Low cost, sensitive, selective, and rapid methods for heavy metal ion (HMI) detection are of growing demand, and HMI biosensors have great potential in meeting this need due to their timeliness, cost-effectiveness and convenience in operation. The most widely reported peptide probe for HMI detection is glutathione (GSH), especially in case of lead ion (Pb2+) detection. GSH is highly stable, cost-effective, and easy to immobilize on a sensor.
- heavy metal ions
- glutathione
- biosensor
- nanoparticle
1. Introduction
2. Recognition of HMIs by GSH
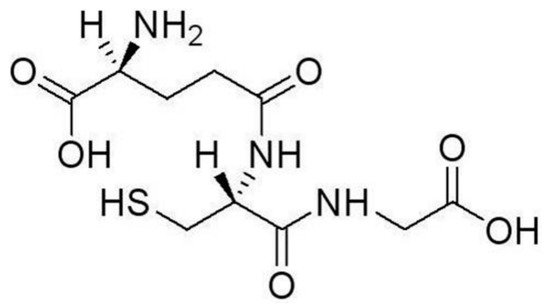
3. Heavy Metal Ion Detection Platforms Using Glutathione
3.1. Optical Techniques
Metallic nanoparticles (NPs) are between 1–100 nm in size with a high surface charge density, a high surface to volume ratio, and, oftentimes, special optical properties due to a localized surface plasmon resonance (LSPR) effect. For these reasons, metallic NPs have been widely used in diverse biological and chemical applications, especially for biochemical detection. Various types of biochemical molecules can be easily incorporated onto the NPs while retaining their biochemical activity. For HMI detection, most metallic NPs are made of gold and some are silver, with gold NPs (GNPs) being the most widely used material in HMI sensors. Other shapes of NPs, such as nanorods and nanostars, have been adopted for sensing [41][42][41,42].
As an important discovery originally found in the 1990s, semiconductor quantum dots (QDs) have attracted intense research interest for their excellent luminescence properties. In contrast to traditional fluorescence techniques, QDs have advantages in excitation spectra, photo-luminescence quantum efficiency, and so on [43][44][50,51]. Reports of new QD-based sensors are on the rise. Most of the reported QD-based HMI detection schemes utilized II–VI semiconductor QDs. As an example, using Mn-doped ZnS QDs, [45][52] reported a Pb2+ detection scheme by phosphorescence measurement. Phosphorescence is generated by the energy transfer from the lowest vibrational energy layer of the excited Mn2+ triplet state to the vibrational energy layer of the ground state. Mn–ZnS QDs are a popular II–VI semiconductor QD for biosensor applications. Because Mn–ZnS QDs can be functionalized without using deoxidants and other inducers, they are convenient to work with. Furthermore, Mn–ZnS QDs have a long-lived doped emission, which can set the signal of Pb2+ detection apart from interference by autofluorescence and scattering light.
3.2. HMI Detection Combined with Electrochemical Techniques
3.3. Summary of HMI Detection Platforms Using GSH
In summary, GSH has been adopted as the probe molecule in a variety of HMI detection platforms. These detection platforms have demonstrated good detection performance, such as a low LOD and a fast response. Some of them have been applied to practical samples with acceptable outcomes. The platforms discussed above present commonly reported detection methods, and are not an exhaustive list of all existing GSH-based HMI sensors. Table 1 gives a more comprehensive summary of recently reported HMI detection platforms using GSH. In addition to the examples discussed in the review, Table 1 includes some less-used detection methods with their operational characteristics. As every application has its own unique requirements, different considerations go into choosing a certain detection platform. For example, it is difficult to find a sensor with high sensitivity and a very fast response at the same time. Some trade-off is needed when choosing a detection method. It is our hope that this summary will provide some guidance for choosing a suitable strategy to detect certain HMIs.Signal Transduction | Sensor Structure | HMI Target | Detection Limit | Linear Range | Response Time | References | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Optical methods | ||||||||||||||||||||||||
Colorimetry | Gold NPs | Pb | 0.1 μM | 0.1~50 μM | 20 ~ 25 min |
[46] |
[43] |
|||||||||||||||||
Silver NPs | Pb | 1 nM | Not mentioned | At least 10 min |
[32] |
|||||||||||||||||||
Gold NPs | Cd | 4.3 pM | 17 pM~16.67 nM | About 17 min |
[47] |
[49] |
||||||||||||||||||
Localized surface plasmon resonance | Gold NPs | Pb | 50 pM | 0.1 nM~10 μM | 15 min |
[33] |
||||||||||||||||||
Fluorescence | Gold NPs | Pb | 0.1 μM | 2~350 μM | About 1 min |
[48] |
[44] |
|||||||||||||||||
Silver NPs | Pb | 0.6 pM (200 ppq) | 60 pM~2.4 nM (20~800 ppt estimated) | Less than 20 min |
[49] |
[45] |
||||||||||||||||||
Surface-enhanced Raman scattering | Silver NPs | As3+ | 10.2 nM (0.76 ppb) | 53.7 nM~4.0 μM (4~300 ppb) | At least 2 min |
[50] |
[46] |
|||||||||||||||||
Whispering gallery mode | Gold NPs | Pb | 0.05 nM | 2.40~48.26 nM | About 40 min |
[35] |
||||||||||||||||||
Spectrophotometry | Mn-doped ZnS QDs | Pb | 2.2 nM (0.45 μg/L) | 4.9 nM~0.49 μM (1.0~100 μg/L) | Not mentioned |
[45] |
[52] |
|||||||||||||||||
Mn-doped ZnS QDs | Pb, Cr, Hg | 0.93 μM for mixed HMIs | 1 μM~1 mM | Not mentioned |
[51] |
[53] |
||||||||||||||||||
CdTe QDs | Pb | 0.26 nM | 0.8~15 nM | About 15 s |
[52] |
[54] |
||||||||||||||||||
Carbon QDs | Hg | 0.05 nM | 1 nM~50 μM | At least 20 min |
[39] |
|||||||||||||||||||
Silver NPs | Ni2+ | 75 μM | About 75 μM~1 mM | Not mentioned |
[53] |
[48] |
||||||||||||||||||
Gold Nanostars | Pb | 0.5 μM | About 0.5~4 μM | About 30 min |
[42] |
|||||||||||||||||||
Dynamic Light Scattering | Gold Nanorod Chains | Pb | 0.025 mM | Not mentioned | Not mentioned |
[41] |
||||||||||||||||||
Electrochemical methods | ||||||||||||||||||||||||
Square Wave Anodic Stripping Voltammetry, SWASV | Magnetic NPs | Cd, Pb | 1.6 nM (0.182 μg/L); | 0.8 nM (0.172 μg/L) | 4.4~879 nM (0.5~100 μg/L), | 2.3~460 nM (0.5~100 μg/L) | 210 s | 210 s |
[40] |
|||||||||||||||
Glassy-Carbon Electrode | Cd | 0.05 nM | 2~20 nM | 120 s |
[54] |
[56] |
||||||||||||||||||
Carbon Paste Electrode | Cd | 8.5 nM (2 ppb) | 4.2~420 nM (1~100 ppb) | Longer than 7 min |
[55] |
[58] |
||||||||||||||||||
Differtial pulsed anodic striping voltammetry, DPASV | Screen-Printed Carbon Nanofiber Electrode | Pb, Cd | ~0.1 nM (~3 μg/L) | About 0.3~4.5 nM (about 10~150 μg/L) | 120 s |
[56] |
[57] |
|||||||||||||||||
FET (Drain current) | Field effect Transistor | Pb | 10 nM | 10 nM~10 μM | 1–2 s |
[34] |
||||||||||||||||||
FET (Pulse-driven capacitance) | Gate Capacitance | Pb | <4.8 nM | (<1 ppb) | 24~96 nM (5~20 ppb) | 1–2 s |
[19] |
|||||||||||||||||
