Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Richard Weiss.
Thiols (RSH) and disulfides (RSSR) are components of many proteins, biopolymers, and biomolecules. Many can be interconverted by simple oxidation and reduction reactions that change drastically their properties and those of host molecules of which they are a part.
- stimulus-responsive materials
- self-healing
- redox-responsive
- drug delivery
- thiol-disulfide exchange
- disulfide-disulfide metathesis
1. Introduction
Thiols (RSH) and disulfides (RSSR) are components of many proteins, biopolymers, and biomolecules. In nature, thiol and disulfide functional groups contribute to key biological functions related to cell signaling, protein conformation and folding processes, redox homeostasis, and biopolymer secondary structure development. Thiol groups and/or disulfide linkages can be incorporated into polymers to produce stimulus-responsive and/or self-healing materials. They owe their stimulus-responsiveness to covalent disulfide bonds, which can be broken and re-formed under mild conditions. The extent of reactivity, and, thus, the stimulus-responsive behavior depends on the nature of the groups flanking the disulfide bond, as well as interactions with stimuli such as light, heat, mechanical force, and changes in the pH or redox state [1]. The ease with which thiols can be converted to disulfides, and vice versa, renders thiol-containing polymers (thiomers) similarly dynamic.
2. Some Important Reactions of Thiols and Disulfides
Thiol-disulfide exchange reactions can occur between deprotonated thiols (thiolates) and disulfides. Thiol-disulfide exchange proceeds via substitution-type pathways, with the thiolate anion (RS−) functioning as the nucleophile (1 in Figure 1). In addition, thiols can be oxidized to disulfides (2 through 10 in Figure 1), and disulfides can be reduced to thiols. Although the products of oxidation or reduction reactions are often the same as those of thiol-disulfide exchange reactions, the pathways are mechanistically different (as shown in Figure 1). There are well-documented multi-step examples of both one electron (7 through 10 in Figure 1) and two electron (2 through 6 in Figure 1) oxidation pathways within cells [7][2]. Thiyl radicals (RS⋅) can be generated from thiols or disulfides using a variety of reagents or stimuli. Thiyl radicals play a crucial role in biological systems and are important reagents in organic synthesis. Thiyl radicals are intermediates in disulfide-disulfide metathesis reactions, also known as disulfide-disulfide exchange reactions, which proceed via a radical pathway (11 in Figure 1). Because disulfide linkages are often the weakest covalent bonds in an organic molecule, they provide an avenue to tune the properties of a material under mild reaction or stimulus conditions. Note that the product distributions of both thiol-disulfide and disulfide-disulfide exchange depend upon the ratios of reactants and the nature of the R groups, which determine product stabilities.
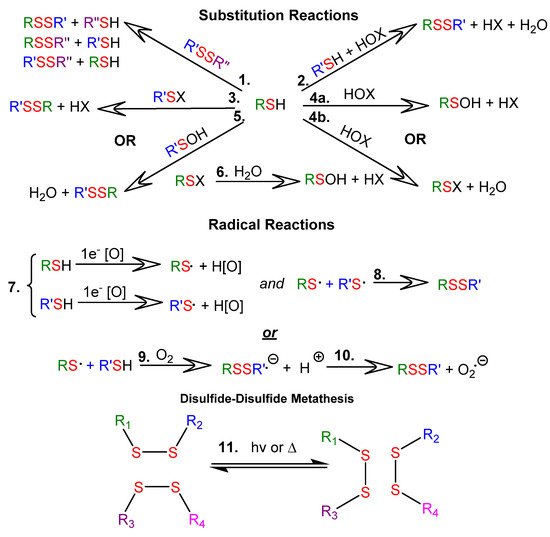

Figure 1. Reactions of thiols (RSH) and disulfides (RSSR): 1.1 SN2-mediated thiol-disulfide exchange; 1.2–1.6. Thiol oxidation with two-electron oxidants, where RSX is a sulfenyl halide, RSOH is a sulfenic acid, HOX is a halohydrin, and HOx is the reduced oxidizing agent; 1.7–1.10 Thiol oxidation with one-electron oxidizing agents; 1.11 disulfide-disulfide metathesis.
3. Biological Relavance of Thiols and Disulfides
Some molecules of biological relevance that exploit the disulfide bond are shown in Figure 2. For example, processes involving cystine (Cys2) and cysteine (Cys) interconversions have structural and functional consequences on the macroscopic and microscopic scale. On a macroscopic level, disulfide bonds in Cys2 stabilize the secondary structure of keratin-based materials, such as hair, wool, and nails. The cleavage of disulfide bonds in keratin results in a dramatic decrease of strength and elasticity of these materials. In that regard, the redox states and pH of intracellular and extracellular environments control the reversible oxidation of Cys to Cys2 in polypeptides. As such, disulfide bonds are keys to the protein folding process, and thus strongly dictate protein conformation.
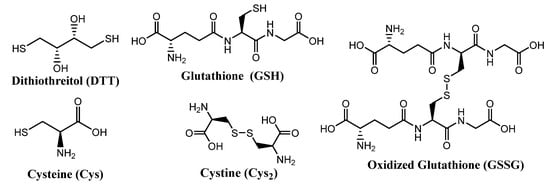

Figure 2.
Structures of some biologically important thiols and disulfides.
4. Thiols and Disulfides in Materials Science
Nature has shown that thiol-disulfide exchange and redox reactions can be used to interconvert thiols and disulfides reversibly in response to changes in pH or redox state. The dynamic nature of the disulfide bond has been extended to materials science to produce a wide range of stimulus-responsive materials. The reviews of Seidi [8][3] and Zhang [9][4] include recent examples of controlled drug release, with an emphasis on stimulus-responsive and redox-triggered drug release. These reviews include several examples of thiol- and disulfide-based materials.
Thiol-disulfide exchange is often used to bind thiomers to disulfide-containing substrates, and to trigger the release of disulfide bound “cargo,” such as drugs or dyes, from a polymer upon exposure to high concentrations of thiols and/or changes in pH. Thiol oxidation and disulfide reduction are commonly used to form and break covalent disulfide crosslinks in polymeric networks, respectively. The scission and reformation of disulfide crosslinks in situ via reduction and oxidation, respectively, is often reported as a means for reversible sol-gel transitions. Commonly, dramatic rheological changes are observed over several reversible sol-gel redox cycles. Self-healing behavior has been widely reported for a variety of disulfide-containing polymers, due to disulfide-disulfide shuffling reactions, which occur after breakage.
5. Applications of Thiomers and Disulfide-Containing Polymers
Drug delivery is among the most common biomedical applications of thiomers and disulfide-containing polymers. The prototypical drug delivery system releases a conjugated drug molecule after being exposed to a cellular trigger. For example, solid tumors have higher levels of GSH and lower pH levels than healthy tissues. Chemotherapy employing a drug delivery system can be tuned to release the drug when exposed to the unique chemical environment of a cancer cell; more targeted drug delivery results in fewer side effects [3][5]. Figure 3 demonstrates the wide variety of drug delivery systems that rely on disulfide bonds for controlled delivery of nucleic acid therapeutics [10][6].
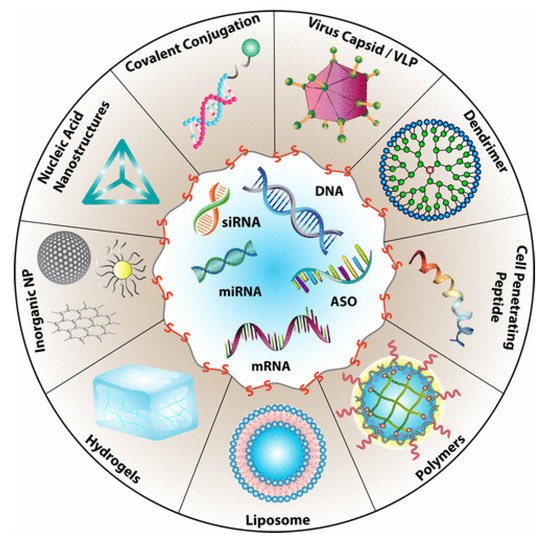

The reviews of Dutta [10][6], Wang [11][7], and Saitoa [12][8] focus on disulfide-based drug delivery systems. Another notable application is in the field of biomedicine, which merges biological and physiological principles within clinical science. Biomedicine often deals with mucoadhesive materials, which contain thiols and/or disulfides that penetrate mucosal membranes by binding with free thiol side chains from Cys residues [2][9].
The applications of thiomers and disulfide-containing polymers extend beyond biomedicine. A wide range of dynamic materials, such as self-healing elastomers [4[10][11],13], shape memory polymers [14][12] and stimulus-responsive polymers [9,15][4][13] have been designed as coatings [16][14], adhesive materials [17][15], and sensors [2,18][9][16]. Incorporation of thiols and/or disulfides into polymers that contain other stimulus-responsive moieties is an emerging technique that has been used to fabricate multi stimulus-responsive materials that may enhance specificity for both biomedical [15][13] and non-biomedical applications [6,14,18][17][12][16] For example, drug delivery systems incorporating multi-stimulus-responsive materials can increase the amount of drug reaching the targeted cells [19,20][18][19]. Some multi stimulus-responsive materials have been designed to release a drug only after the application of two or more stimuli associated with the target environment. Other multi stimulus-responsive drug delivery systems incorporate functional groups designed to enhance targeting specific types of cells.
(References would be added automatically after the entry is online)
References
- Jin, Y.; Yu, C.; Denman, R.J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6334–6654.
- Nagy, P. Kinetics and mechanisms of thiol-disulfide exchange covering direct substitution and thiol oxidation mediated pathways. Antioxid. Redox Signal. 2013, 18, 1623–1641.
- Seidi, F.; Jenjob, R.; Crespy, D. Designing Smart Polymer Conjugates for Controlled Release of Payloads. Chem. Rev. 2018, 118, 3965–4036.
- Zhang, X.; Han, L.; Liu, M.; Wang, K.; Tao, L.; Wan, Q.; Wei, Y. Recent progress and advances in redox-responsive polymers as controlled delivery nanoplatforms. Mater. Chem. Front. 2017, 1, 807–822.
- Bej, R.; Dey, P.; Ghosh, S. Disulfide chemistry in responsive aggregation of amphiphilic systems. Soft Matter. 2020, 16, 11–26.
- Dutta, K.; Das, R.; Medeiros, J.; Thayumanavan, S. Disulfide Bridging Strategies in Viral and Nonviral Platforms for Nucleic Acid Delivery. Biochemistry 2021, 60, 966–990.
- Wang, Q.; Guan, J.; Wana, J.; Li, Z. Disulfide based prodrugs for cancer therapy. RSC Adv. 2020, 10, 24397–24409.
- Saitoa, G.; Swanson, J.A.; Lee, K.-D. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: Role and site of cellular reducing activities. Adv. Drug Deliv. Rev. 2003, 55, 199–215.
- Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 191–221.
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467.
- Kim, S.-M.; Jeon, H.; Shin, S.-H.; Park, S.-A.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior Toughness and Fast Self-Healing and Room Temperature Engineered by Transparent Elastomers. Adv. Mater. 2018, 30, 1705145.
- Ling, L.; Li, J.; Zhang, G.; Sun, R.; Wong, C.-P. Self-Healing and Shape Memory Linear Polyurethane Based on Disulfide Linkages with Excellent Mechanical Property. Macromol. Res. 2018, 26, 365–373.
- Zhuang, J.; Gordon, M.R.; Ventura, J.; Li, L.; Thayumanavan, S. Multi-stimuli responsive macromolecules and their assemblies. Chem. Soc. Rev. 2013, 42, 7421–7435.
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93.
- Lv, C.; Zhao, K.; Zheng, J.A. Highly Stretchable Self-Healing Poly(dimethylsiloxane) Elastomer with Reprocessability and Degradability. Macromol. Rapid Commun. 2018, 39, 17008686.
- Tomei, M.R.; Cinti, S.; Interino, N.; Manovella, V.; Moscone, D.; Arduini, F. Paper-based electroanalytical strip for user-friendly blood glutathione detection. Sen. Actuators B. Chem. 2019, 294, 291–297.
- Utrera-Barrios, S.; Verdejo, R.; Lopez-Manchado, M.A.; Hernandez Santana, M. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902.
- Yin, W.; Ke, W.; Lu, N.; Wang, Y.; Japir, A.A.-W.M.M.; Mohammed, F.; Wang, Y.; Pan, Y.; Ge, Z. Glutathione and Reactive Oxygen Species Dual-Responsive Block Copolymer Prodrugs for Boosting Tumor Site-Specific Drug Release and Enhanced Antitumor Efficacy. Biomacromolecules 2020, 21, 921–929.
- Gao, Y.; Dong, C.-M. Reduction and thermo-sensitive core-cross-linked polypeptide hybrid micelles for triggered and intracellular drug release. Polym. Chem. 2017, 8, 1223–1232.
More
