Magnetic nanoparticles (MNPs) represent an attractive class of nanomaterials due to their unique physical and chemical features that allow them to respond specifically to magnetic fields. Among the magnetic class of materials, iron oxide-based nanoparticles are the only inorganic nanomaterials that have been approved by the Food and Drug Administration (FDA) for medical applications. Magnetic nanomaterials are particularly appealing for cancer immunotherapy due to their unique features, which include (i) the traceability of their signal by magnetic resonance imaging (MRI) or by magnetic particle imaging (MPI) techniques ; (ii) their exploitation as carriers to promote the accumulation and the efficient delivery of biotherapeutic compounds, such as genes and peptides, into a specific target cell or tissue; (iii) their ability to mediate the elimination of cancer cells through the production of a local thermo-ablative effect when exposed to an external alternating magnetic field, referred to as magnetic hyperthermia therapy (MHT); and (iv) their intrinsic immunomodulatory properties that can be harnessed to further promote or modulate the immune function.
- magnetic nanostructures
- surface chemistry
- cancer immunotherapy
- immune therapeutics
- combinatorial immunotherapy
- vaccines
- immunogenic cell death
C1. Introduction
In the past years, a cumulative number of studies have highlighted the critical regulatory role of the immune system in tumour biology [1]. Indeed, it has been proven that the host’s immune system interacts with tumour cells throughout the process of cancer formation and progression, shaping the immunogenicity of tumours, either inhibiting or promoting tumour growth and development [2]. These findings have provided the basis for the development of novel cancer therapeutics; however, such complex mechanisms are still a matter of study and pertain to the medical breakthroughs started in the last decade, but which still hold great promise [3].
T cells have been shown to be major players in the generation of protective immunity and, as pointed out by Galon et al., the presence of tumour-infiltrating CD8+ T cells greatly influences the fate of a tumour [4]. Functional analyses of tumour-infiltrating T cells have contributed to a more detailed tumour stratification, which was found to better represent prognostic tools in the treatment of colorectal carcinoma than standard histopathological classifications [4][5][6][4,5,6].
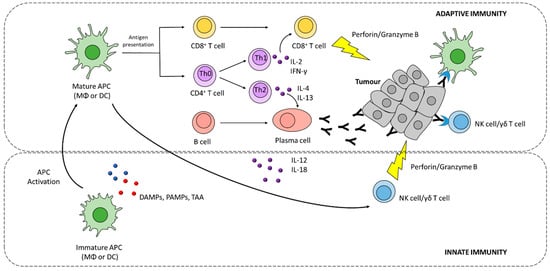
Although the activation of antigen-specific CD8+ T cells is considered a key step for an effective anti-tumour response, it often fails to eradicate cancer cells without a proper activation of the innate immune system [7]. Innate immune cells, such as natural killer (NK) cells, γδ T cells, and macrophages, can recognize and kill tumour cells [8]. Innate immunity is activated in response to a broad variety signals, including pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), which are recognized by pattern recognition receptors (PRRs) expressed by immune cells, such as macrophages, dendritic cells, and NK cells. Particularly relevant in tumour control is the role of NK cells, which together with T cells play a complementary function in contrasting tumour growth and propagation [9][10]. Indeed, NK cells can recognize cells with reduced or absent expression of major histocompatibility complex (MHC) class I molecules, thus ensuring the elimination of cancer cells that evade T cell-mediated killing [9][10].
Besides exerting its effector activity, innate immune cells have a pivotal role in directing and shaping the type and strength of anti-tumour adaptive immune responses, through the release of pro-inflammatory signalling molecules such as interferon gamma (IFN-γ) and interleukin-12 (IL-12) (Figure 1) [10][11][11,12].
Adaptive immunity, involving CD8+, CD4+T cells, and B cells, drives a tumour-specific response aimed at eradicating tumour cells, and contributes to the development of an immunological memory potentially protecting from tumour recurrence.
A series of events are required for the generation of antigen-specific anti-tumour responses, starting with the release of tumour antigens that are taken up by antigen-presenting cells (APCs), such as macrophages (MΦ) and dendritic cells (DCs), and processed into peptides [12][13][13,14]. Processed epitopes are loaded onto MHC I or II molecules for cross-presentation and presentation to CD8+ and CD4+ T cells, respectively (Figure 1) In order to induce effective T cell responses, antigen presentation must be supported by costimulatory signals induced by innate immune cells, such as pro-inflammatory cytokines and costimulatory ligands [14][15]. Furthermore, tumour antigens can also promote B cell activation by binding B cell receptor (BCR) [15][16].
Effector T cells must infiltrate tumour tissues where they recognize tumour antigens presented via MHCI, and selectively kill tumour cells. Tumour cell killing further promotes the release of tumour antigens, which can serve to prime additional T cell responses [16][17]. CD4+ T cells (or T helper cells, Th cells) regulate both cytotoxic cellular immune responses and B cell-dependent antibody production. Th1 cells are characterized by the production and release of IFN-γ, which support tumour cytotoxicity synergistically with TNF-α (Figure 1) [16][17].
Despite the sophisticated and concerted anti-tumour immune response, the protective immunity of cancer patients often fails, as tumour cells have developed multiple mechanisms to evade immune surveillance [17][19]. These mechanisms are ascribable to (i) reduced immune recognition, either by the loss of immunogenic tumour antigens or by the downregulation of antigen-presenting molecules; (ii) increased tumour cell resistance to cytotoxic pathways; (iii) induction of an immunosuppressive tumour microenvironment, through the expression of immunoregulatory molecules (programmed death-ligand 1, cytotoxic T-lymphocyte antigen-4); the recruitment of regulatory cells, including myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and tumour-associated macrophages (TAMs), that will secrete immunosuppressive cytokines, such as IL-10 and transforming growth factor-beta (TGF-β).
The identification of the immune-evading mechanisms of tumours is resulting in novel therapeutic strategies aimed at reversing tumour immune evasion. Particular interest is given to the development of strategies that can enhance the recognition of tumour cells by the immune system, such as therapeutic vaccines, adaptive cell therapy, and immunogenic cell death (ICD)-inducing treatments [5][18][5,20]. Other approaches are aimed at potentiating anti-tumour responses through the employment of immunotherapeutics, targeting immune checkpoint molecules (i.e., ICBs), and immunomodulators, such as immune adjuvants and cytokines, which, in turn, enhance cytotoxic T cell functions [5][18][5,20].
However, standard soluble immunotherapy has often failed to trigger effective cancer immune responses. This lack of effectiveness is due to an inadequate delivery of immunomodulators, as a consequence of their rapid degradation and elimination as free molecules. Likewise, DCs inappropriately uptake soluble vaccine antigens and adjuvants, resulting in an impaired antigen presentation and priming of anti-tumour immune responses [19][20][21][21,22,23].
To overcome the delivery limitations of soluble immunotherapies, nanoparticles have emerged as versatile vectors for the encapsulation, protection, and spatial–temporal-controlled delivery of antigens, adjuvants, and immunomodulators, while allowing, by controlling the structural parameters of the nanoparticles, to increase the uptake efficiency to targeted cells [21][22][23,24].
Magnetic nanoparticles (MNPs) represent an attractive class of nanomaterials due to their unique physical and chemical features that allow them to respond specifically to magnetic fields [23][25]. Among the magnetic class of materials, iron oxide-based nanoparticles are the only nanomaterials that have been approved by the Food and Drug Administration (FDA) for medical applications [24][26]. Magnetic nanomaterials are particular appealing for cancer immunotherapy due to their unique features, which include (i) the traceability of their signal by magnetic resonance imaging (MRI) or by magnetic particle imaging (MPI) techniques [25][27]; (ii) their exploitation as carriers to promote the accumulation and the efficient delivery of biotherapeutic compounds, such as genes and peptides, into a specific target cell or tissue; (iii) their ability to mediate the destruction of cancer cells through the production of a local thermo-ablative effect when exposed to an external alternating magnetic field, referred to as magnetic hyperthermia therapy (MHT)
Progress on the synthesis and functionalization procedures in the last few decades have enabled to obtain MNPs with very-well-controlled physicochemical features, including size, shape, crystallinity, charge, magnetic properties, and surface functionalities [23][26][25,28]. Furthermore, compared to nanoformulations conventionally applied for cancer immunotherapy, such as polymeric and lipid nanoparticles, MNPs can be easily synthesized with inexpensive procedures suitable for large-scale production [27][29].
All these features render MNPs a particularly appealing platform for the development of combinatorial immunotherapies with enhanced therapeutic efficacy, by simultaneously tackling different tumour immune-escape mechanisms [23][26][28][25,28,30].
This review provides an overview of the recent advances in the use of MNP-based nanostructures to enhance the effectiveness of cancer immunotherapy. We highlight the impact of the physicochemical features and surface engineering of magnetic delivery platforms on their therapeutic effect, and describe the use of magnetic nanosystems to enable the development of combinatorial therapeutic approaches for improving the efficacy of cancer immunotherapies.
2. Magnetic Nanomaterials for Cancer Immunotherapy: Synthesis and Properties
MNPs, thanks to their response to a magnetic field of a different nature, show unique advantages compared to other types of nanocarriers, which make them also promising for the field of cancer immunotherapy [23][26][25,28]. In particular, their unique capability as contrast agents in non-invasive molecular imaging techniques, such as MRI and MPI, can assist in the monitoring of the accumulation of magnetic nanoformulations at the target site [29][31]. Likewise, the utilization of MNPs as heat mediators in magnetic hyperthermia enable tumour ablation and the priming of anti-tumour immunity [30][31][32,33]. As the efficiency of MNPs as contrast agents as well as heat mediators depends on their physicochemical properties, the optimization of these properties is required for the synthesis of high-quality MNPs with a tunable size, shape, and composition.
MNPs usually have an overall hydrodynamic size smaller than 100 nm with a typical magnetic core size below 30 nm. Their magnetic properties can be tuned by the choice of size, shape, crystalline structure, and composition, among which iron oxides, such as magnetite (Fe3O4) and maghemite (γ-Fe2O3), or other mixed ferrites, such as zinc-ferrite (ZnFe2O4) or manganese-ferrite (MnFe2O4), are the most relevant for immune applications given the minimized toxicity of the Fe, Zn, and Mn ions of which these ferrites are made. Moreover, the magnetic properties can be fairly modulated by varying other physicochemical features related to surface structure and colloidal stability, or in other words, to the aggregation state of individual MNPs [23][26][25,28]. Indeed, the magnetic properties of iron oxide nanoparticles can be further redesigned by clustering a controlled number of individual superparamagnetic nanoparticles into superparamagnetic nanoparticle clusters, often termed magnetic nanobeads [32][34].
A wide range of methods have been reported for the preparation of high-quality MNPs, including wet chemical techniques (co-precipitation, solvothermal, thermal decomposition, sol-gel synthesis, microemulsion, and chemical redox), physical processes (gas-phase deposition and electron beam lithography), and bacterial and microorganism-based synthesis (Figure 2) [33][34][35][35,36,37]. Among these methods, co-precipitation, solvothermal, and thermal decomposition are the most commonly employed manufacturing processes. Usually, wet chemical methods, such as the thermal decomposition method, involve the decomposition of precursors into liquid media, such as 1-octadecence, at a high temperature and in the presence of capping agents and surfactants, such as oleic acid [34][36]. During the synthesis, the reaction conditions, including temperature and pressure, play important roles in determining the morphology and size of the MNPs, and consequently their magnetic properties [34][36].
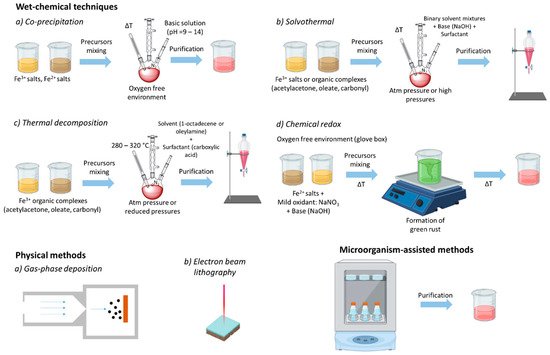
For instance, thermal decomposition and the solvothermal method can deliver MNPs soluble in non-aqueous media, as they are coated by alkylic surfactant molecules (such as oleic acid). On the contrary, MNPs prepared by the co-precipitation method are directly soluble in aqueous media, being coated by tiny polar molecules (such as sodium citrate). In both cases, the MNPs can be stabilized and functionalized by adding/replacing an outer layer of the shell coating, which can have multiple roles. Third, it can also introduce chemical groups feasible for the further functionalization of the MNPs with different biomolecules [26][28].
To optimize the effectiveness of MNP-based immunotherapy, key structural design considerations must be taken into account.
Early studies focused on nanoparticle delivery to tumours exploiting a mechanism known as the enhanced permeability and retention (EPR) effect [36][38]. In particular, a size of less than 100 nm has been identified as optimal to ensure higher accumulation of iron oxide nanoparticles to tumours [36][38]. While these delivery strategies of nanoparticles directly to the tumour are becoming an increasingly appealing option for reshaping the tumour microenvironment, the design of novel nanosystems for cancer immunotherapy is also aimed to trigger tumour-specific responses by harnessing the natural tropism of nanoparticles towards secondary lymphoid organs (including spleen and lymph nodes), where T cell priming occurs. For instance, lipidoid-stabilized iron oxide nanoparticles with a 30 nm core size had approximately 20-fold higher capacity to carry biomolecules such as antigens and adjuvants to lymph nodes via lymphatic drainage compared to smaller (10 nm) or larger (100 nm) nanoparticles [37][39].
Delivery platforms with a large size (>500 nm) lead to a prolonged retention at the injection site and are mostly taken up by local DCs, which after nanoparticle internalization will migrate to the draining lymph nodes [38][40]. Interestingly, a biodistribution study shows that transport through the lymphatic system results in an ~1000-fold increase in accumulation into local draining lymph nodes, which can substantially reduce off-target side effects and improve T cell priming [39][41]; thus, nanoparticles with a size around 30 nm may be preferred for lymph node targeting.
Among the various morphologies of iron oxide nanoparticles, octapod- and plate-shaped nanoparticles with a similar aspect ratio and surface charge showed a higher immunomodulatory potential by inducing inflammasome activation [40][43]. However, spherical nanoparticles diffuse less efficiently through the vascular wall than rod- and bar-shaped nanoparticles with a similar size range [41][44]. It has been reported that reshaping iron oxide nanoparticles from spheres to cubes markedly increases their heating performance [42][46]. In addition, controlled clustering of iron oxide nanocubes into nanoparticle assemblies that are anisotropic in their shape can preferentially increase the MNPs’ heating power [43][47].
Generally, local administration of positively charged MNPs promote a stronger immune response than nanoparticles having a net negative or neutral surface charge [44][45][50,51]. Though, cationic nanoparticles display reduced tissue penetration, probably due to the interaction with the negatively charged components of the ECM [46][52]. Consequently, positively charged nanoparticles are usually retained at the injection site, where they can be more easily taken up by local DCs, compared to neutral and anionic nanoparticles [47][53]. Contrarily, slightly negatively-charged nanoparticles or neutral nanoparticles possess a superior circulation time and therefore may achieve enhanced tumour accumulation when systemically injected.
Besides surface charge, other surface physicochemical properties can affect tremendously the behaviour of MNPs in biological conditions, thus improving targeting efficiency, biocompatibility, therapeutic efficacy, stability, loading capacity, and efficiency [48][56]. After synthesis, most of the MNPs prepared by non-hydrolytic methods are capped by long hydrophobic chains that act as stabilizing agents, making them soluble in organic solvents. Consequently, surface modification of these nanoparticles is firstly required to enable their water solubilisation, making them ready for any further modification. For nanoparticles produced by hydrolytic methods, charged capping molecules, such as citrate molecules, are usually exchanged with other spacer ligands, such as polyethylene glycol derivatives or dextran shells, which help to improve long-term colloidal stability in biological environments [33][34][35,36].
Surface modification has been also exploited to facilitate the loading of immunomodulators that can activate and/or boost the immune responses in patients [49][50][57,58]. The most common surface modification strategies, such as ligand exchange, porous silica, phospholipid, and polymer coating, have been extensively explored to facilitate loading of various immunotherapeutics, including TLR agonists and monoclonal antibodies onto MNPs through non-covalent or covalent interactions, taking advantage of the properties associated with the coating material (e.g., large pore size of the porous silica shell and large number of reactive functional groups of polymers) (Figure 3) [49][50][51][52][57,58,59,60].
A range of surface chemistry strategies has also been explored to facilitate multiple-drug loading. In this regard, the highly porous structures of mesoporous silica-coated ferumoxytol nanoparticles were capable to load both a checkpoint inhibitor (anti-PD-L1 antibody) and chemotherapeutic drug (cabazitaxel) for achieving an anti-tumoural synergistic effect against prostate cancer [49][57]. Likewise, surface modification of MNPs with a lipid shell enabled the co-encapsulation of the α-helix-antigen fusogenic peptide (α-AP) with indocyanine green (ICG), an imaging agent, leading to the development of a theragnostic nanoplatform (α-AP-fmNP) [50][58]. In the context of DC-based vaccines, α-AP-fmNP-loaded DCs were revealed to possess antigen presentation capability and their in vivo migration toward lymph nodes, as confirmed by imaging techniques, was dramatically enhanced upon application of magnetic force, thus preventing anergy and resulting in a significantly improved anti-tumour efficacy [50][58].
In a pioneer study, iron oxide nanoparticles coated by carboxy-dextran were proven to activate the NF-κB pathway in macrophages, which plays important roles in inflammatory responses and immune activation/regulation, promoting M1 macrophage polarization [53][62]. Thus, it appears clear that the coating materials of the iron oxide nanoparticles have a significant influence on mediating the iron oxide nanoparticle’s immunomodulatory properties. Mulens et al., in this regard, reported that the polyethyleneimine (PEI)-coated superparamagnetic iron oxide nanoparticles induced Toll-like receptor 4 (TLR4) activation in both murine and human macrophages, with consequent upregulation of IL-12 production and surface expression of maturation markers such as CD40, CD80, and CD86, indicating M1 polarization [54][65]. Contrarily, treatment with different iron-based formulations (Venofer, Ferinject, and Ferrlecit) reduced the differentiation of monocytes into M1 macrophages and myeloid DCs [55][67], suggesting that the M1-polarization induced by PEI-iron oxide nanoparticles observed in the earlier studies could be influenced by the coating material
Besides the coating material, the chemical composition of the MNP core is another factor that influences the immunomodulatory properties of these nanoparticles. For instance, it has been shown that oppositely to hematite phase (Fe2O3) nanoparticles, magnetite (Fe3O4) iron oxide nanoparticles display a great capacity in promoting macrophage polarization from a pro-tumoural M2 into an anti-tumoural M1 profile [53][56][62,63].
3. Combinatorial Approaches to Potentiate Cancer Immunotherapy
| Magnetic Nanostructure | Surface Chemistry | Immunotherapeutic Drug | Therapeutic Approach |
Remarks | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iron nanoparticles (nano-aAPC) | Dextran functionalized with both MHC-Ig dimer and anti-CD28 antibody | MHC-Ig dimer, anti-CD28 antibody | Adoptive immunotherapy | Application of an external magnetic field induced nano-aAPC aggregation on naive cells, enhancing T cell proliferation in vitro and following adoptive transfer in vivo. | [52] | [60] | ||||||||
| Iron oxide nanoclusters (Magnetosome) |
Cancer cell-derived plasma membrane functionalized with anti-CD205 antibody | TAAs, CpG ODN | Vaccine/Immune adjuvant | Cancer cell membranes serve as a reservoir of TAAs and their co-delivery with TLR9-agonist lead to a great proliferation of T-cells with superior cytotoxic activity. The application of an external magnet enhanced lymph node retention and anti-CD205-mediated CD8 | + | DCs targeting of nanoparticles. | [51] | [59] | ||||||
| Iron oxide nanoclusters (IO-LPMONs) |
Mesoporous organosilica shell having large pore size | OVA antigen | Vaccine/TAMs repolarization | Simultaneous T cell activation and TAMs repolarization induced strong inhibition of tumour growth. | [58] | [138] | ||||||||
| Iron oxide nanospheres (IO@FuDex | 3 | ) | Fucoidan and dextran functionalized with multiple antibodies | Anti-PD-L1, anti-CD3 and anti-CD28 antibodies | T cell activation/Immune checkpoint inhibitor | IO@FuDex | 3 | can directly induce T-cell activation and block the immunosuppressive PD-L1 pathways via intravenous administration. The combination of IO@FuDex | 3 | and magnetic navigation demonstrated a highly improved therapeutic efficacy. | [59] | [116] | ||
| Iron oxide nanoparticle-loaded micelles (poly(I:C)–Pt(IV)–IONP micelles) |
DSPE-PEG(2000)-Pt(IV) prodrug functionalized with poly(I:C) | Poly(I:C) | Immune adjuvant/Chemotherapy | Pt(IV) prodrug synergized with TLR3-agonist inducing a more potent activation of DCs than cisplatin and poly(I:C). | [60] | [139] | ||||||||
| Iron oxide superparticles (Fe | 3 | O | 4 | -R837 SPs) | Poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) copolymer | R837, anti-PD-L1 antibody | ICD/Immune adjuvant/Immune checkpoint inhibitor | Photothermal therapy promotes cancer cells killing, with consequent release of TAAs, and triggers the release of R837 immune adjuvant for a more effective vaccination strategy. Fe | 3 | O | 4 | -R837 SPs efficiently synergize with PD-L1 antibody to eliminate the primary tumours and prevent tumour metastasis to lungs/liver. |
[61] | [140] |
| Core-shell ferrite nanoparticles (CoFe | 2 | O | 4 | @MnFe | 2 | O | 4 | nanoparticles) | Dimercaptosuccinic acid molecule | Anti-PD-L1 antibody | ICD/Immune checkpoint inhibitor | Magnetic hyperthermia induced TAAs release eliciting a systemic immune response affecting distant metastatic tumours. The combined MHT and checkpoint inhibitor demonstrate the great potentials in inhibiting the growth of both primary and metastatic tumours. | [62] | [141] |
| FePt/MoS | 2 | -FA nanocomposites (FPMF NCs) | FePt capped by dimercaptosuccinic acid, MoS | 2 | modified by thiol-polyethylene glycol-folate | CpG ODN, anti-CTLA-4 antibody | ICD/Immune checkpoint inhibitor | PDT act as ICD inducer and its ability to inhibit primary tumours and prevent metastasis was significantly improved when combined with chemotherapy drug/immunotherapeutics. | [63] | [142] | ||||
| Janus nanobullets integrating chlorine e6 (Ce6) loaded, disulfide-bridged mesoporous organosilica bodies with magnetic heads(M-MONs@Ce6) |
Asymmetric mesoporous silica growth, coated with cancer cell membrane | Anti-CTLA-4 antibody | ICD/Immune checkpoint inhibitor | The combination of PDT and magnetic hyperthermia elicits ICD, resulting in tumour-specific immune responses. When combined with anti-CTLA-4 antibody, synergistically enables the eradication of primary and deeply metastatic tumours. |
[64] | [143] | ||||||||
| Iron nanoparticles (FeNPs) | Poly(acrylic acid) (PAA) co-grafted with dopamine (DA) and amine-terminated PEG (5 kDa) | R837 | ICD/Immune adjuvant/Immune checkpoint inhibitor | The combination of MNP-based MHT with local injection of nanoformulated TLR7-agonist and systemic injection of anti-CTLA4 antibody resulted in systemic immune responses that inhibited tumour metastasis and recurrence. | [65] | [144] |
