1. Introduction
Lipid bilayers are integral structural components of cells, allowing the compartmentalization of biomolecules and reactions that make life possible. Different intracellular compartments have distinct lipid compositions that define their identity
[1]. The correct intracellular trafficking and targeting of proteins, nucleic acids, ions and metabolites, as well as replication of viruses all depend on the delicate balance and maintenance of this composition, and thus necessarily on lipid transport
[1][2][3][1,2,3]. In addition, lipids play vital roles as signaling molecules, and rapid, dynamic, and sometimes highly localized changes in lipid composition can trigger downstream effects that allow cells to respond and adapt to daily challenges. While vesicular fission and fusion permit bulk flux and exchange of lipid between different compartments, lipid transfer proteins (LTPs) that sequester lipids in a hydrophobic pocket in order to allow their transit through the cytosol in a non-vesicular mechanism, have been increasingly recognized over the last two decades as playing an integral role in lipid metabolism and signaling
[2].
The canonical oxysterol binding protein (OSBP) first identified in the early 80’s
[4][5][6][4,5,6] is the founding member of the OSBP and OSBP-like (OSBPL) (also called OSBP-related, ORP) family of LTPs. As the name implies, OSBP was initially identified due to its high-affinity binding to the oxysterol 25-hydroxycholesterol and was found to be conserved across diverse species including human, insects, plants and yeast
[6][7][6,7]. Based on homology mapping of the lipid binding domain of OSBP, called the OSBP homology domain (OHD) or OSBP-related domain (ORD), a multi-gene family of 12 members including OSBP and ORP1-11 was identified in mammals
[7][8][9][7,8,9], and 7 members in the yeast
Saccharomyces cerevisiae, termed Osh proteins
[10][11][10,11]. Initial studies on Osh/ORPs focused on their role as oxysterol sensors, and they were thought to exert their influence on cellular functions ultimately through transcriptional control, albeit likely in an indirect manner that was poorly understood
[10][12][13][10,12,13]. However, it was also clear even from early studies that Osh/ORPs could not only bind lipids other than sterols, notably phosphoinositides (PIPs), but were also involved in regulating their subcellular localization, hinting at a transport role beyond sensing.
[10][11][13][10,11,13].
Notably, several Osh/ORP proteins were found early on to localize to structures now termed membrane contact sites (MCS) which are sites of close (<20 nm) apposition between membranes of two different organelles that are held together by protein tethers
[13][14][15][16][13,14,15,16]. These sites provide a short cytosolic transit space ideal for non-vesicular lipid transfer. They are now recognized to serve as platforms for localized signaling, as well as accumulation and exchange of a multitude of biomolecules in addition to lipids, rendering them focal points for communication between organelles
[14][15][14,15]. The solution of the structure of Osh4 revealed a resemblance to other LTPs, with a hydrophobic pocket and a lid
[17]. Shortly after, its role in sterol transport was demonstrated
[18], followed by a pioneering study revealing that phosphatidylinositol-4-phosphate (PI(4)P) was also transported in exchange for sterol, where it was suggested that this transport could be powered by the dephosphorylation of PI(4)P by the endoplasmic reticulum (ER)-resident phosphatase Sac1
[19]. OSBP was then was shown to transfer sterols across ER-Golgi MCS, and the dephosphorylation by Sac1 was confirmed to contribute
[20]. A 4-step cycle of membrane tethering, forward sterol transfer, backward PI(4)P transfer, and PI(4)P hydrolysis was proposed to drive sterol transport up a concentration gradient, a model that was later confirmed for Osh4
[20][21][20,21]. This counter-exchange mechanism was demonstrated for other ORPs, including oxysterol binding related proteins 5 and 8 (ORP5 and ORP8) and Osh6 as discussed in further detail below.
The proteins ORP5 and ORP8 form a subgroup within the family as their ORD sequences are most similar to each other (). They are also the only ORPs to include a C-terminal transmembrane domain that anchors them to the membrane of the ER
[7][22][7,22]. The ORP8 protein has an N-terminal acidic track that is followed by a polybasic segment and then a pleckstrin homology (PH) domain, whereas ORP5 is similar but lacks the acidic track
[7][8][9][23][24][25][7,8,9,23,24,25]. These N-terminal motifs comprise distinct sites for membrane interactions. The PH domains of both ORP5/8 are atypical as they contain a 20 amino acid insertion in variable loop 3 as compared with prototypical PH domains of proteins such as AKR mouse thymoma (AKT) serine/threonine kinase and phospholipase C-gamma (PLCγ), but also of more similar proteins such a OSBP, ORP1 and ceramide transferase (CERT) as shown by sequence alignment (F)
[26]. In particular, the ORP8 PH domain lacks critical Lys and Arg residues, rendering its binding to PIPs rather weak
[25][26][25,26]. The ORD, defined by its signature motif EQVSHHPP, has the form of a barrel with a hydrophobic pocket and a lid that protects an extracted lipid during transfer within an aqueous environment, typical of LTPs
[17][27][17,27]. In addition, ORDs have also been shown to be able to bind two membranes simultaneously thus mediating organelle contact formation
[25][26][25,26]. Interestingly, a sequence identified as an amphipathic helix (AH) within the ORD domain of ORP5 was targeted to lipid droplets (LDs) when expressed alone
[26]. However, the sequence is embedded deeply inside the ORD and thus it is unclear whether it can mediate ORP5 binding to LD membranes
in vivo. Thus, ORP5 and 8 can sense, target to membranes, and interact with lipids at multiple sites within their structure. It should be noted that there is an important distinction between lipid binding and lipid transport. Here we refer to lipid binding as any interaction between proteins and lipids that is strong or stable enough to mediate the recruitment of the proteins to a membrane. The site of this binding interaction may be on the outside surface of the protein, or within the binding pockets of lipid interaction domains such as the PH domain or the ORD. Lipid transport requires lipid binding as a prerequisite, but involves furthermore the extraction of the lipid from the membrane and transit of the bound lipid through the cytosol. The binding of PH domains to membranes generally occurs within the membrane and do not lead to extraction of the lipid, whereas lipid transport is exclusively the function of the ORD in this context. Both ORP5 and 8 are widely if not ubiquitously expressed
[7][8][22][28][7,8,22,28], where ORP5 has high expression in thymus
[8] and ORP8 in brain, liver, spleen, kidney, testis and placenta
[7][28][7,28]. The protein ORP5 has 2 described isoforms: the longest is the canonical sequence, also called ORP5A, and the second is ORP5B, which lacks residues 134-201, disrupting part of the PH domain and thus reducing membrane association and rendering this isoform more reticular
[26]. Similarly, ORP8 has 2 described splice variants, ORP8L, the canonical sequence, and a shorter ORP8S which lacks residues 1-42, rendering its N-terminus much more basic, promoting membrane association
[23][24][25][23,24,25]. For both ORP5 and 8 tissue distribution of alternative splice forms have not been investigated, although in many studies two bands (or even three in the case of ORP8) are discerned in western blots
[22][23][28][29][30][31][32][22,23,28,29,30,31,32], indicating that both splice forms act in concert in many tissues.
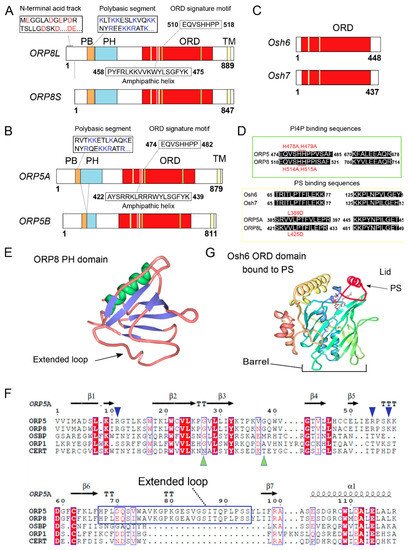
Figure 1. Structure of ORP5 and ORP8 isoforms. (
A–
C) Domain structure of (
A) human ORP8L (canonical form) and ORP8S (missing residues 1-43), (
B) ORP5A (canonical form) and ORP5B (missing residues 134-201 within the PH domain), and (
C)
S. cerevisiae Osh6, Osh7 proteins. Acidic amino acids within the N-terminal domain of ORP8L are highlighted in red. Basic amino acids inside the polybasic segments are highlighted in blue. The position of PI(4)P or PS binding motifs within the ORD domains of ORP5, ORP8, Osh6, Osh7 is indicated by green (PI(4)P) or yellow (PS) strips. The positions of the putative amphipathic helices within the ORD domains of ORP5, ORP8 are indicated by pink strips.
(d) Residues involved in the recognition of head groups of PI(4)P or PS within the ORD domains of ORP5, ORP8, Osh6, and Osh7. (
E) Ribbon representation of the crystal structure of the ORP8 PH domain showing alpha helices (green) and β-sheets (blue), as well as the atypical extended loop 3 (arrow) (PDB id: 1V88). (
F) Multiple sequence alignment of the PH domains from human ORP5A, ORP8, OSBP, ORP1 and CERT highlighting the atypical nature of the ORP5 and ORP8 PH domains. Secondary structure elements of ORP5A are indicated above the alignment. Residues comprising the atypical extended loop present in ORP5A and ORP8 but absent in other PH domains are highlighted by the blue box. Basic amino acids found only in the PH domains of ORP5 and ORP8 proteins but absent in other PH domains are indicated by blue arrows. The amino acids mediating PI(4)P binding which are present in CERT but absent in ORP5 and ORP8 are indicated by green triangles
[26]. Alignments were made with ESPript version 3.0
[33]. (
G) Ribbon representation of the crystal structure of Osh6 ORD bound to PS, the latter shown as a ball-and-stick model (PDB id:4B2Z). The lid covering the barrel is indicated in red. PB: Polybasic segment, PH: Pleckstrin homology domain, ORD: OSBP related domain, TM: transmembrane domain.
2. ORP5/8 as Oxysterol Sensors
Oxysterols are cholesterol metabolites that exert negative feedback on cholesterol synthesis and uptake, as well as influence other biological processes such as apoptosis
[6][12][6,12]. As OSBP was initially identified as an oxysterol binding protein, early studies on mammalian and
S. cerevisiae (Osh1-7) ORP family members largely focused on the effects of genetic manipulation of these proteins on cholesterol metabolism
[34][35][34,35]. Indeed, in yeast, deletion of Osh proteins resulted in a disruption of sterol metabolism
[11][36][11,36]. The Osh6 and Osh7 ORDs are most closely related to ORP5 and 8
[23][37][23,37] (see also ). Osh6 deletion led to an overproduction and aberrant intracellular distribution of sterols while its overexpression had the opposite effect
[11][36][38][39][11,36,38,39]. It was perhaps these defects in sterol metabolism that lead Suchanek and colleagues to investigate whether mammalian ORPs bound sterols
[40]. Both ORP5 and ORP8 bound to the oxysterol photo-25-hydroxycholesterol and to photo-cholesterol in photo-crosslinking experiments, both in vitro and when applied in living cells. Notably, the ORD domains expressed alone were insufficient to mediate sterol binding, and the C-terminal transmembrane domain (residues 266-879) or the full-length protein were required for ORP5 and ORP8, respectively. ORP8 bound strongly and ORP5 moderately to both sterols as compared to other ORPs, although actual binding affinities were not provided.
For ORP8, numerous studies subsequently pointed to a role in sterol control. Indeed, in vitro binding of its ORD to oxysterols and a role in sterol metabolism was confirmed in macrophages
[28]. ORP8 was overexpressed in atherosclerotic plaques and downregulated cholesterol efflux through a transcriptional effect on liver X receptor (LXR) elements within the promoter of the cholesterol transporter ATP binding cassette subfamily A member 1 (ABCA1). In line with a role in oxysterol sensing, in liver cells, ORP8 knockdown and overexpression had opposing effects on cholesterol biosynthesis, and when administered
in vivo, ORP8 regulated serum lipids levels
[41]. The ORP8 ORD was again shown to bind cholesterol
in vitro, and additionally to be capable of extracting cholesterol from liposomes. The effects on cholesterol correlated with the nuclear localization of sterol regulatory binding protein (SREBP) and were dependent on interaction of ORP8 with a nuclear pore protein nucleoporin 62 (NUP62). Similarly, ORP8 knockout mice harbored defects consistent with an increased biosynthesis and secretion of high-density lipoprotein (HDL), as well as gender-specific alterations in serum lipid profiles including triglycerides and cholesterol
[31]. When bone marrow of ORP8 knockout mice was transplanted into wild-type animals no difference in HDL was observed, indicating the HDL defect maybe liver-specific
[42]. Instead an increase was detected in pro-atherogenic very low-density lipoprotein (VLDL) in LDL-receptor knockout model of atherosclerosis. On the other hand, ORP8 knockout transplant recipients had smaller atherosclerotic lesions and more macrophage infiltration, attributed to the fact that ORP8 null macrophages had decreased capacity to form foam cells and produced lower amounts of proinflammatory cytokines. A lipidomics study on a macrophage cell line stably expressing an shRNA against ORP8 provided some clues
[43]. Here, under basal conditions cholesterol and cholesterol esters were increased upon ORP8 knockdown. However, when cells were treated with the inflammatory toll-like receptor 4 (TLR4) agonist lipopolysaccharide (LPS), known to increase oxysterol production, there was a reduction in PIP species containing arachidonic acid, the precursor for proinflammatory two-series eicosanoids prostaglandins and leukotrienes. In addition, lipid species containing docosahexaenoic acid (DHA), an omega -3 fatty acid that serves as a precursor for anti-inflammatory resolvins, were higher upon ORP8 knockdown, again predicting that macrophages may have reduced inflammatory potential. It is interesting to note that in this study in addition to sterols, changes in ceramides and especially phospholipids were also detected, providing early hints that ORP8 may have functions beyond oxysterol sensing and cholesterol metabolism.
Another series of studies that investigated the role of ORP8 as an oxysterol sensor were spurred by the discovery that ORP8 is silenced by miR-143, a micro-RNA associated with obesity, diabetes and cancer
[30][44][30,44]. Oxysterols had been previously shown to dampen AKT signaling, which normally promotes phosphatidylinositol-3 kinase (PI3K) activity, growth and proliferation, and ORP8 reduction impaired insulin mediated AKT activation in hepatocytes. Oxysterol sensing was presumed to contribute but this was not shown directly. The link between miR-143, ORP8 and AKT signaling was then confirmed in cardiomyocytes
[45] and vascular smooth muscle cells
[46]. Additionally, in hepatic cells the ability of 25-hydroxycholesterol to reduce mitotic rate was dependent on ORP8 interaction with the spindle protein sperm associated antigen 5 (SPAG5)
[47], indicating that ORP8 may display both pro- and anti-proliferative activities. In line with these findings, a subsequent report showed that 25-hydroxycholesterol-induced apoptosis was dependent on ORP8 and particularly on the ability of ORP8 ORD to trigger ER stress
[48]. Other more recent studies have confirmed the link between ORP8, ER stress and apoptosis in the context of cancer, suggesting ORP8 to hinder rather than favor cancer progression
[49][50][49,50], despite its promotion of AKT signaling. However, the exact mechanisms through which ORP8 induces ER stress remain to be described.
In contrast to ORP8, early studies on ORP5 associated its overexpression with cancer progression. In one study, ORP5 was found to be highly expressed in a metastatic pancreatic cancer hamster cell line
[29]. Knockdown and overexpression in human and hamster cancer cells revealed ORP5 promotes cell migration, and ORP5 expression correlated with poor prognosis in clinical samples of pancreatic cancer. In a follow-up study, SREBP2 and genes associated with the cholesterol synthesis pathway were found to be upregulated by ORP5 overexpression
[51]. SREBP2 upregulation was linked to histone deacetylase 5 (HDAC5) induction, which in turn reduced the expression of the tumor suppressor and phosphatidylinositol-(3,4,5)-trisphosphate (PI(3,4,5)P3) phosphatase and tensin homolog (PTEN). Thus, ORP5 expression lowered PTEN while its knockdown had the reverse effect. In addition, the suppression of cell migration by statins, drugs that block a step of cholesterol synthesis, depended on ORP5 expression. Based on effects on SREBP and levels of the cholesterol precursor hydroxymethylglutaryl coenzyme A (HMG-CoA)-synthase, the authors concluded that ORP5 enhances cholesterol synthesis, although cholesterol levels were not shown directly. Whether oxysterol sensing or cholesterol transport play a role in controlling these functions remains an open question.
3. ORP5/8 in the Control of Vesicular Trafficking
Sterol content has been known to profoundly affect vesicular trafficking and various ORPs including OSBP have been linked to the regulation of secretory and endocytic trafficking
[52][53][54][55][56][52,53,54,55,56]. Indeed, early yeast studies showed severely impaired endocytosis
[36] and defects in polarized exocytosis
[57] upon deletion of OSH genes, in addition to altered intracellular sterol distribution. Notably, a yeast-two-hybrid screen identified a novel Osh6 and Osh7 interactor Vps4p, an ATPase involved in multi-vesicular body (MVB) sorting that catalyzes the dissociation and disassembly of endosomal sorting complexes required for transport (ESCRT) complexes from endosomal membranes
[38]. Overexpression of residues 366-437 of Osh7, thought initially to be a coiled-coil domain but later shown to be a part of the ORD, and which strongly interacted with Vps4p, led to an MVB sorting defect, a dominant negative effect possibly resulting from Vps4p inhibition. In a parallel study however, the same authors reported that although Osh6 partly colocalized with endosomes, its deletion was not essential for endocytosis, MVB sorting, trafficking to the vacuole (yeast lysosome) or secretion of carboxypeptidase Y to the vacuole. Interestingly, Osh6 bound to PI(4)P, PI(5)P, PI(3,4)P2 and PI(3,5)P2, where both the full-length protein and the ORD domain showed similar binding characteristics, providing another early clue to the role of these ORPs in phospholipid transfer
[39].
In mammalian cells, a transcriptomic study found surprisingly little effects on lipid-related pathways upon ORP8 knockdown in murine macrophages, and instead revealed major changes related to cytoskeletal and secretory functions
[58]. Cells depleted of ORP8 exhibited an abnormal distribution of its previously identified interactor, the nucleoporin NUP62, which enhanced migration and altered microtubule organization
[41]. Interestingly, ORP8 was found to compete with the exocyst complex protein Exo70 for NUP62 interaction, suggesting a role of ORP8 in controlling secretory function. Perhaps the most important contribution to our understanding of how sterol control by ORP5 can impact the secretory as well as endocytic pathway came from the pioneering study by the group of Yang, where the critical role of ORP5 in regulating endosomal cholesterol trafficking was discovered
[22]. Immunoprecipitation experiments followed by mass spectrometry identified an interaction between Osh6 and Osh7 and the yeast protein Niemann-Pick disease, type C1 (NPC1, Ncr1 in yeast), a key player regulating the exit of LDL cholesterol from late-endosome/lysosomes. This interaction, which was also confirmed in vivo with a split-ubiquitin yeast-two-hybrid assay, was abolished under conditions of sterol depletion. In mammalian cells, co-immunoprecipitation experiments confirmed the interaction between ORP5 and NPC1, but here it did not depend on NPC1 cholesterol binding. Interestingly, the purified ORD of ORP5 (residues 266 to 826) was able to transfer a fluorescent cholesterol analog dehydroergosterol (DHE) between liposomes in vitro, which was partially inhibited by PI(4)P in the donor membrane, providing another early clue to ORP5’s phospholipid-related function. Moreover, knockdown of ORP5, but not of ORP8 resulted in cholesterol accumulation in the limiting membrane of endo-lysosomes, impaired cholesterol transport to the ER and reduced the rate of cholesterol esterification. While ER and lipid droplet morphology were unaltered in ORP5-depleted cells, trans-Golgi network proteins were mislocalized and trapped in cholesterol-filled endo-lysosomal compartments, a phenotype that was not found in ORP8 knockdown cells, but that could be reversed by ORP5 overexpression. Furthermore, knockdown of ORP5 affected trafficking events through early and late endosomes, defects that likely resulted from the accumulation of cholesterol in endosomal compartments. The authors proposed a role for ORP5 in non-vesicular transport of cholesterol from the endo-lysosome limiting membrane to the ER, presumably at ER-endo-lysosome MCS, in response to increased cholesterol and possibly with the assistance of NPC1. This study was perhaps the first groundwork to position ORP5 as a lipid transfer rather than sensor protein, although whether the cholesterol transfer activity in cells was truly direct requires further investigation.