Polyphenolic molecules can be found in different stages during the brewing process and react with proteins: during wort boiling, they form the hot break; during cooling, they form the cold break; and during post-fermentation, they are involved in the formation of chill haze and permanent hazes and facilitate the removal of undesirable compounds with filtration. However, they tend to react with proteins in packaged beer and form undesirable haze after the expiration date. As mentioned before, polyphenols can enter beer from hop and malt.
- bitterness
- beer
- aftertaste
- off-flavor
1. Introduction
Flavor stability is, besides colloidal stability, one of the most important indicators of beer quality. Some types of beer (i.e., Indian Pale Ale) are bound to contain strong hoppy notes and bitterness originating from polyphenols in hops or barley. However, the emergence of undesirable notes can be a result of a faulty lead brewing process, whether due to the over-dosage of hop (acids), low temperatures while dry hopping, polyphenols, or simply staling. The ongoing craft beer boom has aimed to present different types of beer to the broader public, and this includes hoppy and bitter beers (mostly ales) that differ from the industrial lagers by increased bitterness [1].
Flavor stability is, besides colloidal stability, one of the most important indicators of beer quality. Some types of beer (i.e., Indian Pale Ale) are bound to contain strong hoppy notes and bitterness originating from polyphenols in hops or barley. However, the emergence of undesirable notes can be a result of a faulty lead brewing process, whether due to the over-dosage of hop (acids), low temperatures while dry hopping, polyphenols, or simply staling. The ongoing craft beer boom has aimed to present different types of beer to the broader public, and this includes hoppy and bitter beers (mostly ales) that differ from the industrial lagers by increased bitterness [2].
As such, polyphenols can provide both bitterness and astringency, depending on their degree of isomerization [2]. The consumers’ perception of bitterness depends on different factors and is very complex. The range of molecules that elicit bitter responses is very wide [3][4]; for example, the reactivity of flavanols with metal ions and molecular oxygen results in flavanol polymers.
As such, polyphenols can provide both bitterness and astringency, depending on their degree of isomerization [4]. The consumers’ perception of bitterness depends on different factors and is very complex. The range of molecules that elicit bitter responses is very wide [5,6]; for example, the reactivity of flavanols with metal ions and molecular oxygen results in flavanol polymers.
Astringency, on the other hand, is much easier to explain. It is described as a mouthfeel characterized by a drying, puckering, or rough sensation across the oral cavity, especially on the tongue [5]. It occurs when large molecular weight polyphenols react with proline-rich proteins found in saliva. Upon their interaction, they precipitate onto the surface of the mouth, which leads to the feeling of a coating dryness [6].
Astringency, on the other hand, is much easier to explain. It is described as a mouthfeel characterized by a drying, puckering, or rough sensation across the oral cavity, especially on the tongue [7]. It occurs when large molecular weight polyphenols react with proline-rich proteins found in saliva. Upon their interaction, they precipitate onto the surface of the mouth, which leads to the feeling of a coating dryness [8].
[7][8], and flavor is comprised of four different components: odor, aroma, taste, and mouthfeel. By definition, the odor is the perception of volatiles by the olfactory mucous membrane in the nasal cavity, after sniffing through the nose and entering the nasal passage. Aroma is also connected with volatile compounds, which volatilize by body heat after putting the beverage in the mouth. Soluble substances in the mouth recognized by the receptors located on the surface of the tongue form the reception of taste [8][9][10].
[10,11], and flavor is comprised of four different components: odor, aroma, taste, and mouthfeel. By definition, the odor is the perception of volatiles by the olfactory mucous membrane in the nasal cavity, after sniffing through the nose and entering the nasal passage. Aroma is also connected with volatile compounds, which volatilize by body heat after putting the beverage in the mouth. Soluble substances in the mouth recognized by the receptors located on the surface of the tongue form the reception of taste [11,12,13].
Mouthfeel is a sensation of the haptic perception of the food product on the surface of the oral cavity (the sparkling of carbon dioxide, the oiliness of fats, and astringency) [9][11][12][13]. All of these components intertwine and sum up the perceived flavor as a result of complex reactions between the senses, as can be seen from
Mouthfeel is a sensation of the haptic perception of the food product on the surface of the oral cavity (the sparkling of carbon dioxide, the oiliness of fats, and astringency) [12,14,15,16]. All of these components intertwine and sum up the perceived flavor as a result of complex reactions between the senses, as can be seen from
Figure 1. A good example is when beers with higher CO2content taste more sour but less astringent.
2. A good example is when beers with higher CO2content taste more sour but less astringent.
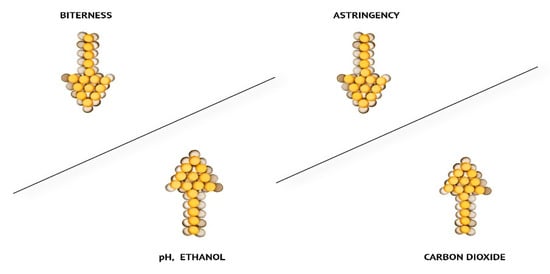
Figure 1. Changes in taste perception (higher levels of CO2 in beer decrease astringency; higher C2H5OH concentration and higher beer pH increase the bitterness perception) [12].
2. Raw Materials and Polyphenols
Even though beer is produced from four basic components, water, malt, hops, and yeast, phenolic compounds in beer commonly originate from hops (30%), malt (70%) [14], or can form during chemical transformations in the brewing process.
Even though beer is produced from four basic components, water, malt, hops, and yeast, phenolic compounds in beer commonly originate from hops (30%), malt (70%) [17], or can form during chemical transformations in the brewing process.
Phenolic acids are not as responsible for beer flavor, as they influence flavor precursors. Bottom-fermented light beers do not withstand heavy phenolic notes, which are, in this case, noted as off-flavor. Top-fermented beers, such as German Weizen or Belgian white beers, are designated by the volatile monophenols [15][16][17]. However, raw material also represents an important parameter [18].
Phenolic acids are not as responsible for beer flavor, as they influence flavor precursors. Bottom-fermented light beers do not withstand heavy phenolic notes, which are, in this case, noted as off-flavor. Top-fermented beers, such as German Weizen or Belgian white beers, are designated by the volatile monophenols [18,19,20]. However, raw material also represents an important parameter [21].
According to [19][20], phenolic compounds quantified in beer are somewhat higher than in white wine and lower than in red wine. This inconsistency can be attributed to the varying quality of raw materials, yeast strains, and brewing process parameters [18].
According to [22,23], phenolic compounds quantified in beer are somewhat higher than in white wine and lower than in red wine. This inconsistency can be attributed to the varying quality of raw materials, yeast strains, and brewing process parameters [21].
Common phenolic compounds that can be found in barley (in free, esterified, or bound form) are benzoic and cinnamic acid derivatives, proanthocyanidins, quinines, flavonols, chalcones, flavones, flavanones, and amino phenolic compounds [21][22][23][24][25][26][27][28][29][30]. Ferulic and p-coumaric acid are the low-molecular weight (LMW) phenolic acids in barley grains. They can be detected in the husk, pericarp, testa, and aleurone, and even in the endosperm matrix. Phenolic acids such as vanillic, sinapinic, and p-hydroxybenzoic acids [23][28][31][32][33][34]; flavan-3-ols from monomers ((+)-catechin and (−)-epicatechin), dimers (prodelphinidin B3 and procyanidin B3), and trimers (procyanidin C2); and higher-molecular weight flavonoid-derived tannins are common in barley as well [23][35][36].
Common phenolic compounds that can be found in barley (in free, esterified, or bound form) are benzoic and cinnamic acid derivatives, proanthocyanidins, quinines, flavonols, chalcones, flavones, flavanones, and amino phenolic compounds [24,25,26,27,28,29,30,31,32,33]. Ferulic and p-coumaric acid are the low-molecular weight (LMW) phenolic acids in barley grains. They can be detected in the husk, pericarp, testa, and aleurone, and even in the endosperm matrix. Phenolic acids such as vanillic, sinapinic, and p-hydroxybenzoic acids [26,31,34,35,36,37]; flavan-3-ols from monomers ((+)-catechin and (−)-epicatechin), dimers (prodelphinidin B3 and procyanidin B3), and trimers (procyanidin C2); and higher-molecular weight flavonoid-derived tannins are common in barley as well [26,38,39].
Polyphenols are secondary plant metabolites and play an important role as protective agents. There are many classes of polyphenols, but only prenylflavonoids are characteristically present in the hop plant. Bitter acids (multifidol glucosides) are also indigenous to hop plants [37]. The most common classes are presented in the following sections adapted from an extensive review by Knez Hrnčič et al.
Polyphenols are secondary plant metabolites and play an important role as protective agents. There are many classes of polyphenols, but only prenylflavonoids are characteristically present in the hop plant. Bitter acids (multifidol glucosides) are also indigenous to hop plants [40]. The most common classes are presented in the following sections adapted from an extensive review by Knez Hrnčič et al.
Prenylflavonoids represent a class of flavonoids with at least one prenyl or geranyl substituent in the ring [38]. It is presumed that the desmethylxanthohumol represents a precursor for the majority of flavonoids in hops [39]. It has been studied as an anticancer agent, but poses many other positive properties against pathogenic fungi, malaria, and HIV-1 viruses [40]. Another chemical compound with anticarcinogenic properties belonging to this group is 8-prenylnaringenin [41].
Prenylflavonoids represent a class of flavonoids with at least one prenyl or geranyl substituent in the ring [41]. It is presumed that the desmethylxanthohumol represents a precursor for the majority of flavonoids in hops [42]. It has been studied as an anticancer agent, but poses many other positive properties against pathogenic fungi, malaria, and HIV-1 viruses [43]. Another chemical compound with anticarcinogenic properties belonging to this group is 8-prenylnaringenin [45].
)-catechin is the third most abundant compound in hop cones possessing antioxidative and vasodilative features [40]. Flavanol(−)-epicatechin and (+)-gallocatechin can be found in hops as well [42]. Flavanols catechin and epicatechin show antioxidative and anti-inflammatory properties [43]. A mixture of hop proanthocyanidins shows more pronounced antioxidative properties than individual flavanols and proanthocyanidins [40].
)-catechin is the third most abundant compound in hop cones possessing antioxidative and vasodilative features [43]. Flavanol(−)-epicatechin and (+)-gallocatechin can be found in hops as well [46]. Flavanols catechin and epicatechin show antioxidative and anti-inflammatory properties [47]. A mixture of hop proanthocyanidins shows more pronounced antioxidative properties than individual flavanols and proanthocyanidins [43].
Quercetin and kaempferol are the most mentioned antioxidant flavanols that can be found in fruits and vegetables, but also in hops [40][42]. Plants usually contain flavonols in the form of glycosides [44], which, in the case of quercetin, presents its bioavailable form, and the least bioavailable form of quercetin is rutin, the most common form of quercetin in hops It is widely recognized for its anti-aging properties [45]. Both, quercetin and kaempferol can inhibit the growth of various cancer cells [46].
Quercetin and kaempferol are the most mentioned antioxidant flavanols that can be found in fruits and vegetables, but also in hops [43,46]. Plants usually contain flavonols in the form of glycosides [48], which, in the case of quercetin, presents its bioavailable form, and the least bioavailable form of quercetin is rutin, the most common form of quercetin in hops It is widely recognized for its anti-aging properties [49]. Both, quercetin and kaempferol can inhibit the growth of various cancer cells [50].
1-(2-methyl propanoyl)phloroglucinol-glucopyranoside, multifidol glucosides, 1-(3-methyl butyryl) phloroglucinol, and 5-(2 Methylpropanoyl) phloroglucinol are constituents of hops. Authors [47] have investigated the human recognition threshold concentrations and the lowest recognitions levels for 11 bitter tastants from the hop hard resin fraction, and the lowest concentration was determined for co-multifidol glucopyranoside (5 μmol/L) showing that minor hop compounds may act as significant taste-carriers [47].
1-(2-methyl propanoyl)phloroglucinol-glucopyranoside, multifidol glucosides, 1-(3-methyl butyryl) phloroglucinol, and 5-(2 Methylpropanoyl) phloroglucinol are constituents of hops. Authors [51] have investigated the human recognition threshold concentrations and the lowest recognitions levels for 11 bitter tastants from the hop hard resin fraction, and the lowest concentration was determined for co-multifidol glucopyranoside (5 μmol/L) showing that minor hop compounds may act as significant taste-carriers [51].
Ferulic acid from hops is designated as a highly antioxidative polyphenolic compound that prevents lipid peroxidation, apoptotic cell death of healthy cells, and acts as a free radical scavenger [42][48]. It is important for the brewing industry because it retards the degradation of iso-α-acids and actively prevents beer spoilage [49]. Ferulic acid has many properties that enhance the toxicity of certain chemicals, carcinogenic agents, and ionizing radiation. Resveratrol is reportedly an anti-inflammatory and anticancer agent, and also acts preventively on cardiovascular diseases development [50][51].
Ferulic acid from hops is designated as a highly antioxidative polyphenolic compound that prevents lipid peroxidation, apoptotic cell death of healthy cells, and acts as a free radical scavenger [46,52]. It is important for the brewing industry because it retards the degradation of iso-α-acids and actively prevents beer spoilage [53]. Ferulic acid has many properties that enhance the toxicity of certain chemicals, carcinogenic agents, and ionizing radiation. Resveratrol is reportedly an anti-inflammatory and anticancer agent, and also acts preventively on cardiovascular diseases development [55,56].
The malting process itself induces modifications in the composition of barley grain including the degradation of endogenous phenolic compounds [23][29][32][52][53][54][55]. According to several authors, the concentration of phenolic compounds in malt can be higher than in barley. However, the main groups remain the same, which indicates that the extraction of flavonoids and phenolic acids in malt is more pronounced [28][29][32]. Behavior of polyphenols after malting and brewing is described in the sections below.
The malting process itself induces modifications in the composition of barley grain including the degradation of endogenous phenolic compounds [26,32,35,57,58,59,60]. According to several authors, the concentration of phenolic compounds in malt can be higher than in barley. However, the main groups remain the same, which indicates that the extraction of flavonoids and phenolic acids in malt is more pronounced [31,32,35]. Behavior of polyphenols after malting and brewing is described in the sections below.
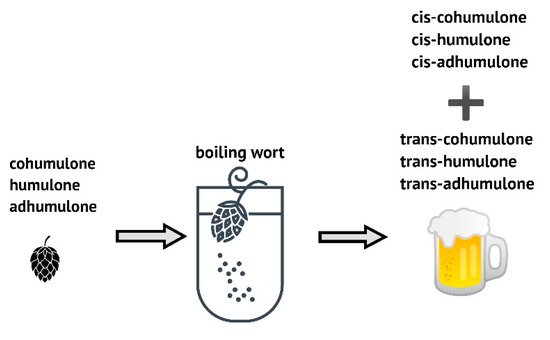
Hops are used as a spice in the brewing process and only 200–600 g/hL of beer. Many hop varieties contribute to beer with two main characteristics: bitterness and aromas. Among many different compounds, resins and essential oils are the most important compounds for the brewing industry, but phenolic compounds, important for the flavor of the finished beer, are significant as well [56]. The α-acids get extracted during wort boiling when they undergo oxidative isomerization to iso-α-acids (iso-humulones), also recognized as bitter compounds of beer (
Hops are used as a spice in the brewing process and only 200–600 g/hL of beer. Many hop varieties contribute to beer with two main characteristics: bitterness and aromas. Among many different compounds, resins and essential oils are the most important compounds for the brewing industry, but phenolic compounds, important for the flavor of the finished beer, are significant as well [61]. The α-acids get extracted during wort boiling when they undergo oxidative isomerization to iso-α-acids (iso-humulones), also recognized as bitter compounds of beer (
Figure 2).
3).
Beer contains from 20–60 mg/L of iso-humulones, and oxidized β-acids sum up to the rest of the bitterness sensation. Besides resins, hops contain 0.5–3.0% of essential oils, which provide certain beer flavors. Phenolic compounds in beer are mostly tannins, flavonoids, and polyphenols. A significant portion of phenolic compounds are in the monomeric form (p-coumaric, ferulic, chlorogenic, caffeic acids, and gallic).
Humulones and linalool, the two-hop constituents, tend to prevent beer gushing [64] if added during wort boiling [65].
Figure 2. Scheme of α-acids isomerization to trans- and cis-forms.
Polyphenolic molecules can be found in different stages during the brewing process and react with proteins: during wort boiling, they form the hot break; during cooling, they form the cold break; and during post-fermentation, they are involved in the formation of chill haze and permanent hazes and facilitate the removal of undesirable compounds with filtration. However, they tend to react with proteins in packaged beer and form undesirable haze after the expiration date [66].
Beer contains from 20–60 mg/L of iso-humulones, and oxidized β-acids sum up to the rest of the bitterness sensation. Besides resins, hops contain 0.5–3.0% of essential oils, which provide certain beer flavors. Phenolic compounds in beer are mostly tannins, flavonoids, and polyphenols. A significant portion of phenolic compounds are in the monomeric form (p-coumaric, ferulic, chlorogenic, caffeic acids, and gallic).
Mashing-in time also plays an important factor in phenolic acid release, but viscosity and grist coarseness are important as well [68,70]. Decoction results with worts with higher total polyphenol content [74]. Several authors [75] reported that using lauter tun can result in lower phenolic compounds levels in wort after separation. Research by [76] noted a significant increase of total polyphenols during lautering, probably due to their extraction from phenol-rich spelt material.
Humulones and linalool, the two-hop constituents, tend to prevent beer gushing [57] if added during wort boiling [58].
It is predicted that during wort boiling, about 70% of polyphenols are extracted from hops [17,79], but according to their polarity and their tendency to form complexes with wort proteins, this number is variable [79]. Hot trub contains 40–70% of proteins, 7–32% of bitter substances, 20–30% of organic substances, and 5% of ash [81]. Higher oligomeric phenolic compounds are prone to form complexes with proteins, and small phenolic molecules like phenolic acids easily get adsorbed to hot trub [82]. However, several phenolic acids and catechin, except for ferulic acid (35% decrease during warm rest), were not affected [76].
Polyphenolic molecules can be found in different stages during the brewing process and react with proteins: during wort boiling, they form the hot break; during cooling, they form the cold break; and during post-fermentation, they are involved in the formation of chill haze and permanent hazes and facilitate the removal of undesirable compounds with filtration. However, they tend to react with proteins in packaged beer and form undesirable haze after the expiration date [59].
Prenylflavanones showed higher levels in beer than prenylchalcones, even though raw hops contain very low amounts of prenylflavanones [86,87,88]. Xanthohumol losses commonly occur due to adsorption to hot trub, yeast, and cold trub. Xanthohumol isomerization assumedly occurs because xanthohumol binds to higher molecular weight (300 to 600 kDa) roasted substances [91,92,93,94,95], which can serve as xanthohumol carriers, and reduce its losses during fermentation, filtration, and stabilization process [95]. Lower levels of PVPP remove phenolic compounds with higher degrees of hydroxylation and oligomerization, and at higher levels, PVPP removes all polyphenols [99,100].
Mashing-in time also plays an important factor in phenolic acid release, but viscosity and grist coarseness are important as well [60][61]. Decoction results with worts with higher total polyphenol content [62]. Several authors [63] reported that using lauter tun can result in lower phenolic compounds levels in wort after separation. Research by [64] noted a significant increase of total polyphenols during lautering, probably due to their extraction from phenol-rich spelt material.
Storage time can significantly impact beer quality, such as colloidal stability and flavor. In a study that followed changes during a six-month storage time, the concentrations of (+)-catechin, (−)-epicatechin, proanthocyanidin B3, and prodelphinidin showed a significant reduction [77], but monomeric flavanols showed increased stability over dimers during six months of storage [77,98]. A study on the influence of acetaldehyde from beer on haze formation and stability of beer phenolics reported that beer pH is influenced by acetaldehyde, which results in a reduction of catechin content and haze formation [105]. Prenylated flavonoids exhibit stability during beer storage, resulting in almost no change in beer stored for 10 years at 20 °C in a brown glass bottle [103].
It is predicted that during wort boiling, about 70% of polyphenols are extracted from hops [14][65], but according to their polarity and their tendency to form complexes with wort proteins, this number is variable [65]. Hot trub contains 40–70% of proteins, 7–32% of bitter substances, 20–30% of organic substances, and 5% of ash [66]. Higher oligomeric phenolic compounds are prone to form complexes with proteins, and small phenolic molecules like phenolic acids easily get adsorbed to hot trub [67]. However, several phenolic acids and catechin, except for ferulic acid (35% decrease during warm rest), were not affected [64].
Phenolic acids show high sensory thresholds. For example, cinnamic acid has a threshold of 67 to 139 mg/L and benzoic acid derivatives are 206 to 315 mg/L for [108]; remaining decarboxylation products exhibit significant flavor activity [19]. Some volatile monophenols bring about the spicy, clove-like, sweet, and vanilla-like notes to a beer, but at higher concentrations, they become unpleasant [19]. However, yeast strain and fermentation conditions influence and amplify the phenolic flavor of wheat beers more than temperature conditions [18,72].
Prenylflavanones showed higher levels in beer than prenylchalcones, even though raw hops contain very low amounts of prenylflavanones [68][69][70]. Xanthohumol losses commonly occur due to adsorption to hot trub, yeast, and cold trub. Xanthohumol isomerization assumedly occurs because xanthohumol binds to higher molecular weight (300 to 600 kDa) roasted substances [71][72][73][74][75], which can serve as xanthohumol carriers, and reduce its losses during fermentation, filtration, and stabilization process [75]. Lower levels of PVPP remove phenolic compounds with higher degrees of hydroxylation and oligomerization, and at higher levels, PVPP removes all polyphenols [76][77].
Storage time can significantly impact beer quality, such as colloidal stability and flavor. In a study that followed changes during a six-month storage time, the concentrations of (+)-catechin, (−)-epicatechin, proanthocyanidin B3, and prodelphinidin showed a significant reduction [78], but monomeric flavanols showed increased stability over dimers during six months of storage [78][79]. A study on the influence of acetaldehyde from beer on haze formation and stability of beer phenolics reported that beer pH is influenced by acetaldehyde, which results in a reduction of catechin content and haze formation [80]. Prenylated flavonoids exhibit stability during beer storage, resulting in almost no change in beer stored for 10 years at 20 °C in a brown glass bottle [81].
Phenolic acids show high sensory thresholds. For example, cinnamic acid has a threshold of 67 to 139 mg/L and benzoic acid derivatives are 206 to 315 mg/L for [82]; remaining decarboxylation products exhibit significant flavor activity [16]. Some volatile monophenols bring about the spicy, clove-like, sweet, and vanilla-like notes to a beer, but at higher concentrations, they become unpleasant [16]. However, yeast strain and fermentation conditions influence and amplify the phenolic flavor of wheat beers more than temperature conditions [15][83].
3. Bitterness
Bitterness is an important property of beers and according to several authors, about 80% of bitterness originates from hops during boiling [84][85][86]. The ratio of trans/cis stereoisomers for standard beers is close to 3:7 beers [87], or 68:32 in favor of the cis-compounds. However, bitter taste in beer is modified with residual sugars and results in pleasant bitterness for the consumer [88]. Isomerized α-acids have tensioactive properties, which stabilize the beer foam, and they act inhibitory to Gram-positive bacteria, while lactic acid bacteria in beer exhibit resistance to iso-α-acids.
Bitterness is an important property of beers and according to several authors, about 80% of bitterness originates from hops during boiling [109,110,111]. The ratio of trans/cis stereoisomers for standard beers is close to 3:7 beers [113], or 68:32 in favor of the cis-compounds. However, bitter taste in beer is modified with residual sugars and results in pleasant bitterness for the consumer [114]. Isomerized α-acids have tensioactive properties, which stabilize the beer foam, and they act inhibitory to Gram-positive bacteria, while lactic acid bacteria in beer exhibit resistance to iso-α-acids.
Bittering procedures have evolved and have transferred the use of hops into almost all brewing stages, such as post-fermentation bittering, or dry hopping. The availability of the hop extends to different forms (cones, pellets, plugs) that can be added at different stages of the brewing process. Dry-hopping is a method of soaking hops in beer during fermentation or conditioning in order to add different aromas and flavors to the finished beer. Moreover, they are recognized as important acceptance factors in different beverages, including beer [89].
Bittering procedures have evolved and have transferred the use of hops into almost all brewing stages, such as post-fermentation bittering, or dry hopping. The availability of the hop extends to different forms (cones, pellets, plugs) that can be added at different stages of the brewing process. Dry-hopping is a method of soaking hops in beer during fermentation or conditioning in order to add different aromas and flavors to the finished beer. Moreover, they are recognized as important acceptance factors in different beverages, including beer [120].
4. Astringency
As described in the introduction, astringency is a complex sensory property, characterized by drying, roughing, and puckering of the skin or mucosal surface in the mouth. Immediate perception is not always possible; however, it is usually recognized in the mouth after swallowing the content [90]. According to Siebert and Chassy [91], several compounds provide this sensation: salts of multivalent metallic cations (alum), dehydrating agents (ethanol or acetone), mineral and organic acids, and plant tannins (polyphenols). The reaction between saliva proteins that lubricate the mouth and polyphenols derived from foods results in an astringent sensation.
As described in the introduction, astringency is a complex sensory property, characterized by drying, roughing, and puckering of the skin or mucosal surface in the mouth. Immediate perception is not always possible; however, it is usually recognized in the mouth after swallowing the content [123]. According to Siebert and Chassy [124], several compounds provide this sensation: salts of multivalent metallic cations (alum), dehydrating agents (ethanol or acetone), mineral and organic acids, and plant tannins (polyphenols). The reaction between saliva proteins that lubricate the mouth and polyphenols derived from foods results in an astringent sensation.
Certain phenolic compounds (ferulic acid, p-coumaric acid, and protocatechuic acid) reportedly aid astringency [92]. On the other hand, flavanols, catechin, and epicatechin add bitterness [2][89].
Certain phenolic compounds (ferulic acid, p-coumaric acid, and protocatechuic acid) reportedly aid astringency [106]. On the other hand, flavanols, catechin, and epicatechin add bitterness [4,120].
Research conducted by [93] investigated the addition of purified polyphenols and oxidized counterparts to water and beer. The conducted sensory analysis confirmed that the addition of polyphenols to beer induced harsh bitterness and increased astringency. A similar report was submitted by [5]. Sensory panelists graded beer with iso-α acids and 100 or 200 mg/L of polyphenols from spent hop as more bitter.
Research conducted by [127] investigated the addition of purified polyphenols and oxidized counterparts to water and beer. The conducted sensory analysis confirmed that the addition of polyphenols to beer induced harsh bitterness and increased astringency. A similar report was submitted by [7]. Sensory panelists graded beer with iso-α acids and 100 or 200 mg/L of polyphenols from spent hop as more bitter.
Removal of acrospires from malt by polishing reduced the astringent components, resulting in smooth-tasting beer [94][95][96]. If subcritical water is applied to malt, from which beer was produced, such beer had reduced astringent components and aftertaste.
Removal of acrospires from malt by polishing reduced the astringent components, resulting in smooth-tasting beer [128,129,130]. If subcritical water is applied to malt, from which beer was produced, such beer had reduced astringent components and aftertaste.
Lower pH values result in lesser polyphenols (tannins) that get extracted to beer, and this beer is then less astringent. Mg2+in concentration over 15 mg/L can affect a sour or bitter astringency found in beer [56].
Lower pH values result in lesser polyphenols (tannins) that get extracted to beer, and this beer is then less astringent. Mg2+in concentration over 15 mg/L can affect a sour or bitter astringency found in beer [61].
