess of nutrients in form of lipids. Excessive expansion of WAT followed by capillary rarefaction triggers a cascade of the biological processes including, ER-stress, mitochondrial dysfunction, hypoxia, changes in extracellular matrix mobility, and adipocyte death which are thought to contribute to inflammation [378][52]. Activation of the inflammatory response leads to the secretion of several pro-inflammatory factors TNFα, Il-1b, Il-6, and monocyte chemoattractant protein (Mcp-1) from adipocytes [379,380][53][54]. This is accompanied by infiltration of immune cells such as M1 macrophages [381][55], Cd8+ T cells [382][56], B cells [383][57], and eosinophils [384][58], thereby enhancing local and systemic inflammation [385][59]. The chronic low-grade inflammatory state observed in obesity is an important contributor to AT insulin resistance (IR). This is important because impaired insulin signaling in adipocytes leads to uncontrolled basal lipolysis, which can induce cell death, and also increase the circulating levels of FFAs. In turn, this leads to lipids accumulation in non-adipose organs inducing systemic IR and increasing the risk to develop cardiovascular disease and T2D [386,387,388][60][61][62].
3. Activation of Thermogenesis as Therapy for Obesity-Associated Metabolic Diseases
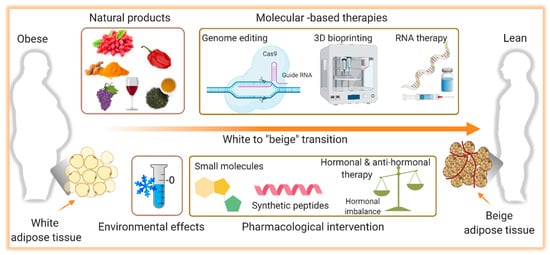
Over the years, the development of drugs to treat obesity was mainly focused on weight loss, primarily due to a reduction in food intake. Many of these molecules failed to meet the desired efficacy and some of them were even withdrawn from the market because of their limited success and harmful side effects [389,390][63][64]. This, with the observation that adult humans have BAT with the capability to dissipate energy, activation of BAT and thermogenesis began to be envisioned as therapy. Ever since the development of interventions that can stimulate browning of WAT as well as BAT mass increase and activation have gained greater attention and will be discussed here. A summary of the potential therapeutic interventions for obesity and metabolic disorders accompanied by aging is shown in Figure 2.
Figure 2. Illustration of the potential therapeutic interventions for the therapy of obesity. Induction of the browning process, the transition from white to brown-like or beige adipocytes, holds a promising therapeutic potential to combat obesity and its complications. Several pharmacological (small molecules, synthetic peptides, hormonal analogs) and non-pharmacological (natural products) interventions are known to induce browning. The role of environmental challenges such as cold exposure on white adipose tissue browning and thermogenesis is also identified. In addition, molecular-based therapies including CRIPR-based genome editing, RNA therapy, and 3D bioprinting are evolving approaches to alter the white adipocytes as a therapeutic target in obesity. Figure created with ©BioRender.io.
3.1. Cold-Induced Thermogenesis
Currently, cold exposure is the most effective intervention to activate BAT in obese humans improving whole-body insulin sensitivity and weight loss [391,392][65][66]. Some candidates have been strongly suggested to mediate the metabolic effect of BAT activation. One of the most well-investigated molecules is FGF21. This protein is mainly present in the liver, but it is also expressed in, skeletal muscle, pancreas, WAT, and BAT. Upon short-term cold exposure, FGF21 expression in adipocytes is significantly increased [393,394][67][68]. FGF21 induces browning of WAT in an autocrine manner [395][69] and enhances insulin signaling in the same cell [396][70]. Moreover, cold exposure also increases the circulating levels of FGF21 [397,398][71][72] which activates the SNS enhancing EE and weight loss [398][72].
Most recently, another member from the FGF family, FGF9, was also demonstrated to be upregulated in the scWAT and BAT of cold-exposed mice. Exerting an autocrine-paracrine regulation, FGF9 binds to FGFR3 receptor in adipocytes to regulate Ucp1 expression [399][73]. In addition to proteins, cold exposure induces the secretion of lipid species from BAT including 12,13-diHOME and 12-HEPE, which enhance BAT fatty acids [6] and glucose uptake [400][74] respectively. Altogether, cold exposure triggers an intricate metabolic network between the central nervous system (CNS) and AT which redirects the utilization of circulating glucose and FFAs to support heat production ultimately improving WAT and BAT function and whole-body metabolism.
3.2. Natural Thermogenic Compounds
3.2.1. Berberine
Berberine is a plant-based alkaloid compound traditionally used in Chinese medicine to treat diarrhea and some infectious diseases [401][75]. Berberine has been extensively studied due to its potential as a cardioprotective, anti-hyperlipidemic, and antidiabetic compound [402,403][76][77]. Most recently, berberine was shown to induce Ucp1 gene expression in brown and white adipocytes through activation of 5′ AMP-activated protein kinase (AMPK) leading to an increased BAT activity, improved EE, and decreased weight gain in db/db mice [404][78]. More importantly, 1 month of berberine supplementation increased BAT volume and activity, reduced body weight, improved insulin sensitivity in patients with non-alcoholic fatty liver [405][79].
3.2.2. Capsaicin and Capsinoids
Capsaicin and its analog capsinoids are compounds found in red peppers [406][80]. Several studies have shown the anti-obesity, anti-diabetic, and anti-inflammatory effects of these compounds. In rodents, capsinoids supplementation improves glucose metabolism, hepatic lipid content and enhances cold-induced EE and WAT browning [407][81]. In humans, chronic supplementation with capsinoids over six weeks decreased body weight and enhanced cold-induced thermogenesis in healthy adult men lacking detectable BAT, suggesting that cold exposure in combination with capsinoid ingestion recruits the activation of brown and beige adipocytes [392][66]. These adaptations occur through activation of the transient receptor potential cation channel subfamily V member 1 TRPV1 receptor (transient receptor potential cation channel subfamily V member 1) in the gut which sends signals to the CNS leading to β2-AR signaling activation in AT [407][81].
3.2.3. Curcumin
Curcumin is a well-known flavonoid found in turmeric root. It has many therapeutic properties including, antioxidant, anti-inflammatory, anti-diabetic, and anti-obesity [408][82]. This is corroborated by the observation that curcumin supplementation reduces BMI, percentage of body fat, lower circulating leptin, and increased adiponectin levels in obese humans [409][83]. Part of these effects may be explained by the induction of browning in WAT via AMPK activation [410,411][84][85] and inhibition of preadipocyte differentiation by downregulating the Pparγ and C/ebpα [412][86]. In mice, supplementation with curcumin for 50 days induces higher expression of mitochondrial and thermogenic genes, higher NE levels, increased β3-AR expression in scWAT, improved cold tolerance, and lower body fat [411][85].
3.2.4. Green Tea
Green tea is made from the leaves of Camellia sinensis and contains several different catechins, especially epigallocatechin gallate (EGCG), which accounts for about 50% to 70% of green tea catechins, and caffeine [413][87]. Green tea extract has several metabolic properties such as antioxidant, anti-hypertensive, anti-carcinogenic, hypocholesterolemia, and has also been shown to induce weight loss [414,415][88][89]. This evidence is supported by the reduction of body weight, mainly due to loss of vWAT mass, in obese women and men subjects submitted to catechins supplementation [414,416][88][90]. There are several potential mechanisms proposed to explain the anti-obesity effects of green tea compounds such as inhibition of de novo lipogenesis, increased FA oxidation, browning of WAT, and activation of BAT [415,417][89][91]. The effect of thermogenesis seems to be dependent on the interaction between catechins, caffeine, and NE. At the cellular level, catechins inhibit catechol-O-methyltransferase, one of several enzymes that degrade catecholamines, and caffeine inhibits phosphodiesterase resulting in higher levels of cyclic AMP (cAMP). This results in higher levels of NE and cAMP leading to fat oxidation and thermogenic activation [418][92].
3.2.5. Resveratrol
3,5,4′-trihydroxy-trans-stilbene (Resveratrol) is a natural compound that belongs to polyphenols’ group. It is found in more than 70 different plants including grapes and has gained greater attention over the years due to its biological properties including the weight loss effect [419][93]. Consistent with this, resveratrol supplementation was shown to reduce the weight gain in diet-induced obese mice. This effect was mediated by improved oxidative capacity in muscle and AT and increased EE [420][94]. Moreover, resveratrol inhibits adipocyte differentiation and lipid accumulation [421][95] and induces browning of WAT [422][96]. The molecular effect of resveratrol is not completely understood, but some evidence suggests that interaction between AMPK activation and NAD-dependent protein deacetylase sirtuin-1 (Sirt1) leads to increased expression of Pgc1α, thereby inducing mitochondrial biogenesis [422][96]. In humans, the effect on weight loss and thermogenesis is not clear and differences in dose and duration of resveratrol supplementation across studies have yielded inconsistent results. Despite this limitation, some beneficial effects including improved HOMA-index have been observed 30 days after resveratrol supplementation, suggesting positive effects on insulin sensitivity [423][97].
3.3. Pharmacological Intervention
3.3.1. Beta 3-Agonist Drugs
In mice, pharmacological activation of BAT using β3-adrenoreceptor agonist drugs increases EE, reduces circulating insulin levels and body fat [424,425,426][98][99][100]. However, the translational potential of this approach is debatable since human β3-adrenoceptor have different binding characteristics compared to rodents and drug bioavailability also varies across species, which limits the capacity to effectively activate BAT [427,428][101][102]. Despite these limitations, a new FDA-approved drug, referred to as Mirabegron, developed to treat overreactive bladder, has been shown to improve glucose tolerance and FA oxidation. At its maximal concentration (200 mg), a single dose of Mirabegron increased BAT glucose uptake and WAT lipolysis [429][103]. Moreover, chronic Mirabegron treatment enhances BAT activity, induces WAT loss, increases HDL, and improves insulin sensitivity in lean and obese subjects [13,430][13][104]. Nevertheless, a recent study performed by Blondin et al. raises some concerns regarding the use of Mirabegron [431][105]. According to the authors, in human adipose tissue, Mirabegron seems to work mainly through b2-adrenoceptor, since b3-adrenoceptor is quite low expressed. This suggesting that this drug lacks receptor selectivity [431][105] and may explain some of its effects on heart rate and blood pressure [429][103].
3.3.2. GLP-1 Receptor Agonist
Glucagon-like peptide 1 is a molecule secreted in response to the absorption of nutrition by the L-cells in the gastrointestinal tract. Innumerous clinical studies have demonstrated its capacity to reduce food intake, enhance insulin secretion, inhibit gluconeogenesis and improve skeletal muscle IR. Besides, recent evidence suggests that GLP-1 increased browning of WAT and BAT activation via GLP-1 binding to its receptor GLP-1R in the hypothalamus [432,433,434][106][107][108]. Since GLP-1 has a short half-life, GLP-1 analogs have been developed and approved as therapies to treat obesity and T2D [432][106]. In mice, GLP-1 analogs have the potential to induce WAT browning and BAT activation [435][109]. In obese and T2D humans, GLP-1 analogs enhance body weight loss and improve overall metabolism, whether this is dependent on decreased food intake or increased BAT activation yet needs to be addressed.
3.4. Gene Therapy
3.4.1. Ex Vivo Gene Therapy
The revolutionary approach of cellular-based therapy combined with gene editing has been considered an alternative to treat metabolic diseases and a pre-clinical study performed has shown promising results. Wang et al. used the CRISPR-Cas9 system (CRISPR-SAM) to overexpress Ucp1 in human white preadipocytes to generate the human beige/brown-like adipocytes (HUMBLE). These cells exhibit gene signatures and metabolic function similar to human brown adipocytes. Upon transplantation into mice, the HUMBLE cells differentiate into mature and functional adipocytes. Importantly, transplantation of HUMBLE cells into diet-induced obese mice resulted in increased heat production, decreased weight gain, improved insulin sensitivity, and glucose tolerance. Most strikingly, these metabolic effects were induced by the communication between the HUMBLE cells and the endogenous BAT via nitric oxide [14]. Looking forward one could envision the generation of personalized HUMBLE cells, where adipocyte progenitor cells would be isolated from the patient’s scWAT, cultivated in vitro, transformed into HUMBLE, and placed back into the patient.
3.4.2. In Vivo Gene Therapy
A more straightforward alternative to modulating the expression of a gene or a protein is the delivery of nucleotides (DNA or RNA species) to the cell of interest. Over the years, a variety of viral and non-viral methods have been developed to deliver DNA, RNA, or protein to human cells to treat different types of diseases. Currently, 12 gene therapy-based drugs are available in the market and many others are being tested in clinal trials [436][110], however, none of them were developed with the intent to treat obesity and its associated disease.
Hopefully, in the near future, with the use of viral vectors, we will be able to target specific tissues and overexpress a protein of interest. In line with this, one could envision the transfection of white and brown AT with the Ucp1 mRNA. A second approach will be to use the same CRISPR-Cas9 system used to generate the HUMBLE cells [14] to induce endogenous Ucp1 overexpression. The advantage of this technique compared to the others discussed earlier relies on the fact that it can be personalized, it may induce more persistent therapeutic outcomes reducing or eliminating the need for medication and avoiding any complication related to the cell transplantation.
3.5. 3D Bioprinting
3D bioprinting technology, allowing the construction of biological tissue in an accurate and reproducible manner is a potential approach for tissue engineering and regenerative medicine. AT bioprinting has particular needs, including morphology, composition, and heterogeneity, as well as the microenvironment, and crosstalk with other cells such as immune cells, vascularization, and ECM. 3D bioprinting of brown and beige AT aiming to create an optimal size and function and transplanting it to the patients seems like a potential strategy in the treatment of obesity and metabolic diseases. This could also be used for testing chemical and pharmaceutical products as well as evaluating the toxicity of the new drugs. Kuss et al. used 3D printed gels to test the effects of stiff vs. soft gels on immortalized human white and brown AT precursor cells and showed that white progenitors prefer soft gels to differentiate as compared to brown progenitors that their differentiation reaches an optimal level interacting with stiffer gels [437][111]. The feasibility of bioprinting the breast structure including the AT and mammary glands has been discussed by Chen et al., and despite several challenges including poor vascularization, it is a promising strategy to count on for the treatment of patients with breast cancer [438][112]. Nonetheless, most of the bioprinted tissue and organs are yet at the level of laboratory uses and there is a long way till they will be clinically applicable.
References
- Reaven, G. Insulin resistance, hypertension, and coronary heart disease. J. Clin. Hypertens. 2003, 5, 269–274.
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689.
- Seale, P.; Lazar, M.A. Brown fat in humans: Turning up the heat on obesity. Diabetes 2009, 58, 1482–1484.
- Ikeda, K.; Maretich, P.; Kajimura, S. The Common and Distinct Features of Brown and Beige Adipocytes. Trends Endocrinol. Metab. 2018, 29, 191–200.
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014.
- Yu, Y.H.; Ginsberg, N.H. Adipocyte signaling and lipid homeostasis: Sequelae of insulin-resistant adipose tissue. Circ. Res. 2005, 96, 1042–1052.
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 1995, 270, 26746–26749.
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432.
- Lihn, A.S.; Pedersen, S.B.; Richelsen, B. Adiponectin: Action, regulation and association to insulin sensitivity. Obes. Rev. 2005, 6, 13–21.
- Goody, D.; Pfeifer, A. MicroRNAs in brown and beige fat. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2019, 1864, 29–36.
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99.
- Lessing, M.P.A.; Hyman, N.M. Intracranial haemorrhage caused by amphetamine abuse. J. R. Soc. Med. 1989, 82, 766–767.
- O’Mara, A.E.; Johnson, J.W.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Fletcher, L.A.; Fink, Y.A.; Kapuria, D.; Cassimatis, T.M.; Kelsey, N.; et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Investig. 2020, 130.
- Wang, C.H.; Lundh, M.; Fu, A.; Kriszt, R.; Huang, T.L.; Lynes, M.D.; Leiria, L.O.; Shamsi, F.; Darcy, J.; Greenwood, B.P.; et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci. Transl. Med. 2020, 12.
- Dominguez, L.J.; Barbagallo, M. The biology of the metabolic syndrome and aging. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 5–11.
- Cañadas-Lozano, D.; Marín-Aguilar, F.; Castejón-Vega, B.; Ryffel, B.; Navarro-Pando, J.M.; Ruiz-Cabello, J.; Alcocer-Gómez, E.; Bullón, P.; Cordero, M.D. Blockade of the NLRP3 inflammasome improves metabolic health and lifespan in obese mice. GeroScience 2020, 42, 715–725.
- Di Angelantonio, E.; Bhupathiraju, S.N.; Wormser, D.; Gao, P.; Kaptoge, S.; de Gonzalez, A.B.; Cairns, B.J.; Huxley, R.; Jackson, C.L.; Joshy, G.; et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016, 388, 776–786.
- Jura, M.; Kozak, L.P. Obesity and related consequences to ageing. Age 2016, 38, 23.
- Tam, B.T.; Morais, J.A.; Santosa, S. Obesity and ageing: Two sides of the same coin. Obes. Rev. 2020, 21, e12991.
- Reis, F.C.G.; Branquinho, J.L.O.; Brandão, B.B.; Guerra, B.A.; Silva, I.D.; Frontini, A.; Thomou, T.; Sartini, L.; Cinti, S.; Ronald Kahn, C.; et al. Fat-specific Dicer deficiency accelerates aging and mitigates several effects of dietary restriction in mice. Aging (Albany. N. Y.) 2016, 8, 1201.
- Caron-Debarle, M.; Lagathu, C.; Boccara, F.; Vigouroux, C.; Capeau, J. HIV-associated lipodystrophy: From fat injury to premature aging. Trends Mol. Med. 2010, 16, 218–229.
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diab. Rep. 2018, 18, 1–10.
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119.
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.-H.; Doria, A.; et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. N. Engl. J. Med. 2009, 360, 1509–1517.
- Jespersen, N.; Andersen, M.; Jensen, V.; Stærkær, T.; Severinsen, M.; Peijs, L.; Soares, R.; Forss, I.; Andersen, E.; Hahn, C.; et al. Thermogenic genes are blunted whereas brown adipose tissue identity is preserved in human obesity. BioRxiv 2020.
- Link, J.C.; Chen, X.; Arnold, A.P.; Reue, K. Metabolic impact of sex chromosomes. Adipocyte 2013, 2, 74–79.
- Lemaître, J.F.; Ronget, V.; Tidière, M.; Allainé, D.; Berger, V.; Cohas, A.; Colchero, F.; Conde, D.A.; Garratt, M.; Liker, A.; et al. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl. Acad. Sci. USA 2020, 117, 8546–8553.
- White, U.A.; Tchoukalova, Y.D. Sex dimorphism and depot differences in adipose tissue function. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 377–392.
- Bloor, I.D.; Symonds, M.E. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm. Behav. 2014, 66, 95–103.
- Balasubramanian, P.; Hall, D.; Subramanian, M. Sympathetic nervous system as a target for aging and obesity-related cardiovascular diseases. GeroScience 2019, 41, 13–24.
- Gaidhu, M.P.; Anthony, N.M.; Patel, P.; Hawke, T.J.; Ceddia, R.B. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL and AMPK. Am. J. Physiol. Cell Physiol. 2010, 298, C961–C971.
- Smith, M.M.; Minson, C.T. Obesity and adipokines: Effects on sympathetic overactivity. J. Physiol. 2012, 590, 1787–1801.
- Guarino, D.; Nannipieri, M.; Iervasi, G.; Taddei, S.; Bruno, R.M. The role of the autonomic nervous system in the pathophysiology of obesity. Front. Physiol. 2017, 8, 665.
- Feldstein, C.; Julius, S. The complex interaction between overweight, hypertension, and sympathetic overactivity. J. Am. Soc. Hypertens. 2009, 3, 353–365.
- Van Baak, M.A. The peripheral sympathetic nervous system in human obesity. Obes. Rev. 2001, 2, 3–14.
- Kotzbeck, P.; Giordano, A.; Mondini, E.; Murano, I.; Severi, I.; Venema, W.; Cecchini, M.P.; Kershaw, E.E.; Barbatelli, G.; Haemmerle, G.; et al. Brown adipose tissue whitening leads to brown adipocyte death and adipose tissue inflammation. J. Lipid Res. 2018, 59, 784–794.
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917.
- Özcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461.
- Park, S.; Aintablian, A.; Coupe, B.; Bouret, S.G. The endoplasmic reticulum stress-autophagy pathway controls hypothalamic development and energy balance regulation in leptin-deficient neonates. Nat. Commun. 2020, 11, 1–11.
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68.
- Xu, L.; Spinas, G.A.; Niessen, M. ER stress in adipocytes inhibits insulin signaling, represses lipolysis, and alters the secretion of adipokines without inhibiting glucose transport. Horm. Metab. Res. 2010, 42, 643–651.
- Lee, J.H.; Park, A.; Oh, K.J.; Lee, S.C.; Kim, W.K.; Bae, K.H. The role of adipose tissue mitochondria: Regulation of mitochondrial function for the treatment of metabolic diseases. Int. J. Mol. Sci. 2019, 20, 4924.
- Altshuler-Keylin, S.; Kajimura, S. Mitochondrial homeostasis in adipose tissue remodeling. Sci. Signal. 2017, 10.
- Gao, A.W.; Houtkooper, R.H. Mitochondrial fission: Firing up mitochondria in brown adipose tissue. EMBO J. 2014, 33, 401–402.
- Lu, X.; Altshuler-Keylin, S.; Wang, Q.; Chen, Y.; Sponton, C.H.; Ikeda, K.; Maretich, P.; Yoneshiro, T.; Kajimura, S. Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci. Signal. 2018, 11.
- Rabiee, A. Beige Fat Maintenance; Toward a Sustained Metabolic Health. Front. Endocrinol. 2020, 11, 634.
- Kusminski, C.M.; Scherer, P.E. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 2012, 23, 435–443.
- Woo, C.Y.; Jang, J.E.; Lee, S.E.; Koh, E.H.; Lee, K.U. Mitochondrial dysfunction in adipocytes as a primary cause of adipose tissue inflammation. Diabetes Metab. J. 2019, 43, 247.
- Ejarque, M.; Ceperuelo-Mallafré, V.; Serena, C.; Maymo-Masip, E.; Duran, X.; Díaz-Ramos, A.; Millan-Scheiding, M.; Núñez-Álvarez, Y.; Núñez-Roa, C.; Gama, P.; et al. Adipose tissue mitochondrial dysfunction in human obesity is linked to a specific DNA methylation signature in adipose-derived stem cells. Int. J. Obes. 2019, 43, 1256–1268.
- Perera, U.; Kennedy, B.A.; Hegele, R.A. Multiple Symmetric Lipomatosis (Madelung Disease) in a Large Canadian Family With the Mitochondrial MTTK c.8344A>G Variant. J. Investig. Med. High. Impact Case Rep. 2018, 6.
- Capel, E.; Vatier, C.; Cervera, P.; Stojkovic, T.; Disse, E.; Cottereau, A.S.; Auclair, M.; Verpont, M.C.; Mosbah, H.; Gourdy, P.; et al. MFN2-associated lipomatosis: Clinical spectrum and impact on adipose tissue. J. Clin. Lipidol. 2018, 12, 1420–1435.
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633.
- Blüher, M. Adipose tissue inflammation: A cause or consequence of obesity-related insulin resistance? Clin. Sci. 2016, 130, 1603–1614.
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91.
- Lumeng, C.N.; Delproposto, J.B.; Westcott, D.J.; Saltiel, A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008, 57, 3239–3246.
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920.
- Winer, D.A.; Winer, S.; Shen, L.; Wadia, P.P.; Yantha, J.; Paltser, G.; Tsui, H.; Wu, P.; Davidson, M.G.; Alonso, M.N.; et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011, 17, 610–617.
- Wu, D.; Molofsky, A.B.; Liang, H.E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011, 332, 243–247.
- Lumeng, C.N.; Liu, J.; Geletka, L.; Delaney, C.; Delproposto, J.; Desai, A.; Oatmen, K.; Martinez-Santibanez, G.; Julius, A.; Garg, S.; et al. Aging Is Associated with an Increase in T Cells and Inflammatory Macrophages in Visceral Adipose Tissue. J. Immunol. 2011, 187, 6208–6216.
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000.
- Glass, C.K.; Olefsky, J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012, 15, 635–645.
- Feinstein, R.; Kanety, H.; Papa, M.Z.; Lunenfeld, B.; Karasik, A. Tumor necrosis factor-α suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J. Biol. Chem. 1993, 268, 26055–26058.
- Müller, T.D.; Clemmensen, C.; Finan, B.; Dimarchi, R.D.; Tschöp, M.H. Anti-obesity therapy: From rainbow pills to polyagonists. Pharmacol. Rev. 2018, 70, 712–746.
- Coulter, A.A.; Rebello, C.J.; Greenway, F.L. Centrally Acting Agents for Obesity: Past, Present, and Future. Drugs 2018, 78, 1113–1132.
- Hanssen, M.J.W.; Hoeks, J.; Brans, B.; Van Der Lans, A.A.J.J.; Schaart, G.; Van Den Driessche, J.J.; Jörgensen, J.A.; Boekschoten, M.V.; Hesselink, M.K.C.; Havekes, B.; et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015, 21, 863–865.
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408.
- Ameka, M.; Markan, K.R.; Morgan, D.A.; BonDurant, L.D.; Idiga, S.O.; Naber, M.C.; Zhu, Z.; Zingman, L.V.; Grobe, J.L.; Rahmouni, K.; et al. Liver Derived FGF21 Maintains Core Body Temperature During Acute Cold Exposure. Sci. Rep. 2019, 9, 630.
- Chartoumpekis, D.V.; Habeos, I.G.; Ziros, P.G.; Psyrogiannis, A.I.; Kyriazopoulou, V.E.; Papavassiliou, A.G. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol. Med. 2011, 17, 736–740.
- Huang, Z.; Zhong, L.; Lee, J.T.H.; Zhang, J.; Wu, D.; Geng, L.; Wang, Y.; Wong, C.M.; Xu, A. The FGF21-CCL11 Axis Mediates Beiging of White Adipose Tissues by Coupling Sympathetic Nervous System to Type 2 Immunity. Cell Metab. 2017, 26, 493–508.
- BonDurant, L.D.; Ameka, M.; Naber, M.C.; Markan, K.R.; Idiga, S.O.; Acevedo, M.R.; Walsh, S.A.; Ornitz, D.M.; Potthoff, M.J. FGF21 Regulates Metabolism Through Adipose-Dependent and -Independent Mechanisms. Cell Metab. 2017, 25, 935–944.
- Hanssen, M.J.W.; Broeders, E.; Samms, R.J.; Vosselman, M.J.; Van Der Lans, A.A.J.J.; Cheng, C.C.; Adams, A.C.; Van Marken Lichtenbelt, W.D.; Schrauwen, P. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci. Rep. 2015, 5, 1–8.
- Bina, H.A.; Kharitonenkov, A.; Antonellis, P.J.; Flier, J.S.; Maratos-Flier, E.; Fisher, F.M.; Chui, P.C.; Lin, Z.; Tian, H.; Lam, K.S.S.L.L.; et al. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2008, 20, 670–677.
- Shamsi, F.; Xue, R.; Huang, T.L.; Lundh, M.; Liu, Y.; Leiria, L.O.; Lynes, M.D.; Kempf, E.; Wang, C.H.; Sugimoto, S.; et al. FGF6 and FGF9 regulate UCP1 expression independent of brown adipogenesis. Nat. Commun. 2020, 11, 1–16.
- Bank, H.V.; Hurtado-Thiele, M.; Oshimura, N.; Simcox, J. Mitochondrial Lipid Signaling and Adaptive Thermogenesis. Metabolites 2021, 11, 124.
- Xu, L.; Zhao, W.; Wang, D.; Ma, X. Chinese medicine in the battle against obesity and metabolic diseases. Front. Physiol. 2018, 9, 850.
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717.
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharmacother. 2021, 133, 110984.
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 1–15.
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 1–18.
- El Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Food ingredients involved in white-to-brown adipose tissue conversion and in calorie burning. Front. Physiol. 2019, 10, 1954.
- Ohyama, K.; Nogusa, Y.; Shinoda, K.; Suzuki, K.; Bannai, M.; Kajimura, S. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes 2016, 65, 1410–1423.
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92.
- Akbari, M.; Lankarani, K.B.; Tabrizi, R.; Ghayour-Mobarhan, M.; Peymani, P.; Ferns, G.; Ghaderi, A.; Asemi, Z. The effects of curcumin on weight loss among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2019, 10, 649.
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202.
- Wang, S.; Wang, X.; Ye, Z.; Xu, C.; Zhang, M.; Ruan, B.; Wei, M.; Jiang, Y.; Zhang, Y.; Wang, L.; et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun. 2015, 466, 247–253.
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009, 139, 919–925.
- Okla, M.; Kim, J.; Koehler, K.; Chungn, S. Dietary factors promoting brown and beige fat development and thermogenesis. Adv. Nutr. 2017, 8, 473–483.
- Kajimoto, O.; Kajimoto, Y.; Yabune, M.; Nakamura, T.; Kotani, K.; Suzuki, Y.; Nozawa, A.; Nagata, K.; Unno, T.; Yuko, M.S.; et al. Tea catechins with a galloyl moiety reduce body weight and fat. J. Heal. Sci. 2005, 51, 161–171.
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187.
- Zhang, Y.; Yu, Y.; Li, X.; Meguro, S.; Hayashi, S.; Katashima, M.; Yasumasu, T.; Wang, J.; Li, K. Effects of catechin-enriched green tea beverage on visceral fat loss in adults with a high proportion of visceral fat: A double-blind, placebo-controlled, randomized trial. J. Funct. Foods 2012, 4, 315–322.
- Neyrinck, A.M.; Bindels, L.B.; Geurts, L.; Van Hul, M.; Cani, P.D.; Delzenne, N.M. A polyphenolic extract from green tea leaves activates fat browning in high-fat-diet-induced obese mice. J. Nutr. Biochem. 2017, 49, 15–21.
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045.
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91.
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122.
- Fischer-Posovszky, P.; Kukulus, V.; Tews, D.; Unterkircher, T.; Debatin, K.M.; Fulda, S.; Wabitsch, M. Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am. J. Clin. Nutr. 2010, 127, 1109–1122.
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 2015, 39, 967–976.
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Van De Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622.
- Yoshida, T.; Sakane, N.; Wakabayashi, Y.; Umekawa, T.; Kondo, M. Anti-obesity and anti-diabetic effects of CL 316, 243, a highly specific β3-adrenoceptor agonist, in yellow KK mice. Life Sci. 1994, 54, 491–498.
- Xiao, C.; Goldgof, M.; Gavrilova, O.; Reitman, M.L. Anti-obesity and metabolic efficacy of the β3-adrenergic agonist, CL316243, in mice at thermoneutrality compared to 22 °C. Obesity 2015, 23, 1450–1459.
- Inokuma, K.I.; Okamatsu-Ogura, Y.; Omachi, A.; Matsushita, Y.; Kimura, K.; Yamashita, H.; Saito, M. Indispensable role of mitochondrial UCP1 for antiobesity effect of β3-adrenergic stimulation. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1014–E1021.
- Arch, J.R.S. Challenges in β3-adrenoceptor agonist drug development. Ther. Adv. Endocrinol. Metab. 2011.
- Clapham, J.C.; Arch, J.R.S. Thermogenic and metabolic antiobesity drugs: Rationale and opportunities. Diabetes Obes. Metab. 2007, 9, 259–275.
- Baskin, A.S.; Linderman, J.D.; Brychta, R.J.; McGehee, S.; Anflick-Chames, E.; Cero, C.; Johnson, J.W.; O’Mara, A.E.; Fletcher, L.A.; Leitner, B.P.; et al. Regulation of human adipose tissue activation, gallbladder size, and bile acid metabolism by A b3-adrenergic receptor agonist. Diabetes 2018, 67, 2113–2125.
- Finlin, B.S.; Memetimin, H.; Zhu, B.; Confides, A.L.; Vekaria, H.J.; El Khouli, R.H.; Johnson, Z.R.; Westgate, P.M.; Chen, J.; Morris, A.J.; et al. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Investig. 2020, 130.
- Blondin, D.P.; Nielsen, S.; Kuipers, E.N.; Severinsen, M.C.; Jensen, V.H.; Miard, S.; Jespersen, N.Z.; Kooijman, S.; Boon, M.R.; Fortin, M.; et al. Human Brown Adipocyte Thermogenesis Is Driven by β2-AR Stimulation. Cell Metab. 2020, 32, 287–300.
- González-García, I.; Milbank, E.; Diéguez, C.; López, M.; Contreras, C. Glucagon, GLP-1 and thermogenesis. Int. J. Mol. Sci. 2019, 20, 3445.
- Lockie, S.H.; Heppner, K.M.; Chaudhary, N.; Chabenne, J.R.; Morgan, D.A.; Veyrat-Durebex, C.; Ananthakrishnan, G.; Rohner-Jeanrenaud, F.; Drucker, D.J.; DiMarchi, R.; et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 2012, 61, 2753–2762.
- López, M.; Diéguez, C.; Nogueiras, R. Hypothalamic GLP-1: The control of BAT thermogenesis and browning of white fat. Adipocyte 2015, 4, 141–145.
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Fernø, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358.
- Goswami, R.; Subramanian, G.; Silayeva, L.; Newkirk, I.; Doctor, D.; Chawla, K.; Chattopadhyay, S.; Chandra, D.; Chilukuri, N.; Betapudi, V. Gene therapy leaves a vicious cycle. Front. Oncol. 2019, 9, 297.
- Samuelson, I.; Vidal-Puig, A. Studying Brown Adipose Tissue in a Human in vitro Context. Front. Endocrinol. 2020, 11, 629.
- Chen, Y.; Liu, Y.; Zhang, J.; Liu, H.; Wang, J.; Liu, Q.; Zhang, Y. Three-dimensional bioprinting adipose tissue and mammary Organoids feasible for artificial breast structure regeneration. Mater. Des. 2021.
More
