Low cost, sensitive, selective, and rapid methods for heavy metal ion (HMI) detection are of growing demand, and HMI biosensors have great potential in meeting this need due to their timeliness, cost-effectiveness and convenience in operation. The most widely reported peptide probe for HMI detection is glutathione (GSH), especially in case of lead ion (Pb2+) detection. GSH is highly stable, cost-effective, and easy to immobilize on a sensor.
- heavy metal ions
- glutathione
- biosensor
- nanoparticle
1. Introduction
2. Recognition of HMIs by GSH
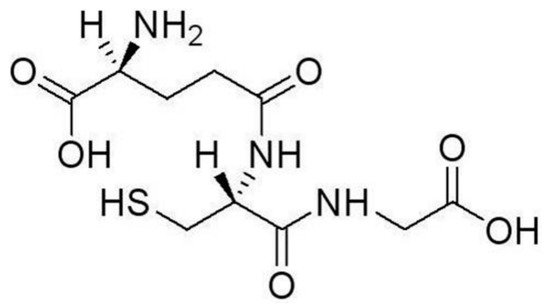
3. Heavy Metal Ion Detection Platforms Using Glutathione
3.1. Optical Techniques
Metallic nanoparticles (NPs) are between 1–100 nm in size with a high surface charge density, a high surface to volume ratio, and, oftentimes, special optical properties due to a localized surface plasmon resonance (LSPR) effect. For these reasons, metallic NPs have been widely used in diverse biological and chemical applications, especially for biochemical detection. Various types of biochemical molecules can be easily incorporated onto the NPs while retaining their biochemical activity. For HMI detection, most metallic NPs are made of gold and some are silver, with gold NPs (GNPs) being the most widely used material in HMI sensors. Other shapes of NPs, such as nanorods and nanostars, have been adopted for sensing [41,42][41][42].
As an important discovery originally found in the 1990s, semiconductor quantum dots (QDs) have attracted intense research interest for their excellent luminescence properties. In contrast to traditional fluorescence techniques, QDs have advantages in excitation spectra, photo-luminescence quantum efficiency, and so on [50,51][43][44]. Reports of new QD-based sensors are on the rise. Most of the reported QD-based HMI detection schemes utilized II–VI semiconductor QDs. As an example, using Mn-doped ZnS QDs, [52][45] reported a Pb2+ detection scheme by phosphorescence measurement. Phosphorescence is generated by the energy transfer from the lowest vibrational energy layer of the excited Mn2+ triplet state to the vibrational energy layer of the ground state. Mn–ZnS QDs are a popular II–VI semiconductor QD for biosensor applications. Because Mn–ZnS QDs can be functionalized without using deoxidants and other inducers, they are convenient to work with. Furthermore, Mn–ZnS QDs have a long-lived doped emission, which can set the signal of Pb2+ detection apart from interference by autofluorescence and scattering light.
3.2. HMI Detection Combined with Electrochemical Techniques
3.3. Summary of HMI Detection Platforms Using GSH
In summary, GSH has been adopted as the probe molecule in a variety of HMI detection platforms. These detection platforms have demonstrated good detection performance, such as a low LOD and a fast response. Some of them have been applied to practical samples with acceptable outcomes. The platforms discussed above present commonly reported detection methods, and are not an exhaustive list of all existing GSH-based HMI sensors. Table 1 gives a more comprehensive summary of recently reported HMI detection platforms using GSH. In addition to the examples discussed in the review, Table 1 includes some less-used detection methods with their operational characteristics. As every application has its own unique requirements, different considerations go into choosing a certain detection platform. For example, it is difficult to find a sensor with high sensitivity and a very fast response at the same time. Some trade-off is needed when choosing a detection method. It is our hope that this summary will provide some guidance for choosing a suitable strategy to detect certain HMIs.Signal Transduction | Sensor Structure | HMI Target | Detection Limit | Linear Range | Response Time | References | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Optical methods | ||||||||||||||||||||||||
Colorimetry | Gold NPs | Pb | 0.1 μM | 0.1~50 μM | 20 ~ 25 min |
[43] |
[46] |
|||||||||||||||||
Silver NPs | Pb | 1 nM | Not mentioned | At least 10 min |
[32] |
|||||||||||||||||||
Gold NPs | Cd | 4.3 pM | 17 pM~16.67 nM | About 17 min |
[49] |
[47] |
||||||||||||||||||
Localized surface plasmon resonance | Gold NPs | Pb | 50 pM | 0.1 nM~10 μM | 15 min |
[33] |
||||||||||||||||||
Fluorescence | Gold NPs | Pb | 0.1 μM | 2~350 μM | About 1 min |
[44] |
[48] |
|||||||||||||||||
Silver NPs | Pb | 0.6 pM (200 ppq) | 60 pM~2.4 nM (20~800 ppt estimated) | Less than 20 min |
[45] |
[49] |
||||||||||||||||||
Surface-enhanced Raman scattering | Silver NPs | As3+ | 10.2 nM (0.76 ppb) | 53.7 nM~4.0 μM (4~300 ppb) | At least 2 min |
[46] |
[50] |
|||||||||||||||||
Whispering gallery mode | Gold NPs | Pb | 0.05 nM | 2.40~48.26 nM | About 40 min |
[35] |
||||||||||||||||||
Spectrophotometry | Mn-doped ZnS QDs | Pb | 2.2 nM (0.45 μg/L) | 4.9 nM~0.49 μM (1.0~100 μg/L) | Not mentioned |
[52] |
[45] |
|||||||||||||||||
Mn-doped ZnS QDs | Pb, Cr, Hg | 0.93 μM for mixed HMIs | 1 μM~1 mM | Not mentioned |
[53] |
[51] |
||||||||||||||||||
CdTe QDs | Pb | 0.26 nM | 0.8~15 nM | About 15 s |
[54] |
[52] |
||||||||||||||||||
Carbon QDs | Hg | 0.05 nM | 1 nM~50 μM | At least 20 min |
[39] |
|||||||||||||||||||
Silver NPs | Ni2+ | 75 μM | About 75 μM~1 mM | Not mentioned |
[48] |
[53] |
||||||||||||||||||
Gold Nanostars | Pb | 0.5 μM | About 0.5~4 μM | About 30 min |
[42] |
|||||||||||||||||||
Dynamic Light Scattering | Gold Nanorod Chains | Pb | 0.025 mM | Not mentioned | Not mentioned |
[41] |
||||||||||||||||||
Electrochemical methods | ||||||||||||||||||||||||
Square Wave Anodic Stripping Voltammetry, SWASV | Magnetic NPs | Cd, Pb | 1.6 nM (0.182 μg/L); | 0.8 nM (0.172 μg/L) | 4.4~879 nM (0.5~100 μg/L), | 2.3~460 nM (0.5~100 μg/L) | 210 s | 210 s |
[40] |
|||||||||||||||
Glassy-Carbon Electrode | Cd | 0.05 nM | 2~20 nM | 120 s |
[56] |
[54] |
||||||||||||||||||
Carbon Paste Electrode | Cd | 8.5 nM (2 ppb) | 4.2~420 nM (1~100 ppb) | Longer than 7 min |
[58] |
[55] |
||||||||||||||||||
Differtial pulsed anodic striping voltammetry, DPASV | Screen-Printed Carbon Nanofiber Electrode | Pb, Cd | ~0.1 nM (~3 μg/L) | About 0.3~4.5 nM (about 10~150 μg/L) | 120 s |
[57] |
[56] |
|||||||||||||||||
FET (Drain current) | Field effect Transistor | Pb | 10 nM | 10 nM~10 μM | 1–2 s |
[34] |
||||||||||||||||||
FET (Pulse-driven capacitance) | Gate Capacitance | Pb | <4.8 nM | (<1 ppb) | 24~96 nM (5~20 ppb) | 1–2 s |
[19] |
|||||||||||||||||
References
- Bansod, B.K.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455.
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 2015, 63, 276–286.
- Pujol, L.; Evrard, D.; Serrano, K.G.; Freyssinier, M.; Cizsak, A.R.; Gros, P. Electrochemical sensors and devices for electrochemical assay in water: The French groups’ contribution. Front. Chem. 2014, 2, 19.
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533.
- Vázquez-González, M.; Carrillo-Carrion, C. Analytical strategies based on quantum dots for heavy metal ions detection. J. Biomed. Opt. 2014, 19, 1–12.
- Ginya, M.A.; Idris, M.B.; Zakariyya, U.A.; Singh, D.; Yakasai, H.; Sani, N. The role of some antioxidants on absorption, desorption and elimination of lead and iron: An in-vivo study. Eur. J. Biomed. Pharm. Sci. 2016, 3, 528–531.
- Afkhami, A.; Soltanifelehgari, F.; Madrakian, T.; Ghaedi, H.; Rezaeivala, M. Fabrication and application of a new modified electrochemical sensor using nano-silica and a newly synthesized schiff base for simultaneous determination of Cd2+, Cu2+ and Hg2+ ions in water and some foodstuff samples. Anal. Chim. Acta 2013, 771, 21–30.
- Sharma, B.; Singh, S.; Siddiqi, N.J. Biomedical implications of heavy metals induced imbalances in redox systems. BioMed. Res. Int. 2014, 2014, 640754.
- Gong, T.; Liu, J.; Liu, X.; Liu, J.; Xiang, J.; Wu, Y. A sensitive and selective platform based on CdTe QDs in the presence of L-cysteine for detection of silver, mercury and copper ions in water and various drinks. Food Chem. 2016, 213, 306–312.
- Turdean, G.L. Design and development of biosensors for the detection of heavy metal toxicity. Int. J. Electrochem. 2011, 2011, 343125.
- Gao, C.; Yu, X.Y.; Xiong, S.Q.; Liu, J.H.; Huang, X.J. Electrochemical detection of arsenic(III) completely free from noble metal: Fe3O4 microspheres-roomtemperature ionic liquid composite showing better performance than gold. Anal. Chem. 2013, 85, 2673–2680.
- Array, G.; Merkoci, A. Nanomaterials application in electrochemical detection of heavy metals. Electrochim. Acta 2012, 84, 49–61.
- Sitko, R.; Janik, P.; Zawisza, B.; Talik, E.; Margui, E.; Queralt, I. Green approach for ultra trace determination of divalent metal ions and arsenic species using totalreflection X-ray fluorescence spectrometry and mercapto-modified graphene oxide nanosheets as a novel adsorbent. Anal. Chem. 2015, 87, 3535–3542.
- Liu, X.; Zhu, Z.; Li, H.; He, D.; Li, Y.; Zheng, H.; Gan, Y.; Li, Y.; Belshaw, N.S.; Hu, S. Liquid spray dielectric barrier discharge induced plasma-chemical vapor generation for the determination of lead by ICPMS. Anal. Chem. 2017, 89, 6827–6833.
- Feng, W.; Xue, X.; Liu, X. One-step, room-temperature, colorimetric detection of mercury (Hg2+) using DNA/nanoparticle conjugates. J. Am. Chem. Soc. 2008, 130, 3244–3245.
- Li, S.; Xu, L.; Ma, W.; Kuang, H.; Wang, L.; Xu, C. Triple raman label-encoded gold nanoparticle trimers for simultaneous heavy metal ion detection. Small 2015, 11, 3435–3439.
- Skotadis, E.; Tsekenis, G.; Chatzipetrou, M.; Patsiouras, L.; Madianos, L.; Bousoulas, P.; Zergioti, I.; Tsoukalas, D. Heavy metal ion detection using dnazyme-modified platinum nanoparticle networks. Sens. Actuators B Chem. 2017, 239, 962–969.
- Kullick, T.; Quack, R.; Röhrkasten, C.; Pekeler, T.; Scheper, T.; Schügerl, H.C.K. Pbs-field-effect-transistor for heavy metal concentration monitoring. Chem. Eng. Technol. 2010, 18, 225–228.
- Maity, A.; Sui, X.; Tarman, C.R.; Pu, H.; Chang, J.; Zhou, G.; Ren, R.; Mao, S.; Chen, J. Pulse-driven capacitive lead ion detection with reduced graphene oxide field-effect transistor integrated with an analyzing device for rapid water quality monitoring. ACS Sens. 2017, 2, 1653–1661.
- Hang, R.; Kang, Y.; Gladwin, E.; Claus, R.O. Selective detection of heavy metal ions by self assembled chemical field effect transistors. Appl. Phys. Lett. 2015, 106, 402647.
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275.
- Blake, D.A.; Jones, R.M.; Ii, R.C.B.; Pavlov, A.R.; Darwish, I.A.; Yu, H. Antibody-based sensors for heavy metal ions. Biosens. Bioelectron. 2001, 16, 799–809.
- Saidur, M.R.; Aziz, A.R.; Basirun, W.J. Recent advances in dna-based electrochemical biosensors for heavy metal ion detection: A review. Biosens. Bioelectron. 2017, 90, 125–139.
- Zhan, S.; Wu, Y.; Wang, L.; Zhan, X.; Zhou, P. A mini-review on functional nucleic acids-based heavy metal ion detection. Biosens. Bioelectron. 2016, 86, 353–368.
- Ritchie, S.M.C.; Kissick, K.E.; Bachas, L.G.; Sikdar, S.K.; Parikh, C.; Bhattacharyya, D. Polycysteine and other polyamino acid functionalized microfiltration membranes for heavy metal capture. Environ. Sci. Technol. 2001, 35, 3252–3258.
- Neupane, L.N.; Oh, E.T.; Park, H.J.; Lee, K.H. Selectively and sensitively detection of heavy metal ions in 100% aqueous solution and cells with a fluorescence chemosensor based on peptide using aggregation induced emission. Anal. Chem. 2016, 88, 3333–3340.
- Farzin, L.; Shamsipur, M.; Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627.
- Oroval, M.; Coll, C.; Bernardos, A.; Marcos, M.D.; Martínez-Máñez, R.; Shchukin, D.G.; Sancenón, F. Selective fluorogenic sensing of As(III) using aptamer-capped nanomaterials. ACS Appl. Mater. Interfaces 2017, 9, 11332–11336.
- Zhou, S.F.; Wang, J.J.; Gan, L.; Han, X.J.; Fan, H.L.; Mei, L.Y.; Huang, J.; Liu, Y.Q. Individual and simultaneous electrochemical detection toward heavy metal ions based on l-cysteine modified mesoporous MnFe2O4 nanocrystal clusters. J. Alloys Compd. 2017, 721, 492–500.
- Zhang, L.; Xu, C.; Li, B. Simple and sensitive detection method for chromium(VI) in water using glutathione-capped CdTe quantum dots as fluorescent probes. Microchim. Acta 2009, 166, 61–68.
- Mah, V.; Jalilehvand, F. Lead(II) complex formation with glutathione. Inorg. Chem. 2012, 51, 6285–6298.
- Anambiga, I.V.; Suganthan, V.; Arunai Nambi Raj, N.; Buvaneswari, G.; Sampath Kumar, T.S. Colorimetric Detection of lead ions using glutathione stabilized silver nanoparticles. Int. J. Sci. Eng. Res. 2013, 4, 710–715.
- Feng, B.; Zhu, R.; Xu, S.; Chen, Y.; Di, J. A sensitive LSPR sensor based on glutathione-functionalized gold nanoparticles on a substrate for the detection of Pb2+ ions. RSC Adv. 2018, 8, 4049–4056.
- Zhou, G.; Chang, J.; Cui, S.; Pu, H.; Wen, Z.; Chen, J. Real-time, selective detection of Pb2+ in water using a reduced graphene oxide/gold nanoparticle field-effect transistor device. ACS Appl. Mater. Inter. 2014, 6, 19235–19241.
- Panich, S.; Wilson, K.A.; Nuttall, P.; Wood, C.K.; Albrecht, T.; Edel, J.B. Label-free Pb(II) whispering gallery mode sensing using self-assembled glutathione-modified gold nanoparticles on an optical microcavity. Anal. Chem. 2014, 86, 6299–6306.
- Beqa, L.; Singh, A.K.; Khan, S.A.; Senapati, D.; Arumugam, S.R.; Ray, P.C. Gold nanoparticle-based simple colorimetric and ultrasensitive dynamic light scattering assay for the selective detection of Pb(II) from paints, plastics, and water Samples. ACS Appl. Mater. Interfaces 2011, 3, 668–673.
- Kim, I.B.; Dunkhorst, A.; Gilbert, J.; Bunz, U.H.F. Sensing of lead ions by a carboxylate-substituted PPE: Multivalency effects. Macromolecules 2005, 38, 4560–4562.
- Vila-Viçosa, D.; Teixeira, V.H.; Santos, H.A.F.; Machuqueiro, M. Conformational Study of GSH and GSSG Using Constant-pH Molecular Dynamics Simulations. J. Phys. Chem. B 2013, 117, 7507–7517.
- Wang, W.; Lu, Y.C.; Huang, H.; Wang, A.J.; Chen, J.R.; Feng, J.J. Solvent-free synthesis of sulfur- and nitrogen-co-doped fluorescent carbon nanoparticles from glutathione for highly selective and sensitive detection of mercury(II) ions. Sens. Actuators B Chem. 2014, 202, 741–747.
- Baghayeri, M.; Amiri, A.; Maleki, B.; Alizadeh, Z.; Reiser, O. A simple approach for simultaneous detection of cadmium(II) and lead(II) based on glutathione coated magnetic nanoparticles as a highly selective electrochemical probe. Sens. Actuators B Chem. 2018, 273, 1442–1450.
- Durgadas, C.V.; Lakshmi, V.N.; Sharma, C.P.; Sreenivasan, K. Sensing of lead ions using glutathione mediated end to end assembled gold nanorod chains. Sens. Actuators B Chem. 2011, 156, 791–797.
- D’Agostino, A.; Taglietti, A.; Bassi, B.; Donà, A.; Pallavicini, P. A naked eye aggregation assay for Pb2+ detection based on glutathione-coated gold nanostars. J. Nanopart. Res. 2014, 16, 2683.
- Nightingale, A.M.; deMelloa, J.C. Improving the ensemble optical properties of InP quantum dots by indium precursor modification. J. Mater. Chem. C 2016, 4, 8454–8458.
- Choi, D.B.; Kim, S.; Yoon, H.C.; Ko, M.; Yang, H.; Do, Y.R. Color-tunable Ag-In-Zn-S quantum-dot light-emitting devices realizing green, yellow and amber emissions. J. Mater. Chem. C 2017, 5, 953–959.
- Chen, J.; Zhu, Y.; Zhang, Y. Glutathione-capped Mn-doped ZnS quantum dots as a room-temperature phosphorescence sensor for the detection of pb2+ ions. Spectrochim. Acta A 2016, 164, 98–102.
- Chai, F.; Wang, C.; Wang, T.; Li, L.; Su, Z. Colorimetric detection of Pb2+ using glutathione functionalized gold nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 1466–1470.
- Li, L.; Liu, B.; Chen, Z. Colorimetric and dark-field microscopic determination of cadmium(II) using unmodified gold nanoparticles and based on the formation of glutathione-cadmium(II) complexes. Microchim. Acta 2019, 186, 37.
- Zhang, H.; Wang, S.; Chen, Z.; Ge, P.; Jia, R.; Xiao, E.; Zeng, W. A turn-on fluorescent nanoprobe for lead(II) based on the aggregation of weakly associated gold(I)-glutathione nanoparticles. Microchim. Acta 2017, 184, 4209–4215.
- Singh, A.K.; Kanchanapally, R.; Fan, Z.; Senapati, D.; Ray, P.C. Synthesis of highly fluorescent water-soluble silver nanoparticles for selective detection of Pb(II) at the parts per quadrillion (PPQ) level. Chem. Commun. 2012, 48, 9047–9049.
- Li, J.; Chen, L.; Lou, T.; Wang, Y. Highly sensitive SERS detection of As3+ ions in aqueous media using glutathione functionalized silver nanoparticles. ACS Appl. Mater. Interfaces 2011, 3, 3936–3941.
- Liu, J.; Lv, G.; Gu, W.; Li, Z.; Tang, A.; Mei, L. A novel luminescence probe based on layered double hydroxides loaded with quantum dots for simultaneous detection of heavy metal ions in water. J. Mater. Chem. C 2017, 5, 5024–5030.
- Wang, H.; Chen, Q.; Tan, Z.; Yin, X.; Lun, W. Electrochemiluminescence of CdTe quantum dots capped with glutathione and thioglycolic acid and its sensing of Pb2+. Electrochim. Acta 2012, 72, 28–31.
- Li, H.; Cui, Z.; Han, C. Glutathione-stabilized silver nanoparticles as colorimetric sensor for Ni. Sens. Actuators B Chem. 2009, 143, 87–92.
- Priya, T.; Dhanalakshmi, N.; Thennarasu, S.; Thinakaran, N. Ultra sensitive detection of Cd (II) using reduced graphene oxide/carboxymethyl cellulose/glutathione modified electrode. Carbohyd. Polym. 2018, 197, 366–374.
- Kaabi, R.; Abderrabba, M.; Gómez-Ruiz, S.; Hierro, I.D. Bioinspired materials based on glutathione-functionalized SBA-15 for electrochemical Cd(II) detection. Microporous Mesoporous Mater. 2016, 234, 336–346.
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Glutathione modified screen-printed carbon nanofiber electrode for the voltammetric determination of metal ions in natural samples. Talanta 2016, 155, 8–13.
