Colorectal cancer is one of the most common gastrointestinal malignancies in humans, affecting approximately 1.8 million people worldwide. This disease has a major social impact and high treatment costs. Animal models allow us to understand and follow the colon cancer progression; thus, in vivo studies are essential to improve and discover new ways of prevention and treatment. Dietary natural products have been under investigation for better and natural prevention, envisioning to show their potential. This manuscript intends to provide the readers a review of rodent colorectal cancer models available in the literature, highlighting their advantages and disadvantages, as well as their potential in the evaluation of several drugs and natural compounds’ effects on colorectal cancer.
- spontaneous models
- induced models
- genetically engineered models
1. Introduction
Worldwide, colorectal cancer is the third most common cancer in men and second in women [1]. Many risk factors have been considered for the development of colorectal cancer, such as the ingestion of processed meat, alcoholic drinks, body fatness, low intake of vegetables and fruits, smoking, and other concomitant diseases, such as inflammatory bowel disease (IBD), Crohn’s disease, and ulcerative colitis [2][3].
Colorectal cancer is characterized by the invasion of neoplastic epithelial cells below the muscularis mucosae of the colorectal wall [4]. Its evolution is slow and characterized by different stages. Progressive changes in the amount or activity of proteins that regulate cell proliferation, differentiation, and cell survival occur, leading to a disorder in cell replication that contributes to the development of proliferative lesions, such as adenoma [5]. Subsequently, the intestinal epithelium undergoes a malignant transformation to invasive carcinoma [4][5]. Besides adenomas, hyperplastic polyps, serrated adenomas, flat adenomas, and dysplastic lesions are also observed in the colon as other types of preneoplastic lesions [5]. In humans, colorectal cancer is histologically classified as an adenocarcinoma [4][6]. In
we can observe the progression from normal intestinal epithelium to carcinoma.
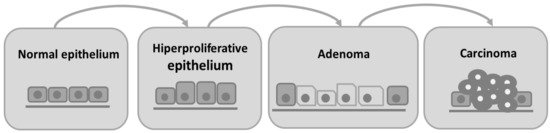
Schematic representation of adenoma–carcinoma multistep model. The normal cells of colon epithelium progress sequentially to a hyperproliferative epithelium, premalignant adenoma, and then carcinoma.
About 97% of colorectal cancers are spontaneous, and the remaining are due to one of two autosomal dominant inherited diseases: hereditary non-polyposis colorectal cancer (HNPCC) and familial adenomatous polyposis (FAP) [4][5]. The genetic mechanisms of spontaneous CRC are present in the adenoma–carcinoma sequence. Carcinogenesis is initiated with inactivating mutations in the tumor suppressor adenomatous polyposis coli (APC) gene, followed by an accumulation of mutations in the genes K-RAS, PI3K, DCC, SMAD2, SMAD4, and lastly the mutation in the tumor suppressor gene TP53 that determines the progression from the non-invasive to the invasive CRC [7].
Laboratory rodents are commonly used as animal models in experimental research because they are easy and cheap to maintain, their physiology and genetics are well studied, and they are mammals like humans [8]. They allow us to understand and follow the progression of diseases, enable the discovery and development of new preventive strategies, which can be later used in clinical trials. An ideal animal model of human disease should be simple, not expensive, and mimic the disease in terms of morphology, biochemical alterations, and biological behavior [4][9].
2. Rat and Mouse Colon and Rectum: Anatomy and Histology
The rat and mouse intestine are similar to that of humans concerning development, structure, and functions [9]. The large intestine comprises the cecum, the colon, the rectum, and anus, and it is responsible for the absorption of water and salt from feces [10] (
).
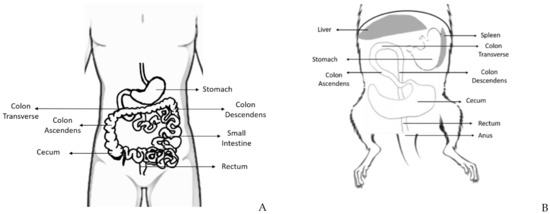
Schematic representation of some parts of the human (
) and rodent (
) digestive system, where is possible to observe the distinct portions of the large intestine: cecum, colon (ascendant, transverse, and descendent), rectum and anus, and its topographic anatomy. Human and rodent in supine position.
The cecum is a curved blind sac responsible for bacterial fermentation and empties into the proximal/right colon. Even though the rodents’ colon and rectum represent a percentage of the total size of the large intestine similar to the humans, the cecum is much bigger in rats, which may be attributed to the high fiber content of their diet [11]. The colon continues toward the pyloric region of the stomach and has the same histological structure of the gastrointestinal tract: mucosa, submucosa, inner circular and outer longitudinal tunica muscularis, and serosa [1]. Despite histological similarities, rats and mice do not have adipose tissue in the submucosa, unlike humans who have it in abundance. The colon can be divided into ascending (it leads cranially to the thoracic cavity), transverse colon (from the left to the right side), and descending colon (on the right side of the abdominal cavity). The rodents’ middle and distal colon corresponds to the human left colon [12]. The rectum is relatively short and indistinct from the distal colon. The anorectal junction has no stratified columnar epithelium, and the anal canal is lined by keratinized stratified squamous epithelium [10].
3. Rodents as Models of Colorectal Cancer
Although there is no ideal animal model that replicates all human disease aspects, the rodents are accepted as good models to study colorectal carcinogenesis because of their physiological similarity with humans, reproducible tumor induction, and the possibility to study the disease biopathology and test strategies for cancer prevention and treatment [4].
An ideal rodent model of colorectal cancer should develop carcinomas in the colon and rectum, with a high incidence in a short period, allow non-invasive monitoring of disease progression, and follow the histological and molecular characteristics of human colorectal cancer [8][12]. The models available to study colorectal cancer include spontaneous, induced, genetically engineered, xenograft, and syngeneic models (
).
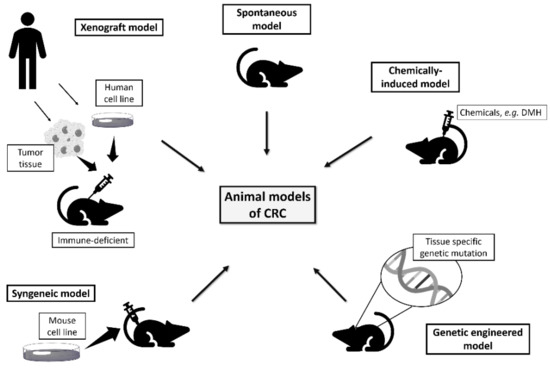
Rodent models available to study human colorectal cancer.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 1–31.
- Diet, Nutrition, Physical Activity and Colorectal Cancer; World Cancer Research Fund: London, UK; American Institute for Cancer Research: Washington, DC, USA, 2018.
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164.
- Kobaek-Larsen, M.; Thorup, I.; Diederichsen, A.; Fenger, C.; Hoitinga, M.R. Review of Colorectal Cancer and Its Metastases in Rodent Models: Comparative Aspects with Those in Humans. Comp. Med. 2000, 50, 16–26.
- Tanaka, T. Colorectal Carcinogenesis: Review of Human and Experimental Animal Studies. J. Carcinog. 2009, 8, 5.
- Machado, V.F.; Feitosa, M.R.; da Rocha, J.J.R.; Féres, O. A Review of Experimental Models in Colorectal Carcinogenesis. J. Coloproctology 2016, 36, 53–57.
- Raskov, H.; Pommergaard, H.-C.; Burcharth, J.; Rosenberg, J. Colorectal Carcinogenesis-Update and Perspectives. World J. Gastroenterol. 2014, 20, 18151.
- Fagundes, D.J.; Taha, M.O. Modelo Animal de Doença: Critérios de Escolha e Espécies de Animais de Uso Corrente. Acta Cirúrgica Bras. 2004, 19, 59–65.
- Johnson, R.L.; Fleet, J.C. Animal Models of Colorectal Cancer. Cancer Metastasis Rev. 2013, 32, 39–61.
- Oliveira, R.C.; Abrantes, A.M.; Tralhão, J.G.; Botelho, M.F. The role of mouse models in colorectal cancer research—The need and the importance of the orthotopic models. Anim. Model Exp. Med. 2020, 3, 1–8.
- Treuting, P.M.; Dintzis, S.M.; Montine, K.S. Comparative Anatomy and Histology: A Mouse, Rat, and Human Atlas; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-802919-0.
- Vdoviaková, K.; Petrovova, E.; Maloveska, M.; Krešáková, L.; Teleky, J.; Elias, M.; Petrášová, D. Surgical Anatomy of the Gastrointestinal Tract and Its Vasculature in the Laboratory Rat. Gastroenterol. Res. Pract. 2016, 2016, 2632368.
