This entry analyzed the recent trend towards, progresses towards the preparation of chemicals of, and value-added biomaterials from marine macroalgae resources, especially green seaweeds and their derived ulvan polysaccharides for various applications. In recent years, ulvan both in pristine and modified forms has gained a large amount of attention for its effective utilization in various areas due to its unique physiochemical properties, lack of exploration, and higher green seaweed production. The pristine form of ulvan (sulfated polysaccharides) is used as a bio-component; food ingredient; or a raw material for the production of numerous chemicals such as fuels, cosmetics, and pharmaceuticals, whereas its modified form is used in the sector of composites, membranes, and scaffolds, among others, because of its physicochemical properties.
1. Introduction
Green tides are large accumulations of green seaweeds that are associated with the eutrophication of the marine environment, mainly referring to coastal waters and estuaries. The macroalgal blooms import detrimental effects on the water chemistry, ecosystem, environment, and economy of coastal areas
[1,2][1][2]. Green tides have been receiving increasing attention around the world. Thus, many investigations on their cause, consequence, and applications have been conducted
[3,4,5,6][3][4][5][6]. It is important to tackle the problems resulting from green tides by mitigating the growth of green seaweeds and utilizing algae-based products. Currently, the development and utilization of sustainable biofuels have obtained abundant concern in the world due to the growing population and energy demand, depleting fossil fuel reserves, global warming, and deteriorating environment
[7]. Algae are third-generation biomass and their potential as a feedstock for biofuel production is intensely growing. The utilization of macroalgae
[8,9][8][9] and microalgae
[10] as feedstock for the production of biofuels such as bioethanol, biodiesel, and biohydrogen offers several advantages, compared with the utilization of first- and second-generation biomass feedstocks
[11]. For instance, seaweeds possess a high level of structural polysaccharides and low lignin contents. In addition to serving as feedstocks for biofuel production, green seaweeds are also an important source of high-value chemicals, such as polyunsaturated fatty acids, carotenoids, phycobilins, and polysaccharides
[12]. Most importantly, green seaweeds are a cheap and important source for biomaterials. For instance, ulvan is a sulfated polysaccharide that can be extracted from green seaweeds. Ulvan has been considered as an attractive material for food, pharmaceutical, agricultural, and medical applications due to its varying physicochemical properties and important biological activities
[13].
Some researchers have reviewed the chemistry and biological activities of green seaweeds
[4]; the extraction, structure, composition and function properties of ulvan
[14,15][14][15]; and the applications of ulvan as a constituent of hybrid biomaterials
[16]. Ulvan mainly consists of sulfated rhamnose, glucuronic acid, iduronic acid, and xylose
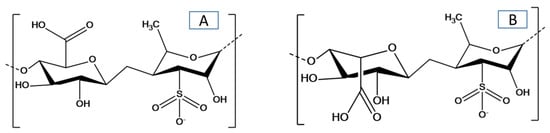
[14]; the structure of ulvan with the major repeating disaccharide units is shown in . The main objective is to highlight recent advances in the field of green seaweed and ulvan polysaccharides. This review discusses the direct utilization of green seaweeds in the production of biofuels and other high-value materials or chemicals (e.g., proteins, lipids, fatty acids, oils, proteins, natural pigments, antioxidants, and biological components) along with the development of ulvan-based materials and their applications. The future direction of the utilization of green seaweeds and the preparation and application of ulvan-based materials will be addressed. To the best of our knowledge, it is the first time there has been a review and discussion of the valorization of green seaweeds and their derived ulvan polysaccharides.
Figure 1. Chemical structure of ulvan with the major repeating disaccharide units: (A) glucuronic acid and rhamnose 3-sulfate, and (B) iduronic acid with rhamnose 3-sulfate.
2. Green Seaweeds and Their Applications
Species of green seaweed from the Genus
Ulva (Phylum Chlorophyta, Class Ulvophyceae, Order Ulvales, Family Ulvaceae) have high growth rates and productivities. This type of seaweeds is easily accumulated in coastal areas around the world. The green seaweeds consist of ≈36% carbohydrate, ≈11% protein, and ≈53% ashes and rich in minerals
[4,6][4][6]. Green seaweeds (
Ulva species) have some unique qualities, such as high growth rates irrespective of geographical location/season and high polysaccharide content
[5]. Most importantly, compared to terrestrial sources, the growth of green seaweeds is faster and it does not require agricultural land/inputs (fertilizer, pesticides, and water), having a possibility for large-scale potential production
[11,17][11][17]. The growth of green seaweeds does not compete with agricultural crops for land and water, which can tackle the conflict between food and fuels compared with corn and sugarcane feedstocks
[11,17][11][17]. Moreover, green seaweeds possess a rapid reproduction rate and high growth rate, which makes them able to be harvested more than once in a year
[9]. Therefore, green seaweeds are potential feedstocks for the production of biofuels, biomaterials, and high-value chemicals.
2.1. Biofuel Production from Green Seaweeds
The utilization of green seaweeds as feedstocks for the production of biobutanol has gained significant interest in science and been reported in the literature
[18]. In this section, the utilization of green seaweeds in the acetone, butanol, and ethanol (ABE) process will be briefly discussed. Van der Wal et al.
[19] used
Ulva lactuca for ABE fermentation. Firstly, a hydrolysate containing 75–93% of the sugars from
Ulva lactuca was prepared by using pretreatments and enzymatic hydrolysis of
Ulva without chemical catalysts. Subsequently, the hydrolysate was used for the production of ABE by using
Clostridium acetobutylicum and
Clostridium beijerinckii. It was found that a high yield of 0.35 g ABE/g sugar was achieved in this process. More interestingly,
Clostridium beijerinckii produced 1,2-propanediol from rhamnose.
Clostridium acetobutylicum produced mostly organic acids. The obtained results demonstrate a great potential of
Ulva lactuca as a feedstock for ABE fermentation
[19]. Potts et al.
[20] also reported butanol production from
Ulva lactuca by using ABE fermentation. The
Ulva lactuca seaweeds were firstly manually and mechanically harvested, dried, and ground. Subsequently, the acid hydrolysis of ground algae was conducted to extract carbohydrates for the algal sugar solution preparation. Finally, butanol was produced by the fermentation of the prepared algal sugar solution using
Clostridium beijerinckii and
Clostridium saccharoperbutylacetonicum. It was found that the butanol concentration in the fermentation broth was 4 g/L, and the removal of the excess solids from the hydrolysate before fermentation resulted in a 75% increase in productivity
[20]. Both examples demonstrate the possibility and potential of the utilization of green seaweeds as feedstocks for the production of biobutanol through ABE fermentation.
Margareta et al.
[21] reported biohydrogen production by using dark fermentation from algal biomass
Ulva sp. for the first time. The acid–thermal combined pretreatment was used to release fermentable sugars from the green algal biomass. The
Clostridium butyricum CGS5 achieved the highest cumulative hydrogen production, equal to 2340 mL/L; the maximum hydrogen productivity, equal to (208.3 mL/L per hour); and hydrogen yield equal to 1.53 mol H
2 per mol of reducing sugar. The hydrogen productivity in this study was better than the hydrogen productivity in Jung et al.’s work
[22], in which
Laminaria japonica was used as feedstock
[21].
Besides the production of biobutanol and biohydrogen from green seaweeds, the production of biogas from green seaweeds is also reported in literature. Akila et al.
[23] investigated the production of biogas and biofertilizer from green seaweed
Ulva sp. by using the anaerobic digestion method. The
Ulva sp. was mixed with organic matter (cow dung) for the biogas production. The solid residue was used as an organic fertilizer for the growth of mung bean. Their research provided an example of maximizing the utilization of green seaweeds in an ecofriendly and sustainable way
[23]. Mhatre et al.
[24] explored strategies for improving biogas production from green seaweed
Ulva lactuca. The individual and sequential extractions were conducted to investigate the influence of removal of sap, which contains valuable minerals that are essential for growth of agricultural crops, ulvan, and protein on methane yields. The biomethane production was enhanced after the extraction treatments, and the highest methane yield (408 mL/g) was obtained in the sap and ulvan-removed residue. This was because high protein and sulfate content are major inhibitors in anaerobic digestion of
Ulva lactuca. The extractions prior to anaerobic digestion not only improved the methane productivity, but also provided high-value products such as sap, protein, and ulvan. The proposed strategy makes the biomethane production process more efficient and sustainable
[24].
Biodiesel can be an alternative and efficient fuel to replace the use of fossil fuels. It is derived as monoalkyl esters of long-chain fatty acids and is usually produced from algal oils, waste cooking oil, and edible and non-edible oils
[25]. Kalavathy et al.
[26] used
Ulva lactuca as feedstock for the production of biodiesel and silica doped with zinc oxide as a heterogeneous nanocatalyst for the transesterification process. The lipid content of algal biomass was extracted by an autoclave followed by the ultra-sonication method, and the extraction of oil was carried out in the Soxhlet apparatus using solvent mixture of n-hexane and methyl tertbutyl ether. It was found that the maximum oil was extracted at optimal conditions of 5% moisture content of algal biomass, 0.15 mm size of biomass, and solvent/solid ratio of 6:1 at 55 °C in 140 min. The maximum biodiesel yield of 97.43% was obtained at optimized conditions of 800 °C calcination temperature, 8% catalyst concentration, 9:1 methanol to oil ratio, 55 °C reaction temperature, and 50 min reaction time
[26]. Khan et al.
[27] used
Ulva fasciata as feedstock for the production of biodiesel. The oil was extracted with n-hexane and the transesterification was carried out by fast stirring using a 9:1 molar ratio of methanol/oil in the presence of waste industrial catalysts for 6 h at 80–100 °C. It was found that the maximum yield of biodiesel equal to 88% was achieved by using the waste brown dust from the steel converter as a catalyst in the transesterification process.
The aforementioned examples demonstrate the possibility and potential of the utilization of green seaweeds as feedstocks for the production of biobutanol, biohydrogen, biogas, and biodiesel. The seaweed hydrolysis methods, such as acid and enzymatic hydrolysis, are crucial to the preparation of sugar solution from seaweeds, which consequently affects the productivity of biobutanol and biohydrogen. The types of bacteria strains are also very important to the productivity of biobutanol and biohydrogen. In the biogas production process, the extraction treatments on seaweeds can enhance methane productivity due to the removal of inhibitors existing in the components of green seaweeds, such as ulvan. In the biodiesel production process, the oil extraction methods and the types of catalysts are crucial to biodiesel productivity because both factors can influence the efficiency of the transesterification process.
2.2. Green Seaweed-Derived Adsorbents
Nowadays, using algae to remove metals is a rapid, reversible, economical, and ecofriendly method
[28,29,30][28][29][30]. Kumar et al.
[28] used green algae
Ulva fasciata sp. as adsorbent to remove copper from its aqueous solution. The copper adsorption behavior of
Ulva fasciata sp. was investigated at different pH values, contact time, initial copper and adsorbent concentrations, and adsorbent size. It was found that the optimum pH value was 5 and the maximum adsorption capacity was 26.88 mg/g
[28]. Ebrahimi et al.
[31] modified the green algae
Cladophora sericioides by L-cysteine to enhance the copper adsorption. The green seaweeds were modified by 7 days cultivation in a reactor containing 20 L of seawater and 3 mg/L-cysteine under illumination cycle 12/12 at an average intensity of 2500 lx at 25 °C. It was found that 65% and 95% of copper ions with initial concentration of 20 mg/L was removed by simple and modified algae, respectively. The maximum adsorption capacities were 13 and 19 mg/g for the simple and modified algae, respectively. The modification significantly improved the copper adsorption capacity
[31].
El-Sikaily et al.
[29] utilized dried green algae
Ulva lactuca and its activated carbon to remove the toxic hexavalent chromium ions from aqueous solution, saline water, and wastewater. It was found that the maximum efficiencies of chromium removal were 92% and 98% for
Ulva lactuca and its activated carbon, respectively. The maximum adsorption capacity was found to be 10.61 and 112.36 mg/g for dried green alga and the activated carbon developed from it, respectively.
Ulva lactuca and its activated carbon are valuable materials for the removal of chromium from waters
[29]. Al-Homaidan et al.
[32] also used dry green algae as a adsorbent to remove hexavalent chromium Cr(VI) from aqueous solution. It was found that the pH significantly affects the adsorption capacity.
Cladophora glomerata showed the maximum of 66.6% removal of Cr(VI) when 1.0 g dried algae was used in 100 mL aqueous solution containing an initial Cr(VI) concentration of 20 mg/L at 45 °C and pH 2 for 60 min of contact time. The maximum adsorption capacity was 1.32 mg/g. These results indicate that
Cladophora glomerata is a potential material for Cr(VI) removal from aqueous solution
[32].
Mwangi et al.
[33] reported the performance of the seaweed (
Caulerpa serrulata) before and after modification with ethylenediamine (EDA) on adsorption of copper, lead, and cadmium in aqueous solution. The adsorption capacities of EDA-modified seaweed for Cu, Cd, and Pb were 5.27, 2.12, and 2.16 mg/g, respectively, and the adsorption capacities of pristine seaweed for Cu, Cd, and Pb were 3.29, 4.57, and 1.06 mg/g, respectively.
Green seaweed-derived adsorbents can be used as low-cost adsorbents to remove metal ions such as copper, chromium, lead, and cadmium in aqueous solutions. Dried Ulva lactuca exhibits desirable adsorption capacities for copper ions and chromium ions. The active carbon derived from Ulva lactuca in particular has shown significantly increased chromium ion adsorption capacities and removal efficiency. Modification on the green seaweeds can improve the adsorption capacity of the green seaweed-based adsorbent.
2.3. Chemicals from Green Seaweeds
The seaweed biorefinery is a sustainable and economical way to fully utilize seaweeds for the production of high value products along with the production of biofuels. The various products obtained from seaweeds such as fatty acids, oils, proteins, natural pigments, antioxidants, and biological components are used in different areas such as food, cosmetics, therapeutics, and biofuels
[34].
The extraction methods are critical for the production of high value products from seaweeds. Gullón et al.
[35] summarized the extraction techniques of bioactive compounds such as polysaccharides, phenolic compounds, fatty acids, pigments, proteins, and vitamins from seaweeds and reviewed the application of algae and their extracts in meat products. Microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), enzyme-assisted extraction (EAE), pressurized liquid extraction (PLE), and supercritical fluid extraction (SFE) have been used to improve the extraction efficiency and to preserve the quality of the final compounds
[35].
Gajaria et al.
[17] integrated the extraction of proteins with recovery of other high value compounds such as seaweed sap, total lipids, ulvan, and cellulose to utilize green seaweed
Ulva lactuca effectively. The extraction processes were conducted in the following order: (1) sap extraction, (2) total lipid extraction, (3) ulvan extraction, (4) protein extraction, and (5) cellulose extraction. Different sap extraction procedures were applied to investigate their influence on the sap composition. It was found that the protein content extracted was 11% on dry weight basis with recovery efficiency of 68.75%. The in vitro digestibility of the protein extracts from green seaweeds was tested by using
o-phthalaldehyde OPA assay
[36] and the high digestibility equal to 85.86% indicated that the recovered protein from green seaweeds is suitable for food supplements. Biorefinery utilizes biomass components that are synthesized as a function of complex photosynthesis and therefore they have to be effectively processed to obtain the products along with bio-energy. The described biorefinery model improves the utilization efficiency of green seaweeds since the valuable products from each extraction process can be applied in different areas
[17].
Trivedi et al.
[37] proposed an integrated process consisting of four extraction process and one fermentation process to fully exploit biomass of the green seaweed
Ulva faciata. Mineral rich liquid extract, lipid, ulvan, and cellulose were sequentially recovered by the extraction processes. In addition to the economically important chemical feedstocks, bioethanol was produced through the enzymatic hydrolysis and fermentation of the final cellulose fraction. The yield of ethanol is comparable to those obtained by direct processing of the individual components from primary biomass. It is believed that this integration of ethanol production and chemical feedstock recovery from green seaweeds could substantially enhance the sustainability of marine biomass utilization
[37].
Besides the biorefinery approach, some value-added chemicals can be directly extracted from green seaweeds. Mzibra et al.
[38] extracted polysaccharides from green seaweeds
Ulva rigida and
Codium decorticatum by using the hot water extraction method under neutral conditions. The extracts were used as biostimulants of tomato seed germination and plant growth. It was found that the compounds in the extract from green seaweeds increased seed germination percentage, plant biomass, and the content of chlorophylls
a and
b. The polysaccharide extract from green seaweeds can be used as a feasible and cost-effective biostimulants for plant growth
[38].
Doh et al.
[39] isolated cellulose nanocrystals (CNCs) from green seaweeds
Ulva lactuca by applying a process consisting of depolymerization, bleaching, acid hydrolysis, and mechanical dispersion. It was found that the CNCs from seaweeds possess rod shape, good thermal stability, and high crystallinity. It is suggested that CNCs from seaweeds have the potential to be used to increase the mechanical properties of polymer materials for food packaging
[39]. Sucaldito and Camacho
[40] isolated CNCs from freshwater green seaweeds
Cladophora rupestris by using hyrobromic acid hydrolysis. The physicochemical properties of CNCs were characterized. It was found that CNCs possess high crystallinity index equal to 94% and high thermal decomposition temperature equal to 381.6 °C. The isolated CNCs were incorporated into starch-based films prepared by solution casting and evaporation method. The mechanical strength of CNC-incorporated starch-based films was found to be significantly improved by 78% when the weight ratio of starch to CNCs was 100:1
[40].
In addition to the valuable products extracted from green seaweeds, some researchers used green seaweed extract in nanoparticle synthesis because the algal extract possesses natural reductants such as pigments and antioxidants
[41,42,43][41][42][43]. For instance, Ishwarya et al.
[44] synthesized zinc oxide nanoparticles by using the extract of green seaweed
Ulva lactuca. The
Ulva lactuca extract was used as a reducing and capping agent and mixed with zinc acetate solution for zinc oxide nanoparticle preparation. In comparison to the conventional synthesis methods of nanoparticles, the synthesis of nanoparticles by using green seaweeds as reducing agents is a green and environmentally friendly method
[42].
3. Ulvan-Based Biomaterials and Their Applications
In recent years there has been a sudden surge in interest to develop and utilize these types of under-exploited marine sources for novel materials. For instance, ulvan possesses attractive physicochemical properties and biological activities, resulting in its applications in different innovative applications
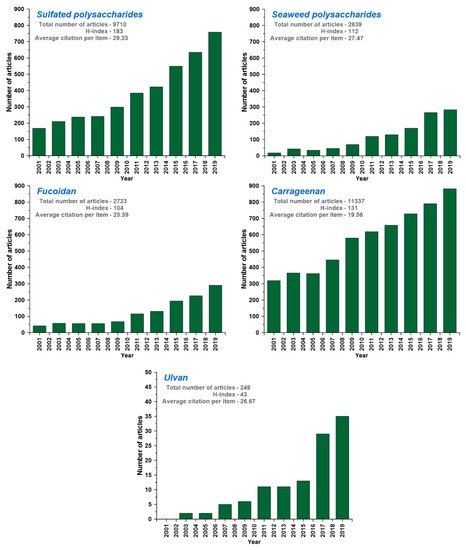
[16,31][16][31]. According to the Web of Science database, distribution of research effect across green seaweed polysaccharides and number of research papers on the subject have increased. In the first 10 years of 21st century, the number of articles related to sulfated polysaccharides, seaweed polysaccharides, and ulvan barely changed in every year. From 2009 to 2019, the number of articles related to sulfated polysaccharides, seaweed polysaccharides, and ulvan increased from 300, 100, and 5 to 800, 300, and 36, respectively (). However, the applications of ulvan retrieved less number of hits in the Institute for Scientific Information (ISI) Web of Index database in comparison with other materials, which shows the need and research opportunity to explore it for diverse applications. Although various seaweed-sulfated polysaccharides are known for their applications, limited literature are available for ulvan ().
Figure 2. Institute for Scientific Information (ISI) Web of Science database for the articles published with the search topic of sulfated polysaccharides, seaweed polysaccharides, carrageenan, fucoidan, and ulvan within the 2001–2019 period (based on 3rd June, 2020 data).
Ulvan is a polysaccharide extracted from cell walls of green seaweeds belonging to
Ulvales, which generally accounts for 9–36% dry weight of the biomass
Ulva [47,48][45][46]. Ulvan is mainly composed of sulfated rhamnose, glucuronic acid, iduronic acid, and xylose
[14,47,49,50][14][45][47][48]. Rhamnose is of interest for its effect on biosynthetic pathways in the dermis and on plant immunity. Uronic acids (glucuronic and iduronic acids) and their sulfate esters are important constituents in mammalian glycosaminoglycans
[14]. In addition, ulvan possesses a repeating disaccharide structure comprised of a uronic acid linked to a sulfated neutral sugar. Therefore, ulvan is a potential candidate for the applications in biomaterial science, for example in wound dressings and tissue engineering, as well as in pharmaceutical and biomedical applications due to its antioxidant activities, biological activities, and peculiar composition and structure
[15,51][15][49].
The investigations on the structure, composition, physicochemical and functional properties, rheology, and gelling properties of ulvan are essential to explore the applications of ulvan
[15,47,50,52,53,54][15][45][48][50][51][52]. The methods of extracting ulvan from green seaweeds have been reported in the literature
[51,55,56,57][49][53][54][55]. Kidgell et al.
[14] reviewed the methods of ulvan extraction from green seaweeds, the characterization of extracted ulvan, and their biological activities. During the extraction process, the subtle changes in pH lead to significant variation in ulvan yield as well as solubility of other macromolecules, which influences its biological functionalities
[14]. Furthermore, the extract conditions affect the structure, thermal properties, and antioxidant properties of ulvan
[58,59][56][57].
Over the last decade, efforts have been attempted to improve life quality through biomaterials
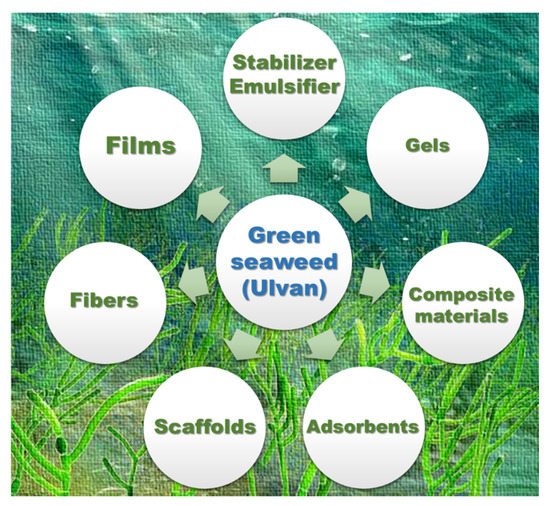
[60][58]. In general, ulvan polysaccharides are utilized for the preparation of certain biomaterials (). In this section, ulvan-derived biomaterials along with their applications are summarized.
Figure 3. Possible bio-based materials using ulvan polysaccharides as a source.
3.1. Ulvan-Based Hydrogel
The use of natural polymers such as polysaccharides in biomedical applications possesses enormous potential due to their advantages over synthetic polymers. Besides their biocompatibility and biodegragability, polysaccharides have a high number of functional groups that can be easily modified or tailored to provide desirable functional properties. Hydrogel made from polysaccharides has found its application in cell encapsulation, drug delivery, and scaffold for tissue engineering
[61][59].
Haug
[62][60] investigated the influence of borate and calcium on the gel formation of ulvan from
Ulva lactuca. It was found that the ulvan hydrogel can be formed with the presence of borate and calcium ions in dialysis solution when the concentration of sulfated polysaccharide is no less than 1 wt % and the pH is above 7.5. The formation of borate–polysaccharide complexes create intermolecular linkages stabilized by calcium ions, which is critical to the gel formation from ulvan
[62][60]. Lahaye and Axelos
[50][48] investigated the gelling properties of water-soluble polysaccharides from green seaweeds (
Ulva spp.). It was found that the investigated ulvan formed a weak hydrogel at a concentration of 1.6 wt % in deionized water. The elastic modulus of hydrogel increased significantly when boric acid and calcium chloride were added. In contrast to the observation of Haug
[62][60], increasing pH to 7.5 and higher than 7.5 is detrimental to the gel formation, which might result from the different structure of ulvan from
Ulva spp.
[50][48]. Both studies found that the presence of borate and calcium ions is important to the formation of ulvan hydrogel.
Kanno et al.
[63][61] reported the preparation and properties of the ulvan–chitosan polyion complex hydrogel. Ulvan was extracted from the green seaweed Chlorophyte
Ulva sp. by using the hot water extraction method. The hydrogel was prepared from the mixture solution of ulvan and chitosan. The formed hydrogel possesses various characteristics such as high stability under acid and base conditions, weak influence on blood coagulation, and high adsorption of CuSO
4, which makes it suitable as a biocompatible ion exchanger as well as other biocompatible materials. The prepared algal-based hydrogel is a renewable organic material and its preparation is simple and green
[63][61].
Curcumin (Cur) is a hydrophobic polyphenolic compound that shows antioxidant, anti-inflammatory, anti-carcinogenic, and anti-cancer activities. However, its medical application is restricted because it is insoluble in water
[64][62]. Bang et al.
[64][62] modified the biocompatible ulvan with acetic anhydride to form amphiphilic polymers and prepared nanogels from acetylated ulvan by using dialysis method. The solubility of Cur in water was improved by 20,000 times by dispersing Cur into the prepared nanogels. It was concluded that the nanogel prepared from hydrophobically modified ulvan can be used to carry and deliver water-insoluble bioactive compounds
[64][62].
Morelli et al.
[65][63] grafted poly(N-isopropylacrylamide) chains onto the backbone of ulvan as a thermosensitive component. The thermosensitive hydrogel was prepared from the modified ulvan by dialysis method. The rheological properties and thermal behavior were investigated. It was found that the sol-gel transition of the prepared material occurred at 31 °C. The results showed that the prepared material is suitable for the in situ gelling systems in biomedical applications
[65][63].
Morelli et al.
[66][64]. prepared an in situ gelling material from ulvan by using enzymatically catalyzed crosslinking reactions. The ulvan was firstly modified with tyramine in order to make it recognizable by horseradish peroxidase enzyme (HRP). The combination of HRP with hydrogen peroxide catalyzed the gel formation through the covalent coupling the grafted phenols. Compared with chemical crosslinking, the enzymatic crosslinking is a straightforward, rapid, and clean method. The results obtained from biological investigations showed that the enzymatically crosslinked ulvan hydrogels are suitable used as vehicle for viable cells in the application of injectable cell delivery systems
[66][64].
Morelli and Chiellini
[61][59] prepared biodegradable hydrogel from functionalized ulvan by using photopolymerization under UV irradiation. Ulvan was functionalized by methacrylic anhydride or glycidyl methacrylate to add unsaturated groups in the ulvan structure. It was found that the UV-induced radical polymerization was not complete after 10 min due to the radical quenching activity of sulfated polysaccharides. This prepared material is suitable for cell encapsulation due to the antioxidant activity. Moreover, this partially crosslinked material is a good base for cytocompatible scaffolds because its softness promotes cell spreading
[61][59].
Yoshimura et al.
[67][65] prepared biodegradable superabsorbent hydrogels from ulvan by crosslinking ulvan with divinylsulfone (DVS) under alkaline aqueous condition. It was found that the maximal water absorbency was 80 g/g when 20 wt % relative to ulvan of DVS was added. The biodegradation speed of hydrogels was dependent on the amount of DVS
[67][65].
The preparation of hydrogels from ulvan can be achieved by using the dialysis method; ulvan–chitosan polyion complex-gel formation; photopolymerization; and physical, chemical, and enzymatic crosslinking. The combination of ulvan modification and the gel formation method confers the ulvan-based gel desirable properties targeting various applications such as injectable cell delivery system, drug delivery, and scaffold for tissue engineering.
3.2. Membranes and Films
The use of biodegradable and active films for packaging has gained intensive attention due to environmental issues. The films based on polymers such as ulvan polysaccharides from natural renewable sources are non-toxic and environmentally friendly. Moreover, they possess antioxidant properties, which is vital for food packaging
[58][56]. Therefore, Guidara et al.
[58][56] developed active films based on ulvan that was extracted from the green seaweed
Ulva lactuca by using acid extraction and enzymatic chemical extraction. The authors prepared 3 wt % ulvan solution by dissolving dried ulvan in distilled water followed by the addition of glycerol or sorbitol as plasticizer. It was found that enzymatic-chemical extraction showed more beneficial impacts on the optical, thermal, structural, and antioxidant properties of the prepared films. The films plasticized with glycerol exhibited better compact structure, lower temperature of transition, and greater antioxidant property than the films with sorbitol. The films prepared with ulvan seemed to be a promising packaging material due to their attractive optical, structural, thermal, and antioxidant properties
[58][56]. Ganesan et al.
[68][66] extracted ulvan from the green seaweed
Ulva fasciata and utilized the extract to prepared edible films for food application. The films prepared from ulvan with glycerol significantly improved the physicochemical and mechanical properties of the films while decreasing the water vapor permeability, which is vital for food packaging. Most importantly, the ulvan polysaccharide-based films showed strong antioxidant activity, which is also very important to food packaging
[68][66].
Wound dressings are widely used for the treatment of different wounds. Alves et al.
[69][67] prepared polymeric membranes from crosslinked polysaccharide ulvan by solution casting for the application of medicated dressing. Firstly, ulvan was crosslinked by 1,4-butanediol diglycidyl ether (BDDE) to render the membrane insoluble in water and stable at physiological conditions. Subsequently, the pristine ulvan membranes and the crosslinked ulvan membranes incorporated with dexamethasone as model drug were prepared. It was found that the prepared membranes revealed remarkable water uptake ability and increased mechanical performance due to crosslinking. As the drug release results showed, 49% of the drug was released initially from membranes in 8 h. Afterwards, a slower release of the drug was detected and at day 14, wherein around 72% of dexamethasone was released from ulvan membranes. On the basis of these results, the prepared crosslinked ulvan membranes have great potential for use as drug delivery systems in medicated wound dressings
[69][67].
Toskas et al.
[70][68] synthesized ulvan, chitosan, and ulvan/chitosan polyelectrolyte membranes for the cultivation of osteoblasts. The combination of anionic ulvan and the cationic chitosan formed supramolecular structures and stabilized membranes due to the electrostatic interactions. The structure and porosity can be altered by changing the weight ratio of the two polysaccharides. The excellent attachment and proliferation of 7F2 osteoblasts on the prepared ulvan and ulvan/chitosan membranes were attributed to the nanofibrous structure mimicking the fibrous part of the extracellular matrix structure. It is concluded that the ulvan/chitosan membranes were potential materials for the development of scaffolds
[70][68].
Bigot et al.
[71][69] proposed a simple and an alternative procedure for the grafting of bioactive polysaccharide ulvan onto a polyvinyl chloride (PVC) surface. Firstly, isothiocyanate groups (NCS) were introduced onto the PVC surface using potassium isothiocyanate in a water/DMSO mixture. Subsequently, the polysaccharides were directly grafted onto the PVC-NCS surface using 1-ethyl-3-methyl-imidazolium phosphate ionic liquid as solvent for polysaccharides and as catalyst for the grafting reaction. The ulvan/PVC membranes have great potential in medical applications due to the wide application of PVC as biomaterial and the pharmacological activities of ulvan
[71][69].
The ulvan film solubility in water and the water vapor permeability of ulvan film have been investigated by Gurdara et al.
[72][70]. In their work, ulvan films were prepared from ulvan extracted from the green seaweed
Ulva lactuca by varying the glycerol or sorbitol in specific plasticizer concentration. It was found that the increase of the plasticizers’ concentration in film resulted in a significant enhancement of the water solubility of films. The incorporation of glycerol into the composition of films showed a better performance on the enhancement of the solubility when compared to the films with sorbitol. On the contrary, the increase of the plasticizers’ concentration in film resulted in a decrease of water vapor permeability, and the incorporation of sorbitol into the composition of films showed a better performance on reduction of water vapor permeability compared with films with glycerol.
According to the aforementioned discussion, ulvan-based membranes and films have found their applications in food packaging, wound dressings, cultivation of osteoblasts, and in the medical field. The physicochemical properties of ulvan-based membranes and films can be tailored by plasticization with glycerol, crosslinking, and blending. In addition to the polymer solution casting method, the formation of ulvan membranes on a substrate made from synthetic polymers, for example, in PVC by surface grafting, could be a versatile method to combine the advantages of conventional synthetic polymers and natural polymers.
3.3. Nanofibers
Electrospinning is a versatile way to fabricate nanofibers from polymeric solution. The fabrication of fibers by using natural polymers for the applications in the biomedical area has received increasing attention due to their high porosity, high surface area-to-volume ratio, and architectural similarity to natural extracellular matrixes
[73][71]. The poor rheological properties of the ulvan solution and the limited solubility of ulvan in various solvent systems restrict the formation of nanofibers from ulvan by using electrospinning. Thus, the improvement of the rheological properties and the charge-carrying capacity of the ulvan solution are important to fiber formation
[16].
Toskas et al.
[74][72] improved the spinnability of ulvan extracted from
Ulva rigida by blending with poly(vinyl alcohol) (PVA) and successfully fabricated the ulvan-based nanofibers by electrospinning the ulvan/PVA solution. The prepared nanofibers had an average diameter down to 84 nm and a highly ordered crystalline structure. The spinnability of ulvan in combination with its biological and physicochemical properties can lead to new biomedical applications such as drug release systems
[74][72]. Kikionis et al.
[73][71] prepared novel composite nanofibers comprising ulvan and polycaprolactone (PCL) or ulvan and polyethylene oxide (PEO) by using an electrospinning technique. It was found that ulvan was incorporated well with the biocompatible polymers, providing the collective properties of these materials. The average diameters of the prepared fibers decreased when the ulvan content increased. The ulvan/PEO nanofibers can impart antithrombogenic properties and can be used as a drug release and wound healing medium. The ulvan/PCL nanofibers can be used as tissue engineering scaffolding materials due to the long biodegradation period of PCL
[73][71].
3.4. 3D Porous Scaffolds
Scaffold design is crucial for tissue engineering applications. An ideal scaffold should possess suitable porosity and interconnectivity, mechanical properties, good biocompatibility, and biodegradability
[75,76][73][74]. The sulfonated polysaccharide-based scaffolds for orthopedic tissue engineering were reviewed by Dinoro et al.
[77][75].
The combination of both synthetic and natural materials can enhance the performance of these materials and broaden their applicability. Therefore, Alves et al.
[75][73] combined ulvan with poly-D,L-lactic acid (PDLLA) to produce a novel scaffold for bone tissue engineering applications. The scaffolds of PDLLA loaded with ulvan particles were prepared by subcritical fluid sintering with carbon dioxide at 40 °C and 50 bar. Pristine ulvan particles and ulvan particles loaded with dexamethasone were dispersed within the PDLLA matrix. It was found that the prepared scaffold possesses appropriate morphologic features, suitable mechanical performance, and adequate cytocompatibility. Furthermore, the prepared scaffold can also be used as a drug delivery system
[75][73].
Dash et al.
[78][76] proposed an approach of producing photo-crosslinked polymeric ulvan scaffolds that are enzymatically treated for calcium phosphate deposition. Ulvan was firstly modified with methacrylic anhydride to obtain photoreactive groups, and subsequently the modified ulvan was used to prepare the photo-crosslinked scaffolds by using UV light. The crosslinked ulvan scaffolds were treated with alkaline phosphatase (ALP) to induce the mineral formation. It was found that the ulvan scaffolds were homogeneously mineralized at ambient temperature and the formed minerals contained apatite. The mineralized scaffolds were nontoxic and the formed minerals improved the osteogenic cell activity on the scaffolds. The biofunctionalized scaffolds could be potentially used as resorbable bone graft substitutes
[78][76].
The polymer scaffolds possess high osteoconductivity and osteoinductivity when they are mineralized with apatite, which is very important to their medical applications. Therefore, Dash et al.
[79][77] prepared ulvan-based scaffold from polyelectrolyte complexes of chitosan (40 wt %) and ulvan (60 wt %) and investigated the formation of apatitic minerals mediated by alkaline phosphatase (ALP). It was found that the calcium phosphate minerals were successfully deposited on the ALP-treated scaffold. The globular structure of the deposited minerals is beneficial to the cell attachment, proliferation, and extracellular matrix formation. In addition, the mineralized scaffolds are nontoxic. Therefore, it is a green way to fabricate scaffold from polyelectrolyte complexes. The prepared scaffold can be used as a resorbable materials for tissue engineering
[79][77].
In addition to the ulvan-derived materials, ulvan was investigated as a stabilizing and emulsifying agent in colloidal formulation containing functional agents for food and cosmetic applications due to its edibility and amphiphilic character
[80][78]. Ulvan was also used as a reducing and stabilizing agent for the synthesis of silver nanoparticles under mild conditions
[81][79]. Furthermore, ulvan was used to prepare lysozyme/ulvan complexes to improve the antibacterial activity of lysozyme. The prepared lysozyme/ulvan can be used as a promising nanocarrier for positively charged bioactive molecules
[13].
Additionally, their potential antioxidant activity has drawn tremendous attention to their applications in the cosmetic industry
[14]. The sulfated polysaccharide containing extracts from
Ulva rigida has been shown to protect HeLa cells from hydrogen peroxide-induced oxidative stress in vitro
[82][80]. Furthermore, ulvans are rich in rhamnosyl residues, which are reported to promote cell proliferation and collagen biosynthesis, and the presence of glucuronic acids confers moisturizing properties that help to prevent skin damage from dry environments
[83][81]. The unique chemical composition of ulvan provides distinct rheological properties and gelling characteristics to its suspensions at varying temperatures, pH values, and various cations, which enables ulvan to be used as a stabilizer and emulsifier in variety of applications including the food and cosmetics industries
[15,80][15][78].
The peculiar structure, the strong bioactivity, and the presence of both hydrophilic (hydroxyl, carboxyl, sulfate) and hydrophobic (methyl) groups make ulvan a unique biopolymer for the preparation of functional materials. The reported literature on the preparation of ulvan-based materials, their characterization, and possible applications are summarized in .
Table 21. Ulvan-derived biomaterials and their reported applications.
| Source Materials |
End Products |
Preparation Method |
Applications |
Reference |
| Ulvan from Ulva lactuca |
Hydrogel |
The hydrogel was formed when dialyzed against seawater. |
- |
[62][60] |
| Ulvan from Ulva spp. |
Hydrogel |
The ulvan hydrogel was formed in distilled water and water containing borate and calcium ions. |
- |
[50][48] |
| Ulvan from Ulva spp. |
Hydrogel |
The hydrogel was prepared from the mixture solution of ulvan and chitosan. |
Biocompatible ion exchanger as well as other biocompatible materials |
[63][61] |
| Ulvan from Ulva lactuca |
Hydrogel |
Ulvan was modified with acetic anhydride to form amphiphilic polymers.
Nanogels were prepared from acetylated ulvan by using the dialysis method. |
Carrier and delivery of water-insoluble bioactive compounds |
[64][62] |
| Ulvan from Ulva spp. |
Hydrogel |
The thermosensitive hydrogel was prepared from the modified ulvan with thermal-sensitive group by using the dialysis method. |
In situ gelling systems in biomedical applications |
[65][63] |
| Ulvan from Ulva spp. |
Hydrogel |
The thermosensitive hydrogel was prepared from modified ulvan by using enzymatically catalyzed crosslinking reactions. |
Vehicle for viable cells in the application of injectable cell delivery systems |
[66][64] |
| Ulvan from Ulva armoricana |
Hydrogel |
The biodegradable hydrogel was prepared from functionalized ulvan by using photopolymerization. |
Cell encapsulation
Cytocompatible scaffolds |
[61][59] |
| Ulvan from Ulva spp. |
Hydrogel |
Hydrogels were prepared by crosslinking ulvan with divinylsulfone (DVS) under alkaline aqueous conditions. |
- |
[67][65] |
| Ulvan from Ulva lactuca |
Film |
Glycerol or sorbitol was used as a plasticizer.
Film was prepared by casting solution into a plastic Petri disk. |
Packaging material |
[58][56] |
| Ulvan from Ulva fasciata |
Film |
Glycerol was used as a plasticizer.
Film was prepared by casting solution in a framed glass plate. |
Food packaging |
[68][66] |
| Ulvan |
Film |
Film was prepared by casting solution in Petri dishes. |
Drug delivery systems
Medicated wound dressings |
[69][67] |
| Ulvan/chitosan |
Film |
Film was prepared by casing solution on flat glass. |
Cultivation of osteoblasts
Potential materials for the development of scaffolds |
[70][68] |
| Ulvan |
Film |
The ulvan film was formed by grafting of bioactive polysaccharide ulvan onto PVC surface. |
Medical applications |
[71][69] |
| Ulvan from Ulva rigida |
Fiber |
The ulvan-based nanofibers were prepared by electrospinning ulvan/PVA solution. |
Drug release systems |
[74][72] |
| Ulvan from Ulva fasciata/PEO |
Fiber |
The ulvan-based nanofibers were prepared by electrospinning ulvan/PEO solution. |
Drug release and wound healing medium |
[73][71] |
| Ulvan from Ulva fasciata/PCL |
Fiber |
The ulvan-based nanofibers were prepared by electrospinning ulvan/PCL solution. |
Long-term drug release and tissue engineering scaffolding materials |
[73][71] |
| Ulvan from Ulva lactuca/PDLLA |
Scaffolds |
The scaffolds of PDLLA loaded with ulvan particles were prepared by subcritical fluid sintering with carbon dioxide at 40 °C and 50 bar. |
Bone tissue engineering applications |
[75][73] |
| Ulvan from Ulva armoricana |
Scaffolds |
The ulvan scaffold was prepared by using photo-crosslinking. |
Resorbable bone graft substitutes |
[78][76] |
| Ulvan from Ulva armoricana |
Scaffolds |
The ulvan scaffold was prepared by the formation of ulvan–chitosan polyelectrolyte complexes. |
Tissue engineering |
[79][77] |