Breast cancer (BC) is the most frequent cancer among women and represents the second leading cause of cancer-specific death. A subset of patients with metastatic breast cancer (MBC) presents limited disease, termed ‘oligometastatic’ breast cancer (OMBC). The oligometastatic disease can be managed with different treatment strategies to achieve long-term remission and eventually cure. Several approaches are possible to cure the oligometastatic disease: locoregional treatments of the primary tumor and of all the metastatic sites, such as surgery and radiotherapy; systemic treatment, including target-therapy or immunotherapy, according to the biological status of the primary tumor and/or of the metastases; or the combination of these approaches. Encouraging results involve local ablative options, but these trials are limited by being retrospective and affected by selection bias. Systemic therapy, e.g., the use of CDK4/6 inhibitors for hormone receptor-positive (HR+)/HER-2 negative BC, leads to an increase of progression-free survival (PFS) and overall survival (OS) in all the subgroups, with favorable toxicity. Regardless of the lack of substantial data, this subset of patients could be treated with curative intent; the appropriate candidates could be mostly young women, for whom a multidisciplinary aggressive approach appears suitable.
- oligometastatic breast cancer
- locoregional therapy
- CDK4/6 inhibitors
- multidisciplinary
1. Introduction
2. Options for Treatment of Oligometastatic Breast Cancer
The oligometastatic disease can be managed with different treatment strategies to achieve long-term remission and eventually cure. In Figure 1 a flow chart of treatment options is presented.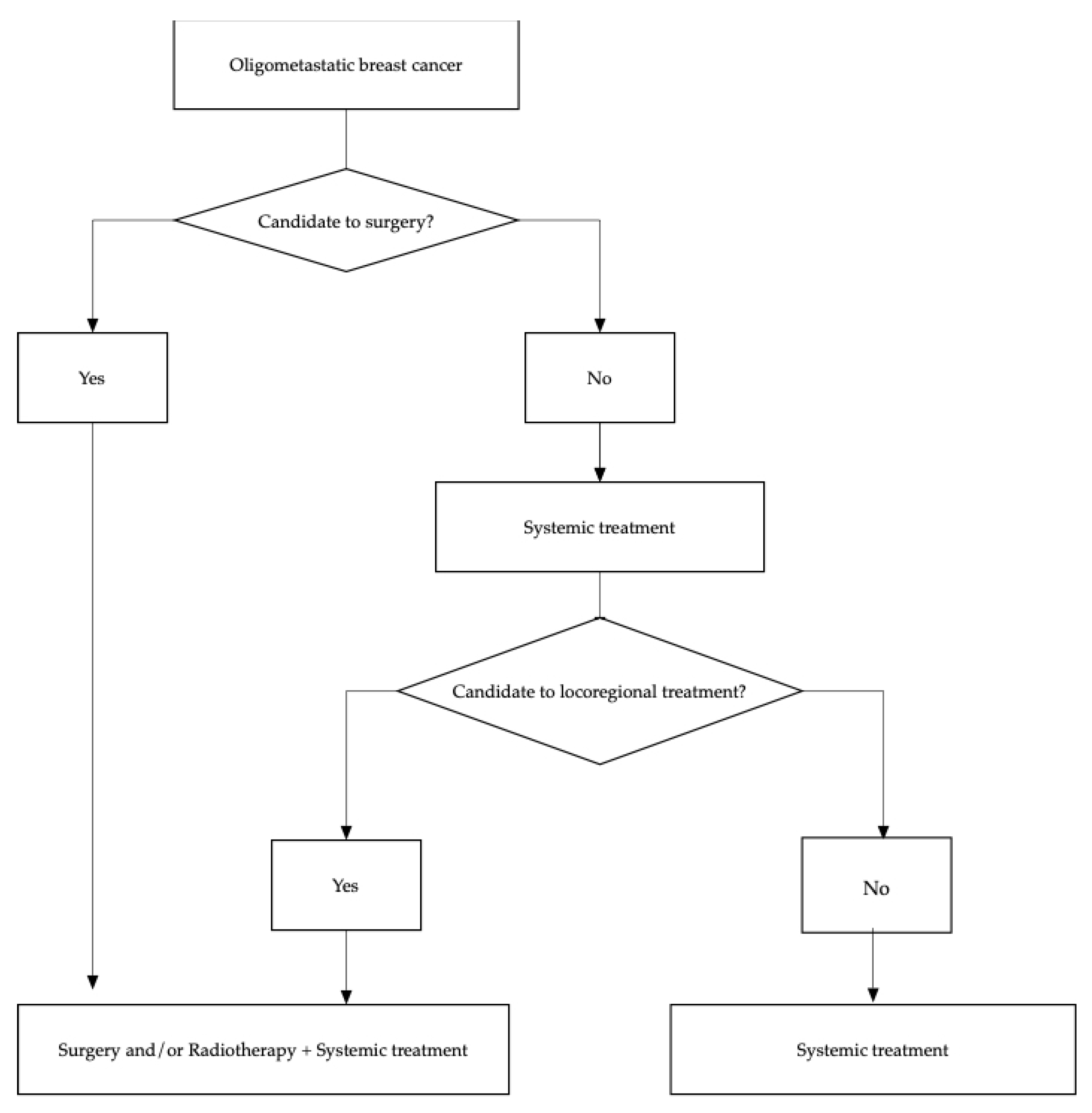 Figure 1. Diagram flow of therapeutic options in oligometastatic breast cancer.
Figure 1. Diagram flow of therapeutic options in oligometastatic breast cancer.2.1. Surgery
| Trial | Number of Patients | Site of Metastases | Biological Subtype | Site of Surgery |
Outcome |
|---|---|---|---|---|---|
| Tata Memorial, NCT00193778 |
350 | Bone and/or visceral | HR+ /HER2− HR+/HER2+ |
|
2.3. Systemic Treatments
Systemic treatment remains a milestone in the management of metastatic breast cancer. Considering hormone receptor (HR) positive, HER2-negative metastatic breast cancers, certainly CDK4/6 inhibitors in combination with endocrine therapy have changed the paradigm of the treatment [22]. Concerns about the difference among the three CDK4/6 inhibitors involve the significant OS improvement, demonstrated from MONALEESA-3, MONALEESA-7, and MONARCH-2 trials, but not reported in PALOMA-1, PALOMA-3, and MONALEESA-2 trials [23][24][25][26][27][28]. As a result, a meta-analysis of all these randomized controlled trials evaluated the OS improvement among Palbociclib, Ribociclib and Abemaciclib, and focused on the efficacy of these compounds in some relevant subgroups of patients. Of 5862 patients from MONALEESA-2, MONALEESA-3, and MONALEESA-7 trials, 2429 presented visceral (lung or liver) disease, 929 had bone-only disease, and 2504 had visceral and bone disease. Of 2845 patients, grouped by the number of metastases, 782 had only one metastatic site, 635 two, and 1428 three or more. The pooled results of the meta-analysis showed no heterogeneity for all these subgroups, with a statistically significant improvement in PFS with a similar hazard ratio [29]. Therefore, this meta-analysis demonstrates that CDK4/6 inhibitors plus endocrine therapy are beneficial in terms of PFS, regardless of the presence of visceral metastases, the number of metastatic sites, and the length of the treatment-free interval. Consequently, the pooled estimate for the overall population is also feasible for OMBC patients [29].2.4. Combination of Radiotherapy and Systemic Treatment
| Trial | Number of Patients | CDK4/6-I | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hans et al. | 5 | Palbociclib | 5 pain relief 1 stable disease |
| 1. No differences in OS 2. Better locoregional PFS FOR surgery 3. Worse DPFS for surgery |
|||||
| MF0701, NCT00557986 |
274 | Bone and/or lung and/or liver | HR+ 85.5% HER2+ 30.4% TN 7.3% |
| ||||||
| Meattini et al. |
|
| 1. Increase in median survival for surgery upfront 2. Superior survival for locoregional treatment in women with luminal tumors, age < 55 years, and solitary bone metastases |
|||||||
| 5 | Ribociclib | 3 stable disease | 2 partial response |
ECOG-ACRIN E 2108, NCT01242800 |
258 | Bone and/or any organ system, including CNS |
HR+/HER2− 60% HER2+ 26% TN 15% |
|||
| Chowdary et al. |
|
|
16 |
| Palbociclib | 16 pain relief 0 local failures | 1. No difference in OS and PFS 2. Possible detrimental effect of locoregional treatment in TN mBC 3. Increase of 2.5x risk of locoregional progression in patients who received systemic therapy without locoregional treatment |
|||
| TBCRC 013, NCT00941759 |
127 | Bone and/or any organ system, including CNS |
HR+/HER2– HR+/HER2+ HR−/HER2+ HR−/HER2− |
| 1. No improvement of PFS and OS for surgery in patients who have responded to first-line treatment |
2.2. Radiotherapy
| Ippolito et al. | |||
| 16 | Palbociclib | Ribociclib |
16 pain relief 2 complete responses 2 partial responses 1 stable disease |
| Mudit et al. | 16 | Palbociclib | 16 pain relief 0 local failures |
| Guerini et al. | 18 | Palbociclib Ribociclib Abemaciclib |
16 pain relief 0 pain recurrence 17 local control 1 local recurrence |
3. Conclusions
References
- Henry, N.L.; Shah, P.D.; Haider, I.; Freer, P.E.; Jagsi, R.; Sabel, M.S. Cancer of the Breast. Abeloff’s Clin. Oncol. 2020, 12, 1560–1603.
- O’Shaughnessy, J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncology 2005, 10, 20–29.
- Makhlin, I.; Fox, K. Oligometastatic Breast Cancer: Is This a Curable Entity? A Contemporary Review of the Literature. Curr. Oncol. Rep. 2020, 22, 1–10.
- Al-Shafa, F.; Arifin, A.J.; Rodrigues, G.B.; Palma, D.A.; Louie, A.V. A Review of Ongoing Trials of Stereotactic Ablative Radiotherapy for Oligometastatic Cancers: Where Will the Evidence Lead? Front. Oncol. 2019, 9, 543.
- Palma, D.A.; Louie, A.V.; Rodrigues, G.B. New Strategies in Stereotactic Radiotherapy for Oligometastases. Clin. Cancer Res. 2015, 21, 5198–5204.
- Huang, F.; Wu, G.; Yang, K. Oligometastasis and oligo-recurrence. Radiat. Oncol. 2014, 9, 230.
- Pagani, O.; Senkus, E.; Wood, W.; Colleoni, M.; Cufer, T.; Kyriakides, S.; Costa, A.; Winer, E.P.; Cardoso, F.; Force, E.-M.T. International guidelines for management of metastatic breast cancer: Can metastatic breast cancer be cured? J. Natl. Cancer Inst. 2010, 102, 456–463.
- Cardoso, F.; Costa, A.; Senkus, E.; Aapro, M.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.; Biganzoli, L.; Cardoso, M.J.; et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3). Ann. Oncol. 2017, 28, 16–33.
- Jain, S.K.; Dorn, P.L.; Chmura, S.J.; Weichselbaum, R.R.; Hasan, Y. Incidence and implications of oligometastatic breast cancer. J. Clin. Oncol. 2012, 30, e11512.
- Niibe, Y.; Hayakawa, K. Oligometastases and Oligo-recurrence: The New Era of Cancer Therapy. Jpn. J. Clin. Oncol. 2010, 40, 107–111.
- Correa, R.J.M.; Salama, J.K.; Milano, M.T.; Palma, D.A. Stereotactic body radiotherapy for oligometastasis opportunities for biology to guide clinical management. Cancer J. 2016, 22, 247–256.
- Reyes, D.K.; Pienta, K.J. The biology and treatment of oligometastatic cancer. Oncotarget 2015, 6, 8491–8524.
- Westphal, T.; Gampenrieder, S.P.; Rinnerthaler, G.; Greil, R. Cure in metastatic breast cancer. Memo Mag. Eur. Med. Oncol. 2018, 11, 172–179.
- Divisi, D.; Barone, M.; Zaccagna, G.; Gabriele, F.; Crisci, R. Surgical approach in the oligometastatic patient. Ann. Transl. Med. 2018, 6, 94.
- Criscitiello, C.; Giuliano, M.; Curigliano, G.; Laurentiis, M.D.; Arpino, G.; Carlomagno, N.; Placido, S.D.; Golshan, M.; Santangelo, M. Surgery of the primary tumor in de novo metastatic breast cancer: To do or not to do? Eur. J. Surg. Oncol. 2015, 41, 1288–1292.
- Harris, E.; Barry, M.; Kell, M.R. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann. Surg. Oncol. 2013, 20, 2828–2834.
- Matsushita, H.; Jingu, K.; Umezawa, R.; Yamamoto, T.; Ishikawa, Y.; Takahashi, N.; Katagiri, Y.; Kadoya, N. Stereotactic Radiotherapy for Oligometastases in Lymph Nodes—A Review. Technol. Cancer Res. Treat. 2018, 17, 1–8.
- National Comprehensive Cancer Network. Central Nervous System Cancers (Version 2.2018). Available online: (accessed on 21 February 2019).
- Gondi, V.; Hermann, B.P.; Mehta, M.P.; Tomé, W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 348–354.
- Li, J.; Bentzen, S.M.; Renschler, M.; Mehta, M.P. Regression after Whole-Brain Radiation Therapy for Brain Metastases Correlates with Survival and Improved Neurocognitive Function. J. Clin. Oncol. 2007, 25, 1260–1266.
- Ashworth, A.; Rodrigues, G.; Boldt, G.; Palma, D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013, 82, 197–203.
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Jahanzeb, M.; et al. Endocrine Therapy for Hormone Receptor–Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103.
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936.
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letro-zole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547.
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316.
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524.
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The E_ect of Abemaciclib plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2019, 6, 116–124.
- Finn, R.S.; Boer, K.; Bondarenko, I.; Patel, R.; Pinter, T.; Schmidt, M.; Shparyk, Y.V.; Thummala, A.; Voitko, N.; Bananis, E.; et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res. Treat. 2020, 183, 419–428.
- Rossi, V.; Berchialla, P.; Giannarelli, D.; Nisticò, C.; Ferretti, G.; Gasparro, S.; Russillo, M.; Catania, G.; Vigna, L.; Mancusi, R.L.; et al. Should All Patients with HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor as First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers 2019, 26, 1661.
- Chowdhary, M.; Sen, N.; Chowdhary, A.; Usha, L.; Cobleigh, M.A.; Wang, D.; Patel, K.R.; Barry, P.N.; Rao, R.D. Safety and Efficacy of Palbociclib and Radiation Therapy in Patients with Metastatic Breast Cancer: Initial Results of a Novel Combination. Adv. Radiat. Oncol. 2019, 4, 453–457.
- Huang, C.-Y.; Hsieh, F.-S.; Wang, C.-Y.; Chen, L.-J.; Chang, S.-S.; Tsai, M.-H.; Hung, M.-H.; Kuo, C.-W.; Shih, C.-T.; Chao, T.-I.; et al. Palbociclib enhances radiosensitivity of hepatocellular carcinoma and cholangiocarcinoma via inhibiting ataxia telangiectasia–mutated kinase–mediated DNA damage response. Eur. J. Cancer 2018, 102, 10–22.
- Kawamoto, T.; Shikama, N.; Sasai, K. Severe acute radiation-induced enterocolitis after combined palbociclib and palliative radiotherapy treatment. Radiother. Oncol. 2019, 131, 240–241.
- Messer, J.A.; Ekinci, E.; Patel, T.A.; Teh, B.S. Enhanced dermatologic toxicity following concurrent treatment with palbociclib and radiation therapy: A case report. Rep. Pract. Oncol. Radiother. 2019, 24, 276–280.
- Kwapisz, D. Oligometastatic breast cancer. Breast Cancer 2018, 26, 138–146.
- Kent, C.L.; McDuff, S.G.R.; Salama, J.K. Oligometastatic breast cancer: Where are we now and where are we headed?—A narrative review. Ann. Palliat. Med. 2021, 10, 5954–5968.
