Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Thorsten Steinberg and Version 2 by Amina Yu.
The periodontium is a complex composite tissue consisting of the gingiva, the periodontal ligament (PDL), cementum, and the alveolar bone. Beneath harboring various cell types, amongst others, gingival keratinocytes (GKs), gingival fibroblasts (GFs), periodontal ligament fibroblasts (PDLFs), cementoblasts and osteoblasts, the ECMs of the different periodontal cell and tissue entities are considerably different .
- mechanotransduction (MT)
- nuclear mechanotransduction (NMT)
- YAP/TAZ
- extracellular matrix (ECM)
- gingipain proteases
- periodontitis
- oral squamous cell carcinoma (OSCC)
- regeneration
1. The ECM, Focal Adhesions and Adherens Junctions in Periodontal Health and Disease
The periodontium is a complex composite tissue consisting of the gingiva, the periodontal ligament (PDL), cementum, and the alveolar bone. Beneath harboring various cell types, amongst others, gingival keratinocytes (GKs), gingival fibroblasts (GFs), periodontal ligament fibroblasts (PDLFs), cementoblasts and osteoblasts, the ECMs of the different periodontal cell and tissue entities are considerably different [1][39].
The cementum and the alveolar bone consist of an inorganic hydroxyapatite matrix and collagen type I as the major organic compound [2][40]. Additionally, the glycosaminoglycans (GAGs) decorin and biglycan as well as the glycoproteins (GPs) osteonectin, osteopontin, fibronectin, and osteocalcin can be found in the ECM of both tissues [3][4][41,42]. The PDL harbors the fibrous collagen types I and III, and small amounts of collagen V and VI [5][43]. Above, the basement membrane collagen types IV and VII and collagen type XII, which is important for fibrillar organization, are expressed in the PDL [6][44]. Elastin, fibronectin (FN) and chondroitin-/dermatan-/keratin-sulfate, containing GAGs, also support PDL function [7][45].
The gingiva contributes to periodontal integrity via epithelium and connective tissue. While the GKs form a stratified squamous epithelial layer, GFs are embedded in a lamina propria with collagen type I, III, elastin and many other macromolecules [8][46].
Physical ECM properties are key determinants of cell behavior in vitro and in vivo. The stiffness of the ECM, quantified by the Young’s modulus, as well as viscoelastic properties, spatial arrangement of adhesion points, and other geometric constraints, influence cellular responses through MT and other signaling hubs [9][10][11][14,47,48]. This means that ECM composition, homeostasis and MT are tightly coupled and are, therefore, highly interdependent [12][49]. Cell morphology, migration, proliferation, differentiation, and apoptosis are consequently not only influenced by biochemical signals, but also by the direct mechanical properties of the respective ECM environment [13][50]. Interestingly, cellular responses, namely the actomyosin-derived cell-inherent contraction forces, as discussed below, seem to directly reflect ECM stiffness, meaning that the Young’s modulus of the ECM is encoded within the cell’s response to that specific microenvironment [14][51].
Mechanistically, the above-mentioned ECM constituents directly or indirectly, i.e., mediated through adaptor proteins, interact with neighboring cells via surface receptors. Regarding MT, the family of integrin proteins is especially important as they are the core linking hub between the ECM and the cytosol. Within the plasma membrane, integrins form heterodimers, which are composed of an α- and a β-subunit. Various combinations of heterodimers have been described in different experimental systems and they proved to have different ligand specificity. Table 1 summarizes the most important integrin heterodimers and their corresponding ligands relevant to periodontal MT [15][16][17][18][19][20][21][52,53,54,55,56,57,58]. As can be seen, one heterodimer can sometimes bind more than one ligand (e.g., α1β1-integrin) and one ligand, such as fibronectin, is recognized by multiple heterodimers. Therefore, the tissue- or cell-specific expression pattern of integrins determines its interaction with the ECM. Periodontal cell populations foremostly harbor α2β1, α3β1 and α5β1 integrins. Of interest, the expression pattern of periodontal integrins changes in response to damage or during wound healing or carcinogenesis [22][59].
Table 1. Selected integrin heterodimers and their corresponding ligands.
| Integrin Heterodimers | α1β1 | α2β1 | α3β1 | α5β1 | αVβ1 | αVβ3 | αVβ5 |
|---|---|---|---|---|---|---|---|
| Ligand(s) | Different collagens (e.g. type I) |
Different collagens (e.g. type I) | laminins | fibronectin | fibronectin | fibronectin | vitronectin |
Upon mechanical loading, such as during mastication, occlusion or orthodontic treatment, the ECM of the periodontal tissues is deformed. Exemplarily, occlusion forces that exert compressive load onto a tooth are transmitted to the PDL, which serves as a push-pull transducer [23][60]. This means that the ECM components within the PDL, such as the collagen fibers, are stretched. As they are either directly or indirectly connected to the integrin receptors and these proteins undergo a conformational change, which is herewith the consequence of the initial mechanical stimulus. Therefore, these surface receptors are considered mechanoreceptors, as they transmit the ECM’s physical state into the cell’s interior.
Intracellularly, integrins are linked to various signaling proteins, which function as a molecular clutch that couples integrin–ECM interaction to intracellular biochemical signaling, the centerpiece of MT. Histologically, these integrin-dependent cell-to-matrix connection structures are called FAs [24][15].
Next to the plasma membrane, a plethora of proteins form the “integrin signaling layer”, where focal adhesion kinase (FAK), the head domain of talin and paxillin interact with the cytoplasmic integrin domain. Vinculin and the tail of talin are designated as the “force transduction layer” [25][61]. Finally, these proteins are linked to the actin cytoskeleton through an “actin binding layer”, which consists of α-actinin, vasodilator-simulated phosphoprotein (VASP), and zyxin [26][62]. Beneath the listed proteins, many other cellular key players, such as sarcoma (Src)-family kinases or the extracellular signal-regulated kinases 1/2 (ERK1/2) have been shown to directly interact with FAs and regulate the activity and assembly status of its components (Figure 1A) [24][27][28][15,63,64].
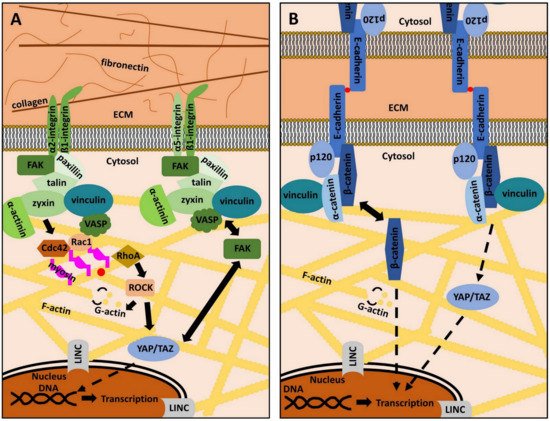
Figure 1. The role of focal adhesions and adherens junctions in mechanotransduction. (A): Focal adhesions (FAs) are adhesion structures that bind extracellular matrix (ECM) ligands via integrin receptors. The latter are composed of varying combinations of an α- and a β-subunit. Each heterodimer has specific ECM ligands (see Table 1). Intracellularly, integrins are linked to various signaling molecules that constitute a molecular clutch, which transmits mechanical information from the ECM into the cell’s interior and vice versa. Focal adhesion kinase (FAK), paxillin, talin, zyxin, vinculin, vasodilator-simulated phosphoprotein (VASP) and α-actinin are examples of important FAs proteins, connecting integrin receptors to the actin cytoskeleton (yellow). The small GTP-binding proteins Ras-related C3 botulinum toxin substrate 1 (Rac1), cell division control protein homologue 42 (Cdc42), and Ras homologue A (RhoA), together with Rho-associated, coiled-coil-containing protein kinase (ROCK) modulate the dynamic de- and repolymerization of globular (G)-actin (yellow dots) and filamentous (F)-actin. FAK activity and subcellular localization of yes-associated protein (YAP) and its cellular homologue transcriptional co-activator with PDZ motif (TAZ) are strongly interconnected. The linker of nucleoskeleton and cytoskeleton (LINC) complex couples the cytoplasmic cytoskeleton to the nucleus. Both mechanisms are important to regulate gene expression in response to mechanical signals. Details are described in the main text. (B): Cell-to-cell adhesion depends on adherens junctions (AJs). Cadherins, as exemplified by E-Cadherin, are transmembrane proteins that bind other cadherins on neighboring cells in a Ca2+-dependent manner (red dots). Intracellularly, cadherins are linked to various proteins, such as p120, α-catenin, β-catenin, and vinculin, which indirectly connect cadherins to the actin cytoskeleton (yellow). YAP/TAZ regulation is also dependent on AJs integrity. β-catenin can also serve as a transcription factor in the nucleus and its subcellular localization contributes to determining cell behavior. Further details are described in the main text.
The actin cytoskeleton is the common downstream target of all MT pathways [29][30][65,66]. It not only consists of filamentous (F) actin, but also of actin-regulating proteins (ARPs), which regulate the dynamic building and destruction of the filaments from globular (G) actin monomers. G-actin binds adenosine-triphosphate (ATP) and hydrolyses ATP to adenosine-diphosphate (ADP) within F-actin. This reaction goes along with conformational changes of actin monomers and contributes to the dynamic turnover of actin-related cytoskeletal structures [31][32][33][67,68,69]. Actin polymerization is regulated by a class of guanosine–triphosphate (GTP) binding proteins, known as Ras homologue A (RhoA), cell division control protein homolog 42 (Cdc42), Ras-related C3 botulinum toxin substrate 1 (Rac1), as well as the Rho-associated, coiled-coil-containing protein kinase 1 (ROCK1) (Figure 1A) [34][35][36][37][70,71,72,73]. These small GTPases are addressed by the mechanotransducing proteins of FAs and their activity state determines the polymerization of G-actin as well as the formation of lamellipodia or filopodia. ROCK1 is even more directly involved in periodontal differentiation, homeostasis, and regeneration, as the inhibition of ROCK1 prevents proper the differentiation of PDL cells into osteoblasts and reduces ECM regeneration via the downregulation of collagen I and fibronectin [38][39][74,75]. These findings underscore the essential role of actin cytoskeleton regulation in the MT of the periodontium.
Actin filaments are additionally stabilized by ARPs, such as Arp2/3, tropomyosin or profilin. Contrarily, severing proteins, such as gelsolin, support F-actin depolymerization or destruction [40][41][76,77]. Altogether, the complex interplay of actin regulatory proteins governs actin’s dynamic de- and repolymerization and enables complex cellular processes, such as cell division or migration [42][78]. Further details of these complex cytoskeletal regulatory principles are beyond the scope of this review and interested readers are referred to other comprehensive discussions on this subject [43][44][79,80].
Thus far, the description of the actin cytoskeleton and its dynamics does not explain how cells maintain their shape during mechanical stimulation and how cellular integrity can be achieved by mechanisms of FAs-related signaling. To this end, cells need the ability to actively generate forces to withstand external deformation or to exert mechanical stimuli on their environment. This is possible through the action of cytoplasmic motor proteins, known as myosins, which are coupled to the actin cytoskeleton. Via the hydrolysis of ATP, these motor proteins can actively move along actin filaments [45][46][47][81,82,83]. Besides functions in cargo transport, myosins can, therefore, generate tension and traction forces through the relative displacement of actin filaments [48][49][50][51][84,85,86,87]. This mechanism immediately explains that FAs are not only outside-in signaling platforms that transmit mechanical ECM signals into the cell, but that actomyosin-generated cytoskeletal forces can also be transmitted to the ECM with the help of integrins and their neighboring adaptor proteins. Therefore, FAs are bidirectional mechanosensitive signaling hubs.
Of interest, this is of enormous importance for ECM homeostasis and regeneration in the PDL, as FN and collagen fibrillogenesis depends on intracellularly derived contractile forces. Actin stress fibers serve as a guide trail for the centripetal movement of α5β1-bound FN, which supports FN-FN interactions [52][53][88,89]. Collagen molecules can then be deposited on the pre-existing FN fibrils. As this process is force-dependent, characteristic ECM structures, such as the parallel arrays of collagen fibers within the PDL, can be explained via this mechanism and are, therefore, a result of bidirectional MT related to FAs. Interestingly, a recent study gave new insights into the actual nonuniformity of the PDL and revealed the mechanical properties of different subregions within the gomphosis. The so-called collar region is characterized by a high proportion of collagen type I, making it resistant to tensile forces due to high mechanical stiffness. Contrary to that, the furcation region is less stiff and contains less type I collagen, which seems to be associated with a dual function in resisting compressive loads [54][30].
In periodontitis, collagen and other ECM components are degraded by proteases, such as MMPs or bacteria-derived gingipains (see Section 4) [55][90]. This changes integrin-dependent MT, leading to a decrease in intracellular actomyosin contractility (outside-in-signaling). Consequently, inside-out signaling is also impaired, which leads to incorrect collagen fibril deposition, worsening the catabolic destruction of the periodontium [56][22][36,59].
FAs related signaling also comprise an important step in (alveolar) osteocyte differentiation and the cell’s response to fluid shear stress. The reduction in the protein sclerostin by mechanical loading is mediated by FAK-dependent phosphorylation of the histone deacetylase 5 (HDAC5), which is translocated into the nucleus in response to this post-translational modification. Sclerostin suppression leads to an increase in bone formation and thus mediates an adaptation process by which shear stress leads to mechanical strengthening of the exposed tissue [57][58][91,92].
Besides cell-to-matrix adhesion, cell-to-cell adhesion is of great importance, especially in epithelial tissues, such as parts of the gingiva. The main constituents of the so-called adherens junctions (AJs) are cadherin (Cad)-family members (Cads), which are Ca2+-dependent, membrane-embedded proteins that connect cells via homophilic interaction [59][93]. In the cytoplasm, Cads are connected to various proteins, amongst others α-catenin, p120, vinculin or β-catenin [60][61][62][63][22,94,95,96]. These adaptor proteins are comparable to the integrin-linked intracellular mechanotransducers, as they connect Cads to the actin cytoskeleton. The same mechanisms and principles as discussed above also apply for AJs and qualify the actin cytoskeleton not only as the common final pathway of AJs and FAs signaling, but also as a crosstalk platform that integrates mechanical cues transmitted through various MT pathways [64][65][97,98]. ERK1/2, YAP, and its cellular homologue transcriptional co-activator with PDZ motif (TAZ), vinculin and FAK, are both addressed by AJs and FAs, underscoring the complex mutuality of cell-to-cell and cell-to-matrix adhesion (Figure 1B) [66][67][68][69][17,99,100,101]. This is the reason why current systemic approaches try to elucidate the tissue- and cell-type specific interplay and fine-regulation of signaling crosstalk related to MT [70][13]. It is of great interest, to shed light into these principles in periodontal tissues.
In the periodontal context, Cads fulfill different functions, ranging from maintenance of cellular differentiation to epithelial barrier function, tumor suppression, and MT-related tissue homeostasis. Specifically, β-catenin, in its function as a transcriptional regulator, is important in PDLF differentiation and simultaneously inhibits the cementoblastic phenotype [71][102].
The epithelial E-Cad, which is expressed in GKs, plays a significant role both in periodontitis and oral carcinogenesis. Patients suffering from periodontitis show decreased protein levels of E-Cad, which is indirect evidence for a dysfunctional epithelial barrier function. This further promotes the inflammatory process [72][103]. Downregulation of E-Cad has also been reported in many carcinomas, where it represents a key step during epithelial-to-mesenchymal transition (EMT). Thereby, epithelial cells detach from their surrounding cells and develop a migratory, fibroblastoid phenotype, which is a prerequisite for tissue invasion and metastasis. Regarding oral carcinogenesis, reduced expression levels of E-Cad and β-catenin are indicators of the progression from dysplasia to cancer and an aggressive OSCC phenotype [73][104]. As chronic inflammation is a risk factor for cancer development, the loss of E-Cad-related barrier function and MT offers valuable insights into the link between periodontitis and OSCC [74][75][105,106].
Beneath E-Cad, Cadherin 11 (Cad11) is another member of the cadherin family expressed in periodontal tissues [76][77][107,108]. Upon mechanical loading, expression of Cad11 and β-catenin decreases in PDLFs, which consequently leads to a reduction in collagen 1 synthesis and changes in cellular morphology [78][109]. These findings clearly show that not only FAs but also AJs are involved in ECM homeostasis and regeneration and that cell-to-cell adhesion is not limited to intercellular information exchange. This hypothesis is supported by other experimental results, where knock-down of Cad11 impairs elastin and collagen synthesis [79][110]. In mice, Cad11 deficiency reduces cell contractility, which again represents the involvement of AJs in both ECM structure and MT [80][111].
Taken together, the findings presented in this chapter show that the ECM and its molecular composition are important determinants of periodontal cell behavior in the context of MT. As the periodontal tissues are affected by diseases, such as periodontitis or OSCC development, the relevance of MT in these pathophysiological processes is of clinical relevance. So far, the convergence of FAs and AJs-mediated signal transduction on the cytoskeleton has been discussed. However, the mechanisms by which the information encoded within the contractility and tension of the actin-cytoskeleton is translated into cellular adaptation, which depend on further cellular key players, such as the mechanoresponsive co-transcriptional activators YAP/TAZ and the nuclear cytoskeleton, will be discussed in the subsequent sections.
2. Conclusions
MT-immanent mechanobiological signals are key determinants of cell behavior and tissue adaptation to the external cell and tissue environment. The interplay of molecular mechanosensors and mechanotransducers is complex and represents the tight interrelationship between different cellular compartments and signaling hubs. FAs, AJs, YAP/TAZ, the cytoskeleton, LINC, and the nucleoskeleton only represent a small subset of cellular players involved in MT and NMT but reveal important principles of how the cell percepts and integrates biophysical mechanical information. Periodontal tissues are of special interest to MT research and are a paradigm of how the above-discussed signaling networks, cascades, and molecules govern tissue development, homeostasis and regeneration, cell proliferation, differentiation, and pathologic processes, such as periodontitis or OSCC. By further elucidating the cell-type specific and spatiotemporal fine-tuning of mechanobiological processes, the future translation of these principles into clinical applications will prove to be a strong tool in the field of oral regenerative medicine.
