Climate change is causing soil salinization, resulting in crop losses throughout the world. The ability of plants to tolerate salt stress is determined by multiple biochemical and molecular pathways. Here we discuss physiological, biochemical, and cellular modulations in plants in response to salt stress. Knowledge of these modulations can assist in assessing salt tolerance potential and the mechanisms underlying salinity tolerance in plants. Salinity-induced cellular damage is highly correlated with generation of reactive oxygen species, ionic imbalance, osmotic damage, and reduced relative water content. Accelerated antioxidant activities and osmotic adjustment by the formation of organic and inorganic osmolytes are significant and effective salinity tolerance mechanisms for crop plants. In addition, polyamines improve salt tolerance by regulating various physiological mechanisms, including rhizogenesis, somatic embryogenesis, maintenance of cell pH, and ionic homeostasis.
- abiotic stresses
- cell membrane stability
- climate change
- osmolytes
- polyamines
1. Introduction
1.1. Overview of Salinity
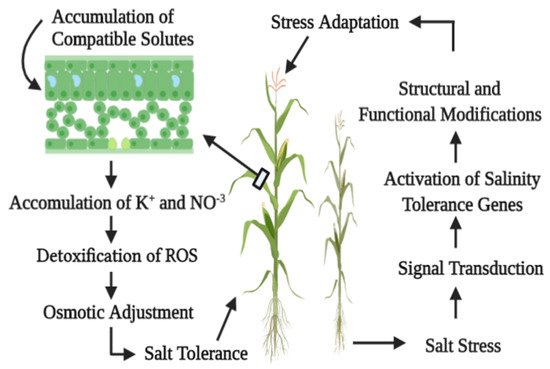
1.2. Salinity and Morphological Attributes
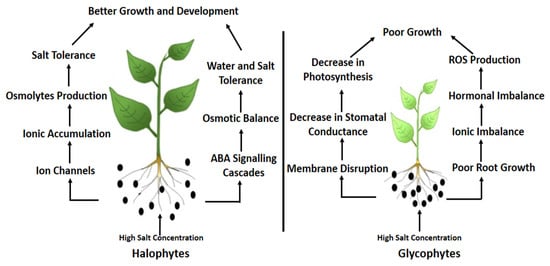
1.3. Salinity and Physiological Attributes

2. Salinity and Biochemical Attributes
2.1. Salinity and Proline
2.2. Salinity and Polyamines
2.3. Salinity and Glycine-Betaine
3. Salinity and Phytohormones
Plant hormones are signaling molecules with the ability to alter the physiological mechanisms of the plant, even if present in very minute quantities [248][212]. Common plant hormones are auxins, gibberellins, cytokinins, abscisic acid, and ethylene [249][213]. Plants growing under salt stress experience imbalances in hormonal homeostasis. Stressed conditions drastically alter physiological mechanisms of the plant, creating massive changes in endogenous hormonal contents. Higher concentrations of toxic ions are negatively correlated with the levels of plant hormones like gibberellins, auxins, and cytokinin and are positively associated with the abscisic acid level [250,251][214][215]. Exogenous plant hormone application on salt stressed plants was found to alleviate the negative effects of salinity on the morphological (leaf area, dry mass), physiological (chlorophyll content, stomatal conductance, photosynthetic rate), and yield characteristics of crops [252,253][216][217]. Abscisic acid (ABA) and ethylene are involved in signaling under stress conditions. An increase in ABA concentration in plant cells has been reported under saline conditions [254][218]. Carotenoids are the precursor for ABA synthesis, with roots and leaves the sites of synthesis [255][219]. Water deficit in the root zone causes ABA generation in roots, and ABA transport to shoots is xylem mediated. The increase in pH of xylem sap increases the transport of ABA to the guard cells, where it regulates the stomatal opening and closing through the involvement of Ca ions [256][220]. Alterations in ABA levels indirectly affect photosynthesis through disruption in stomatal opening and closing. The photosynthetic efficiency of the plant cell declines along with deregulation of translocation and assimilate partitioning of photosynthates [8,257][8][221]. Exogenously applied plant growth regulators have been widely reported to enhance stress tolerance in numerous plant species [258][222]. ABA is reported to be useful in alleviating plant salt stress under low water potential. ABA production in the plant cell is also related to ethylene synthesis under salt stress. The interaction between these two stress hormones is apparent from vegetative growth and seed germination under salt stress. During root inhibition by salt stress, ethylene regulates the ABA concentration. However, the reverse has been reported in the case of seed germination [259][223]. Salicylic acid has displayed a defensive role in plants experiencing stress, signaling the plant to adapt to the stressful environment [260][224].Salinity and Growth Regulation
References
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131.
- Rogel, J.A.; Ariza, F.A.; Silla, R.O. Soil salinity and moisture gradients and plant zonation in Mediterranean salt marshes of Southeast Spain. Wetlands 2000, 20, 357–372.
- Flowers, T.J.; Garcia, A.; Koyama, M.; Yeo, A.R. Breeding for salt tolerance in crop plants—The role of molecular biology. Acta Physiol. Plant. 1997, 19, 427–433.
- Rubio, J.S.; Garcia-Sanchez, F.; Rubio, F.; Martinez, V. Yield, blossom-end rot incidence and fruit quality in pepper plants under moderate salinity are affected by K+ and Ca+2 fertilization. Sci. Hortic. 2009, 119, 79–87.
- Ghassemi, F.; Jakeman, A.J.; Nix, H.A. Salinization of Land and Water Resources; University of New south Wales Press Ltd.: Canberra, Australia, 1995.
- Sonowal, H.; Pal, P.B.; Shukla, K.; Ramana, K.V. Ramana, Aspalatone Prevents VEGF-Induced Lipid Peroxidation, Migration, Tube Formation, and Dysfunction of Human Aortic Endothelial Cells. Oxid. Med. Cell. Longev. 2017, 11, 2017.
- Wang, H.; Tang, X.; Shao, C.; Shao, H.; Wang, L. Molecular cloning and bioinformatics analysis of a new plasma membrane Na+/H+ antiporter gene from the halophyte Kosteletzkya virginica. Sci. World J. 2014, 2014, 141675.
- Xiong, L.; Zhu, J.K. Molecular and genetic aspects of plant responses to osmotic stress. Plant Cell Environ. 2002, 25, 131–139.
- Meng, X.; Zhou, J.; Sui, N. Mechanisms of salt tolerance in halophytes: Current understanding and recent advances. Open Llife Sci. 2018, 13, 149–154.
- Ashraf, M.H.P.J.C.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190.
- Siew, R.; Klein, C.R. The effect of NaCl on some metabolic and fine structural changes during the greening of etiolated leaves. J. Cell Biol. 1969, 37, 590–596.
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savoure, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447.
- Cushman, J.C. Molecular cloning and expression of chloroplast NADP-malate dehydrogenase during crassulacean acid metabolism induction by salt stress. Photosynth. Res. 1990, 35, 15–27.
- Sévin, D.C.; Stählin, J.N.; Pollak, G.R.; Kuehne, A.; Sauer, U. Global Metabolic Responses to Salt Stress in Fifteen Species. PLoS ONE 2016, 11, e0148888.
- Dschida, W.J.; Platt-Aloia, K.A.; Thomson, W.W. Epidermal peels of Avicennia germinans (L.) Stearn: A useful system to study the function of salt glands. Ann. Bot. Lond. 1992, 70, 501–509.
- Robert, I.L.; Frank, W.J.; Summy, K.R.; Hudson, D.Y.; Richard, S. The Biological Flora of Coastal Dunes and Wetlands. Avicennia Germinans J. Coast. Res. 2017, 33, 191–207.
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527.
- Shahid, M.A.; Balal, R.M.; Khan, N.; Rossi, L.; Rathinasabapathi, B.; Liu, G.; Khan, J.; Cámara-Zapata, J.M.; Martínez-Nicolas, J.J.; Garcia-Sanchez, F. Polyamines provide new insights into the biochemical basis of Cr-tolerance in Kinnow mandarin grafted on diploid and double-diploid rootstocks. Environ. Exp. Bot. 2018, 1, 248–260.
- Zhu, J.; Hasegawa, P.M.; Bressan, R.A. Molecular aspects of osmotic stress in plants. Citri. Rev. Plant Sci. 1997, 16, 253–277.
- Serrano, R.; Mulet, J.M.; Rios, G.; Marquez, J.A.; de Larrinoa, I.F.; Leube, M.P.; Mendizabal, I.; Pascual-Ahuir, A.; Proft, M.; Ros, R.; et al. A glimpse of the mechanism of ion homeostasis during salt stress. J. Exp. Bot. 1999, 50, 1023–1036.
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431.
- Khan, N.; Bano, A.; Babar, M.A. The stimulatory effects of plant growth promoting rhizobacteria and plant growth regulators on wheat physiology grown in sandy soil. Arch. Microbiol. 2019, 201, 769–785.
- Price, A.; Hendry, G. Iron catalysed oxygen radical formation and its possible contribution to drought damage in nine native grasses and three cereals. Plant Cell Environ. 1991, 14, 477–484.
- Khan, N.; Bano, A. Effects of exogenously applied salicylic acid and putrescine alone and in combination with rhizobacteria on the phytoremediation of heavy metals and chickpea growth in sandy soil. Int. J. Phytoremed. 2018, 16, 405–414.
- Stevens, R.M.; Pech, J.M.; Grigson, G.J. A Short Non-Saline Sprinkling Increases the Tuber Weights of Saline Sprinkler Irrigated Potatoes. Agronomy 2017, 7, 4.
- Nxele, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. Afr. J. Bot. 2017, 1, 261–266.
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151.
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022.
- Zekri, M.; Parsons, L.R. Calcium influnces growth and leaf mineral concentration of citrus under saline conditions. Hort. Sci. 1990, 25, 784–786.
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537.
- Saha, J.; Brauer, E.K.; Sengupta, A.; Popescu, S.C.; Gupta, K.; Gupta, B. Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 2015, 3, 21.
- Mansour, M.M.F. Cell permeability under salt stress. In Strategies for Improving Salt Tolerance in Higher Plants; Jaiwal, P.K., Singh, R.P., Gulati, A., Eds.; Oxford and IBH: New Delhi, India, 1997; pp. 87–110.
- Ali, S.; Xie, L. Plant Growth Promoting and Stress mitigating abilities of Soil Born Microorganisms. Recent Pat. Food Nutr. Agric. 2019, 15.
- Mansour, M.M.F.; Salama, K.H.A. Cellular basis of salinity tolerance in plants. Environ. Exp. Bot. 2004, 52, 113–122.
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166.
- Al-Amri, S.M. Improved growth, productivity and quality of tomato (Solanum lycopersicum L.) plants through application of shikimic acid. Saudi J. Biol. Sci. 2013, 1, 339–345.
- Mahlooji, M.; Seyed Sharifi, R.; Razmjoo, J.; Sabzalian, M.R.; Sedghi, M. Effect of salt stress on photosynthesis and physiological parameters of three contrasting barley genotypes. Photosynthetica 2017, 56, 549–556.
- Shekari, F.; Abbasi, A.; Mustafavi, S.H. Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J. Saudi Soc. Agric. Sci. 2017, 1, 367–374.
- Dolatabadian, A.; Sanavy, S.A.M.M.; Gholamhoseini, M.; Joghan, A.K.; Majdi, M.; Kashkooli, A. The role of calcium in improving photosynthesis and related physiological and biochemical attributes of spring wheat subjected to simulated acid rain. Physiol. Mol. Biol. Plants. 2013, 19, 189–198.
- Farooq, S.; Azam, F. The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J. Plant Physiol. 2006, 163, 629–637.
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250.
- Dolatabadi, N.; Toorchi, M. Rapeseed (Brassica napus L.) genotypes response to NaCl salinity. J. Biodiv. Environ. Sci. 2017, 10, 265–270.
- Wu, J.; Seliskar, D.M.; Gallagher, J.L. Stress tolerance in the march plant Spartina patens: Impact of NaCl on growth and root plasma membrane lipid composition. Physiol. Plant 1998, 102, 307–317.
- Balal, R.M.; Shahid, M.A.; Vincent, C.; Zotarelli, L.; Liu, G.; Mattson, N.S.; Garcia-Sanchez, F. (Kinnow mandarin plants grafted on tetraploid rootstocks are more tolerant to Cr-toxicity than those grafted on its diploids one. Environ. Exp. Bot. 2017, 140, 8–18.
- Plant, A.L.; Bray, E.A. Regulation of Gene Expression by Abscissic Acid During Environmental Stresses; Marcel Dekker: New York, NY, USA, 1999; pp. 303–331.
- Delphine, S.; Alvino, A.; Zacchini, M.; Loreb, F. Consequences of salt stress on conductance to CO2 diffusion, Rubisco characteristics and anatomy of spinach leaves. Aust. J. Plant Physiol. 1998, 25, 395–402.
- Mehmood, A.; Khan, N.; Irshad, M.; Hamayun, M. IAA Producing Endopytic Fungus Fusariun oxysporum wlw Colonize Maize Roots and Promoted Maize Growth Under Hydroponic Condition. Eur. Exp. Biol. 2018, 8, 24.
- Romero-Aranda, R.; Moya, J.L.; Tadeo, F.R.; Legaz, F.; Primo-Millo, E.; Talón, M. Physiological and anatomical disturbances induced by chloride salts in sensitive and tolerant citrus: Beneficial and detrimental effects of cations. Plant Cell Environ. 1998, 21, 1243–1253.
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. 2000, 51, 463–499.
- Blumwald, E.; Aharon, G.S.; Apse, M.P. Sodium transport in plant cells. Biochim. Biophys. Acta 2000, 1465, 140–151.
- Maser, P.; Gierth, M.; Schroeder, J.I. Molecular mechanisms of potassium and sodium uptake in plants. Plant Soil. 2002, 247, 43–54.
- Khan, N.; Bano, A. Modulation of phytoremediation and plant growth by the treatment with PGPR, Ag nanoparticle and untreated municipal wastewater. Int. J. Phytoremed. 2016, 18, 1258–1269.
- Zribi, L.; Fatma, G.; Fatma, R.; Salwa, R.; Hassan, N.; Nejib, R.M. Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato “Solanum lycopersicum (variety Rio Grande)”. Sci. Hortic. 2009, 120, 367–372.
- Orlovsky, N.; Japakova, U.; Zhang, H.; Volis, S. Effect of salinity on seed germination, growth and ion content in dimorphic seeds of Salicornia europaea L. (Chenopodiaceae). Plant Divers. 2016, 1, 183–189.
- Shaterian, J.; Waterer, D.; De Jong, H.; Tanino, K.K. Differential stress responses to NaCl salt application in early and late maturing diploid potato (Solanum sp.) clones. Environ. Exp. Bot 2005, 54, 202–212.
- Chartzoulakis, K.; Klapaki, G. Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci. Hortic. 2000, 86, 247–260.
- Kaya, C.; Tuna, A.L.; Ashrafa, M.; Altunlu, H. Improved salt tolerance of melon (Cucumis melo L.) by the addition of proline and potassium nitrate. Environ. Exp. Bot 2007, 60, 397–403.
- Giuffrida, F.; Graziani, G.; Fogliano, V.; Scuderi, D.; Romano, D.; Leonardi, C. Effects of nutrient and NaCl salinity on growth, yield, quality and composition of pepper grown in soilless closed system. J. Plant Nutr. 2014, 37, 1455–1474.
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2007, 7, 42039.
- Savvas, D.; Lenz, F. Effect of NaCl or nutrient-induced salinity on growth, yield and composition of egg plants grown in rockwool. Sci. Hortic. 2000, 84, 37–47.
- Abbas, W.; Ashraf, M.; Akram, N.A. Alleviation of salt-induced adverse effects in egg plant (Solanum melongena L.) by glycinebetaine and sugar beet extracts. Sci. Hortic. 2010, 125, 188–195.
- Mahjoor, F.; Ghaemi, A.A.; Golabi, M.H. Interaction effects of water salinity and hydroponic growth medium on eggplant yield, water-use efficiency, and evapotranspiration. Int. Soil Water Conserv. 2016, 1, 99–107.
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Iqbal, Z.; Liu, G.D.; Zotarelli, L.; Khan, N. Chitosan alleviates phytotoxicity caused by boron through augmented polyamine metabolism and antioxidant activities and reduced boron concentration in Cucumis sativus L. Acta Physiol. Plant. 2017, 1, 31.
- Tabatabaie, S.J.; Nazari, J. Influence of nutrient concentrations and NaCl salinity on the growth, photosynthesis, and essential oil content of peppermint and lemon verbena. Turk. J. Agric. For. 2007, 31, 245–253.
- Pardossi, A.; Bagnoli, G.; Malorgio, F.C.; Campiotti, A.; Tognoni, F. NaCl effects on celery (Apium graveolens L.) grown in NFT. Sci. Hortic 1999, 81, 229–242.
- Zheng, Y.; Jia, A.; Ning, T.; Xu, J.; Li, Z.; Jiang, G. Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol. 2008, 165, 1455–1465.
- Saini, S.; Sharma, I.; Pati, P.K. Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Front. Plant Sci. 2015, 6, 950.
- Kalhoro, N.A.; Rajpar, I.; Kalhoro, S.A.; Ali, A.; Raza, S.; Ahmed, M.; Kalhoro, F.A.; Ramzan, M.; Wahid, F. Effect of salts stress on the growth and yield of wheat (Triticum aestivum L.). Am. J. Plant Sci. 2016, 7, 2257.
- Xie, Z.; Duan, L.; Tian, X.; Wang, B.; Eneji, A.E.; Li, Z. Coronatine alleviates salinity stress in cotton by improving the antioxidation defence system and radical-scavenging activity. J. Plant Physiol. 2008, 165, 375–384.
- Sun, C.; Feng, D.; Mi, Z.; Li, C.; Zhang, J.; Gao, Y.; Sun, J. Impacts of Ridge-Furrow Planting on Salt Stress and Cotton Yield under Drip Irrigation. Water 2017, 9, 49.
- Caines, A.M.; Shennan, C. Interactive effects of Ca+2 and NaCl salinity on the growth of two tomato genotypes differing in Ca+2 use efficiency. Plant Physiol. Biochem. 1999, 37, 569–576.
- Evers, D.; Hemmer, K.; Hausman, J.F. Salt stress induced biometric and physiological changes in Solanum tuberosum L. cv. Bintje grown in vitro. Acta Physiol. Plant 1998, 20, 3–7.
- Gao, Y.; Lu, Y.; Wu, M.; Liang, E.; Li, Y.; Zhang, D.; Yin, Z.; Ren, X.; Dai, Y.; Deng, D.; et al. Ability to Remove Na+ and Retain K+ Correlates with Salt Tolerance in Two Maize Inbred Lines Seedlings. Front. Plant Sci. 2016, 7, 1716.
- Hu, Y.; Xia, S.; Su, Y.; Wang, H.; Luo, W.; Su, S.; Xiao, L. Brassinolide Increases Potato Root Growth In Vitro in a Dose-Dependent Way and Alleviates Salinity Stress. Biomed. Res. Int. 2016, 8231873.
- Alom, R.; Hasan, M.A.; Islam, M.R.; Wang, Q.F. Germination characters and early seedling growth of wheat (Triticum aestivum L.) genotypes under salt stress conditions. JCSB 2016, 1, 383–392.
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effect of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007, 59, 173–178.
- De Pascale, S.; Barbieri, G. Effects of soil salinity and top removal on growth and yield of broad bean as a green vegetable. Sci. Hortic 1997, 71, 147–165.
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 2005, 162, 669–677.
- Hu, Y.; Schmidhalter, U. Drought and Salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil. Sci. 2005, 168, 541–549.
- Rahnama, H.; Ebrahimzadeh, H. The effect of NaCl on antioxidant activities in potato seedlings. Biol. Plant. 2005, 49, 93–97.
- Korkmaz, A.; Şirikçi, R.; Kocaçınar, F.; Değer, Ö.; Demirkırıan, A.R. Alleviation of salt-induced adverse effects in pepper seedlings by seed application of glycinebetaine. Sci. Hortic. 2012, 4, 197–205.
- Roy, T.S.; Chakraborty, R.; Parvez, M.N.; Mostofa, M.; Ferdous, J.; Ahmed, S. Yield, dry matter and specific gravity of exportable potato: Response to salt. Univ. J. Agric. Res. 2017, 5, 98–103.
- Mulholland, B.J.; Taylor, I.B.; Jackson, A.C.; Thornpson, A.J. Can ABA mediate responses of salinity have stressed tomato. Environ. Exp. Bot. 2003, 50, 17–28.
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth promoting bacteria confer resistance in tomato plants to salt stress. Plant Sci. Plant Sci. 2004, 42, 565–572.
- Smalle, J.; Van Der Straeten, D. Ethylene and vegetative development. Physiol. Plant. 1997, 100, 593–605.
- Akhtar, S.S.; AkhtarAktas, H.; Abak, K.; Cakmak, I. Genotypic variation in the response of pepper to salinity. Sci. Hortic. 2006, 110, 260–266.
- Delgado, M.J.; Garrido, J.M.; Ligero, F.; Lluch, C. Nitrogen fixation and carbon metabolism by nodules and bacteroids of pea plants under sodium chloride stress. Physiol. Plant. 1993, 89, 824–829.
- Borucki, W.; Sujkowska, M. The effects of sodium chloride-salinity upon growth, nodulation, and root nodule structure of pea (Pisum sativum L.) plants. Acta Physiol. Plant. 2008, 30, 293–301.
- Andrzej, T.; Philip, P. Role of root microbiota in plant productivity. J. Exp. Bot. 2015, 66, 2167–2175.
- Zahran, H.H. Rhizobium-Legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. R. 1999, 63, 968–989.
- Batista-Santos, P.; Duro, N.; Rodrigues, A.P.; Semedo, J.N.; Alves, P.; da Costa, M.; Graça, I.; Pais, I.P.; Scotti-Campos, P.; Lidon, F.C.; et al. Is salt stress tolerance in Casuarina glauca Sieb. ex Spreng. associated with its nitrogen-fixing root-nodule symbiosis? An analysis at the photosynthetic level. Plant Physiol. Biochem. 2015, 1, 97–109.
- Othman, Y.; Al-karaki, G.; Al-Tawaha, A.R.; Al-Horani, A. Variation in germination and ion uptake in barley genotypes under salinity conditions. World J. Agric. Res. 2006, 2, 11–15.
- Kandil, A.A.; Shareif, E.; Gad, M.A. Effect of Salinity on Germination and Seeding Parameters of Forage Cowpea Seed. Res. J. Seed Sci. 2017, 10, 17–26.
- Khan, M.A.; Rizvi, Y. Effect of salinity, temperature and growth regulators on the germination and early seedling growth of Atriplex griffithi Var. Stocksii. Can J. Bot. 1994, 72, 475–479.
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 2, 507–533.
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Minhas, P.S. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172.
- Stepien, P.; Klobus, G. Water relations and photosynthesis in Cucumus sativus L. leaves under salt stress. Biol. Plant. 2006, 50, 610–616.
- Dobrota, C. Energy dependant plant stress acclimation. Rev. Environ. Sci. 2006, 5, 243–251.
- Yeo, A.R.; Capron, S.J.M.; Flowers, T.J. The effect of salinity upon photosynthesis in rice (Oryza sativa L.) Gas exchange by individual leaves relation to their salt content. J. Exp. Bot. 1985, 36, 1240–1248.
- Muradoglu, F.; Gundogdu, M.; Ercisli, S.; Encu, T.; Balta, F.; Jaafar, H.Z.; Zia-Ul-Haq, M. Cadmium toxicity affects chlorophyll a and b content, antioxidant enzyme activities and mineral nutrient accumulation in strawberry. Biol. Res. 2015, 48, 1–7.
- El-Shintinawy, F.; El-Ansary, A. Differential Effect of Cd2+ and Ni2+ on Amino Acid Metabolism in Soybean Seedlings. Biol. Plant. 2000, 43, 79–84.
- Puniran-Hartley, N.; Hartley, J.; Shabala, L.; Shabala, S. Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: In planta evidence for cross-tolerance. Plant Physiol. Biochem. 2014, 1, 32–39.
- Garcia-Legaz, M.F.; Ortiz, J.M.; Garcia-Lindon, A.; Cerda, A. Effect of salinity on growth, ion content and CO2 assimilation rate in lemon varieties on different rootstocks. Physiol. Plant. 1993, 89, 427–432.
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18.
- Hanachi, S.; Van Labeke, M.C.; Mehouachi, T. Application of chlorophyll fluorescence to screen eggplant (Solanum melongena L.) cultivars for salt tolerance. Photosynthetica 2014, 52, 57–62.
- Tiwari, J.K.; Munshi, A.D.; Kumar, R.; Pandey, R.N.; Arora, A.; Bhat, J.S.; Sureja, A.K. Effect of salt stress on cucumber: Na+–K+ ratio, osmolyte concentration, phenols and chlorophyll content. Acta Physiol. Plant. 2010, 1, 103–114.
- Lycoskoufis, I.H.; Savvas, D.; Mavrogianopoulos, G. Growth, gas exchange, and nutrient status in pepper (Capsicum annuum L.) grown in recirculating nutrient solution as affected by salinity imposed to half of the root system. Sci. Hortic. 2005, 106, 147–161.
- Zhang, T.; Gong, H.; Wen, X.; Lu, C. Salt stress induces a decrease in oxidation energy transfer from phycobilisomes to photosystem II but an increase to photosystem I in the cyanobacterium Spirulina platensis. J. Plant Physiol. 2010, 12, 20.
- Wu, X.; He, J.; Chen, J.; Yang, S.; Zha, D. Alleviation of exogenous 6-benzyladenine on two genotypes of eggplant (Solanum melongena Mill.) growth under salt stress. Protoplasma 2014, 251, 169–176.
- Yan, K.; Shao, H.; Shao, C.; Chen, P.; Zhao, S.; Brestic, M.; Chen, X. Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol. Plant. 2013, 1, 2867–2878.
- Hayat, S.; Hasan, S.A.; Yusuf, M.; Hayat, Q.; Ahmad, A. Effect of 28- homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ. Exp. Bot. 2010, 69, 105–112.
- Misra, A.N.; Sahu, S.M.; Misra, M.; Singh, P.; Meera, I.; Das, N.; Kar, M.; Shau, P. Sodium chloride induced changes in leaf growth, and pigment and protein contents in two rice cultivars. Biol. Plant. 1997, 39, 257–262.
- Neelesh, K.; Veena, P. Effect of Salt Stress on Growth Parameters, Moisture Content, Relative Water Content and Photosynthetic Pigments of Fenugreek Variety RMt-1. J. Plant Sci. 2015, 10, 210–221.
- Bethke, P.C.; Drew, M.C. Stomatal and non-stomatal components to inhibition of photosynthesis in leaves of Capsicum annum during progressive exposure to NaCl salinity. Plant Physiol. 1992, 99, 219–226.
- Reddy, M.P.; Vora, A.B. Changes in pigment composition, Hill reaction activity and saccharides metabolism in Bajra (Pennisetum typhoides) leaves under NaCl salinity. Photosynthetica 1986, 20, 50–55.
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2013, 1, 4–18.
- Parida, A.K.; Das, A.B. Salt tolerance and salinity affects on plant. Ecotoxicol. Environ. 2005, 60, 324–349.
- Cao, Y.Y.; Duan, H.; Yang, L.N.; Wang, Z.Q.; Zhou, S.C.; Yang, J.C. Effect of heat stress during meiosis on grain yield of rice cultivars differing in heat tolerance and its physiological mechanism. Acta Agron. Sin. 2008, 34, 2134–2142.
- Elhamid, E.; Sadak, M.; Tawfik, M. Alleviation of Adverse Effects of Salt Stress in Wheat Cultivars by Foliar Treatment with Antioxidant 2—Changes in Some Biochemical Aspects, Lipid Peroxidation, Antioxidant Enzymes and Amino Acid Contents. Agric. Sci. 2014, 5, 1269–1280.
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Adnan Shahid, M.; Yang, J. Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus. Front. Microbiol. 2018, 9, 2507.
- Kartashov, A.V.; Radyukina, N.L.; Ivanov, Y.V.; Pashkovskii, P.P.; Shevyakova, N.I.; Kuznetsov, V.V. Role of antioxidants systems in wild plant adaptation to salt stress. Russ. J. Plant Physiol. 2008, 55, 463–468.
- Katsumi, M. Physiological modes of brassinolide action in cucumber hypocotyl growth. In Brassinosteriods: Chemistry, Bioactivity and Applications; Cutler, H.G., Yokota, T., Adam, G., Eds.; American Chemical Society: Washington, DC, USA, 1991; pp. 246–254.
- Faried, H.N.; Ayyub, C.M.; Amjad, M.; Ahmed, R. Salinity impairs ionic, physiological and biochemical attributes in potato. Pak. J. Agric. Sci. 2016, 53, 17–25.
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578.
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014.
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014, 94, 329–354.
- Ashraf, M.; Bhatti, B. Physiological responses of some salt tolerance and salt sensitive lines of wheat (Crthamus tinctorius L.). Acta Physiol. Plant. 1999, 117, 23–28.
- Adnan, A.; Erkan, Y. Effect of salinity stress on chlorophyll, carotenoid content, and proline in Salicornia prostrata Pall. and Suaeda prostrata Pall. subsp. prostrata (Amaranthaceae). Braz. J. Bot. 2016, 39, 101–106.
- Kamal, A.; Qureshi, M.S.; Ashraf, M.Y.; Hussain, M. Salinity induced some changes in growth and physio-chemical aspects of two soybean [Glycine max (L.) merr] genotype. Pak. J. Bot. 2003, 35, 93–97.
- Parvin, K.; Haque, M.N. Protective Role of Salicylic Acid on Salt Affected Broccoli Plants. J. Agric. Ecol. 2017, 10, 1–10.
- Stepien, P.; Klobus, G. Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol. Plant. 2005, 125, 31–40.
- Chandrasekaran, M.; Kim, K.; Krishnamoorthy, R.; Walitang, D.; Sundaram, S.; Joe, M.M.; Selvakumar, G.; Hu, S.; Oh, S.H.; Sa, T. Mycorrhizal symbiotic efficiency on C3 and C4 plants under salinity stress—A meta-analysis. Front. Microbiol. 2016, 11, 1246.
- Akbar, S.; Sultan, S. Soil bacteria showing a potential of chlorpyrifos degradation and plant growth enhancement. Braz. J. Microbiol. 2016, 47, 563–570.
- Bhantana, P.; Lazarovitch, N. Evapotranspiration, Crop coefficient and growth of two young pomegranate (Punica granatum L.) varieties under salt stress. Agric. Water Manag. 2010, 97, 715–722.
- Gorai, M.; Ennajeh, M.; Khemira, H.; Neffati, M. Combined effect of NaCl-salinity and hypoxia on growth, photosynthesis, water relations and solute accumulation in Phragmite saustralis plants. Flora 2010, 205, 462–470.
- Baby, T.; Collins, C.; Tyerman, S.D.; Gilliham, M. Salinity negatively affects pollen tube growth and fruit set in grapevines and is not mitigated by silicon. Am. J. Enol. Vitic. 2016, 67, 218–228.
- Hernandez, J.A.; Almansa, M.S. Short term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol. Plant. 2002, 115, 251–257.
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, M.; Babar, M.A. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 1997, 52, 2.
- Hong, B.; Barg, R.; Ho, T.D. Developmental and organ specific expression of an ABA and stress induced protein in barley. Plant Mol. Biol. 1992, 18, 663–674.
- Hare, P.D.; Cress, W.A.; Staden, J.V. Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ. 1998, 21, 535–554.
- Galinski, E.A. Compatible solutes of halophilic eubacteria-eubacteria-molecular principles, water-solute interactions, stress protection. Cell Mol. Life Sci. 1993, 49, 487–496.
- Lutts, S.; Lefèvre, I. How can we take advantage of halophyte properties to cope with heavy metal toxicity in salt-affected areas? Ann. Bot. 2015, 1, 509–528.
- Brown, A.D. Microbial Water Stress Physiology, Principles and Perspectives; Wiley: Hoboken, NJ, USA, 1990.
- Solomon, A.; Beer, S.; Waisel, Y.; Jones, G.P.; Paleg, L.G. Effects of NaCl on the Carboxylating activity of Rubisco from Tamarix jordanis in the presence and absence of proline-related compatible solutes. Plant Physiol. 1994, 90, 198–204.
- Papageorgiou, G.; Murata, N. The unusually strong stability effects of glycine betaine on the structure and function of the oxygen evolving photosystem II complex. Photosynth. Res. 1995, 44, 243–252.
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 1, 1241–1257.
- Stoop, J.M.H.; Williamson, J.D.; Pharr, D.M. Mannitol metabolism in plants: A method for coping with stress. Trends Plant Sci. 1996, 1, 139–144.
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 94, 346–352.
- Managbanag, J.R.; Torzilli, A.P. An analysis of trehalose, glycerol, and mannitol accumulation during heat and salt stress in a salt marsh isolate of Aureobasidium pullulans. Mycologia 2002, 1, 384–391.
- Khan, N.; Bano, A.; Zandi, P. Effects of exogenously applied plant growth regulators in combination with PGPR on the physiology and root growth of chickpea (Cicer arietinum) and their role in drought tolerance. J. Plant Interact. 2018, 1, 239–247.
- Tipirdamaz, R.; Gagneul, D.; Duhazé, C.; Aïnouche, A.; Monnier, C.; Özkum, D.; Larher, F. Clustering of halophytes from an inland salt marsh in Turkey according to their ability to accumulate sodium and nitrogenous osmolytes. Environ. Exp. Bot. 2006, 57, 139–153.
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16.
- Sandhu, D.; Cornacchione, M.V.; Ferreira, J.F.; Suarez, D.L. Variable salinity responses of 12 alfalfa genotypes and comparative expression analyses of salt-response genes. Sci. Rep. 2017, 22, 42958.
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011.
- Dar, M.I.; Naikoo, M.I.; Rehman, F.; Naushin, F.; Khan, F.A. Proline accumulation in plants: Roles in stress tolerance and plant development. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: New Delhi, India, 2016; pp. 155–166.
- Ali, B.; Hayat, S.; Ahmad, A. 28-homobrassinolide ameliorates the saline stress in Cicer arietinum L. Environ. Exp. Bot. 2007, 59, 217–223.
- Mantilla, B.S.; Marchese, L.; Casas-Sánchez, A.; Dyer, N.A.; Ejeh, N.; Biran, M.; Silber, A.M. Proline Metabolism is Essential for Trypanosoma brucei brucei Survival in the Tsetse Vector. PLoS Pathog. 2017, 13, e1006158.
- Kahlaoui, B.; Hachicha, M.; Misle, E.; Fidalgo, F.; Teixeira, J. Physiological and biochemical responses to the exogenous application of proline of tomato plants irrigated with saline water. J. Saudi Soc. Agric. Sci. 2018, 1, 17–23.
- Nemoto, Y.; Sasakuma, T. Differential stress responses of early salt-stress responding genes in common wheat. Phytochemistry 2002, 61, 129–133.
- Jaarsma, R.; de Vries, R.S.; de Boer, A.H. Effect of salt stress on growth, Na+ accumulation and proline metabolism in potato (Solanum tuberosum) cultivars. PLoS ONE 2013, 8, e60183.
- Joseph, E.A.; Radhakrishnan, V.V.; Mohanan, K.V. A Study on the Accumulation of Proline—An Osmoprotectant Amino Acid under Salt Stress in Some Native Rice Cultivars of North Kerala, India. Univ. J. Agric. Res. 2015, 3, 15–22.
- Knight, H.; Anthony, J.; Knight, M.R. Calcium signaling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997, 12, 1067–1078.
- Misra, N.; Gupta, A.K. Interactive effects of sodium and calcium on proline metabolism in salt tolerant green gram cultivar. Am. J. Plant Physiol. 2006, 1, 1–2.
- Ashraf, M.; Foolad, M.R. Roles of glycinebetaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216.
- Huang, Z.; Zhao, L.; Chen, D.; Liang, M.; Liu, Z.; Shao, H.; Long, X. Salt Stress Encourages Proline Accumulation by Regulating Proline Biosynthesis and Degradation in Jerusalem Artichoke Plantlets. PLoS ONE 2013, 8, e62085.
- Lutts, S.; Majerus, V.; Kinet, J.M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Int. Rice Res. 1999, 25, 39–40.
- Devnarain, N.; Crampton, B.G.; Chikwamba, R.K.; Becker, J.V.W.; O’Kennedy, M.M. Physiological responses of selected African sorghum landraces to progressive water stress and re-watering. S. Afr. J. 2016, 103, 61–69.
- Yan, H.; Gang, L.Z.; Zhao, C.Y.; Guo, W.Y. Effects of exogenous proline on the physiology of soybean plantlets regenerated from embryo in vitro and on the ultra-structure of their mitochondria under NaCl stress. Soybean Sci. 2000, 19, 314–319.
- Hare, P.D.; Cress, W.A.; Staden, J.V. Disruptive effects of exogenous proline on chloroplast and mitochondrial ultrastructure in Arabidopsis leaves. S. Afr. J. 2002, 68, 393–396.
- Zeyner, A.; Romanowski, K.; Vernunft, A.; Harris, P.; Müller, A.M.; Wolf, C.; Kienzle, E. Effects of Different Oral Doses of Sodium Chloride on the Basal Acid-Base and Mineral Status of Exercising Horses Fed Low Amounts of Hay. PLoS ONE 2017, 12, e0168325.
- Jimenez-Bremont, J.F.; Becerra-Flora, A.; Hernandez-Lucero, E.; Rodriguez-Kessler, M.; Acosta-Gallegos, J.A.; Ramirez-Pimental, J.G. Proline accumulation in two bean cultivars under salt stress and the effect of polyamines and ornithine. Biol. Plant. 2006, 50, 763–766.
- Gill, S.S.; Tuteja, N. Polyamines and abiotic stress tolerance in plants. Plant Signal Behav. 2010, 5, 26–33.
- Griffith, S.M.; Banowetz, G.M. Nitrogen nutrition and flowering. In Nitrogen Nutrition in Higher Plants; Srivastava, H.S., Singh, R.P., Eds.; Associated Publ. Co.: New Delhi, India, 1995; pp. 385–400.
- Kumar, R.; Khurana, A.; Sharma, A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2013, 65, 4561–4575.
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of Polyamines, Abscisic Acid, Nitric Oxide, and Hydrogen Peroxide under Chilling Stress in Tomato (Lycopersicon esculentum Mill.) Seedlings. Front. Plant Sci. 2017, 8, 203.
- Shabala, S.; Cuin, T.A.; Pottosin, I. Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 2007, 581, 1993–1999.
- Rodríguez, A.A.; Maiale, S.J.; Menéndez, A.B.; Ruiz, O.A. Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J. Exp. Bot. 2009, 1, 4249–4262.
- Mishra, S.N.; Sharma, I. Putrescine as a growth inducer and as a source of nitrogen for mustard seedlings under sodium chloride salinity. Indian J. Exp. Biol. 1994, 32, 916–918.
- Lakra, N.; Tomar, P.C.; Mishra, S.N. Growth response modulation by putrescine in Indian mustard Brassica juncea L. under multiple stress. Indian J. Exp. Biol. 2016, 54, 262–270.
- Maruri-López, I.; Jiménez-Bremont, J. Hetero- and homodimerization of Arabidopsis thaliana arginine decarboxylase AtADC1 and AtADC2. Biochem. Biophys. Res. Commun. 2017, 11, 508–513.
- Wani, S.H.; Singh, N.B.; Haribhushan, A.; Mir, J.I. Compatible solute engineering in plants for abiotic stress tolerance-role of glycine betaine. Curr. Genom. 2013, 14, 157.
- Baloda, A.; Madanpotra, S.; Jaiwal, P.K. Transformation of mung bean plants for abiotic stress tolerance by introducing codA gene, for an osmoprotectant glycine betaine. J. Plant Stress Physiol. 2017, 3, 5–11.
- Wei, D.; Zhang, W.; Wang, C.; Meng, Q.; Li, G.; Chen, T.H.; Yang, X. Genetic engineering of the biosynthesis of glycinebetaine leads to alleviate salt-induced potassium efflux and enhances salt tolerance in tomato plants. Plant Sci. 2017, 257, 74–83.
- Park, E.J.; Jeknic, Z.; Chen, T.H.H. Exogenous application of glycine betaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714.
- Park, H.J.; Kim, W.Y.; Yun, D.J. A New Insight of Salt Stress Signaling in Plant. Mol. Cells 2016, 39, 447–459.
- Khan, N.; Bano, A. Rhizobacteria and Abiotic Stress Management. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 65–80.
- Mohammad, N.; Naeem, K.; Zakia, A.; Abdul, G. Genetic diversity and disease response of rust in bread wheat collected from Waziristan Agency, Pakistan. Int. J. Biodivers. Conserv. 2011, 3, 10–18.
- Shanker, A.; Venkateswarlu, B. Abiotic Stress in Plants: Mechanisms and Adaptations. BoD–Books on Demand; IntechOpen: Norderstedt, Germany, 2011; p. 22.
- Nawaz, K.; Ashraf, M. Exogenous application of glycine betaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop Sci. 2009, 196, 28–37.
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Maximova, E.; Fuggi, A.; Carillo, P. Durum Wheat Roots Adapt to Salinity Remodeling the Cellular Content of Nitrogen Metabolites and Sucrose. Front. Plant Sci. 2016, 7, 2035.
- Wyn-Jones, R.G.; Gorham, J.; McDonnell, E. Organic and inorganic solute contents as selection criteria for salt tolerance in the Triticeae. In Salinity Tolerance in Plants: Strategies for Crop Improvement; Staples, R., Toennissen, G.H., Eds.; Wiley and Sons: New York, NY, USA, 1984; pp. 189–203.
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; Carillo, P. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Plant Physiol. 2016.
- Majeed, K.; Al-Hamzawi, A. Effect of Calcium Nitrate, Potassium Nitrate and Anfaton on Growth and Storability of Plastic Houses Cucumber (Cucumis sativus L. cv. Al-Hytham). Am. J. Plant Sci. 2010, 5, 278–290.
- Kong, X.; Luo, Z.; Dong, H.; Eneji, A.E.; Li, W. Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton. J. Exp. Bot. 2012, 63, 2105–2116.
- Wu, H.; Shabala, L.; Liu, X.; Azzarello, E.; Zhou, M.; Pandolfi, C.; Shabala, S. Linking salinity stress tolerance with tissue-specific Na+ sequestration in wheat roots. Front. Plant Sci. 2015, 6.
- Maleka, P.; Kontturi, M.; Pehu, B.; Somersalo, S. Photosynthesis response of drought and salt stressed tomato and turnip plants to foliar applied glycinebetaine. Physiol. Plant. 1999, 105, 45–50.
- Athar, H.U.R.; Zafar, Z.U.; Ashraf, M. Glycinebetaine Improved Photosynthesis in Canola under Salt Stress: Evaluation of Chlorophyll Fluorescence Parameters as Potential Indicators. J. Agron. Crop Sci. 2015, 201, 428–442.
- Luo, J.Y.; Zhang, S.; Peng, J.; Zhu, X.Z.; Lv, L.M.; Wang, C.Y.; Cui, J.J. Effects of Soil Salinity on the Expression of Bt Toxin (Cry1Ac) and the Control Efficiency of Helicoverpa armigera in Field- Grown Transgenic Bt Cotton. PLoS ONE 2017, 12, e0170379.
- Raza, M.A.S.; Shahid, A.M.; Saleem, M.F.; Khan, I.H.; Ahmad, S.; Ali, M.; Iqbal, R. Effects and management strategies to mitigate drought stress in oilseed rape (Brassica napus L.): A review. Zemdirbyste-Agriculture 2017, 104, 85–94.
- Akram, R.; Fahad, S.; Hashmi, M.Z.; Wahid, A.; Adnan, M.; Mubeen, M.; Khan, N.; Rehmani, M.I.; Awais, M.; Abbas, M.; et al. Trends of electronic waste pollution and its impact on the global environment and ecosystem. Environ. Sci. Pollut. Res. Int. 2019, 1, 1–6.
- Bhuiyan, M.S.; Maynard, G.; Raman, A.; Hodgkins, D.; Mitchell, D.; Nicol, H. Salt effects on proline and glycine betaine levels and photosynthetic performance in Melilotus siculus, Tecticornia pergranulata and Thinopyrum ponticum measured in simulated saline conditions. Funct. Plant Biol. 2016, 4, 254–265.
- Demiral, T.; Türkan, I. Does exogenous glycinebetaine affect antioxidative system of rice seedlings under NaCl treatment? J. Plant Physiol. 2004, 18, 1089–1100.
- Talaat, N.B.; Shawky, B.T. Influence of arbuscular mycorrhizae on yield, nutrients, organic solutes, and antioxidant enzymes of two wheat cultivars under salt stress. J. Soil Sci. Plant Nutr. 2011, 174, 283–291.
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 28, 4033–4044.
- Ghosh, B.; Ali, M.N.; Saikat, G. Response of Rice under Salinity Stress: A Review Update. J. Res. Rice 2016, 4, 1–8.
- Mack, G.; Hoffmann, C.M.; Marlander, B. Nitrogen compounds in organs of two sugar beet genotypes (Beta vulgaris L.) during the season. Field Crop. Res. 2007, 102, 210–218.
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.; Babar, M.A. Comparative physiological and metabolic analysis reveals a complex mechanism involved in drought tolerance in chickpea (Cicer arietinum L.) induced by PGPR and PGRs. Sci. Rep. 2019, 9, 1–19.
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93.
- Turan, S.; Tripathy, B.C. Salt and genotype impact on antioxidative enzymes and lipid peroxidation in two rice cultivars during de-etiolation. Protoplasma 2013, 1, 209–222.
- Das, M.K.; Roychoudhury, A. ROS and responses of antioxidant as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 1–3.
- Elstner, E.F. Mechanisms of oxygen activation in different compartments of plant cell. In Active Oxygen/Oxidative Stress and Plant Metabolism; Pell, E.J., Stefen, K.L., Eds.; American society of Plant Physiol: Rockville, MD, USA, 1991; pp. 13–25.
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Sharma, S. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161.
- Mehmood, A.; Hussain, A.; Irshad, M.; Khan, N.; Hamayun, M.; Ismail; Afridi, S.G.; Lee, I.J. IAA and flavonoids modulates the association between maize roots and phytostimulant endophytic Aspergillus fumigatus greenish. J. Plant Interact. 2018, 1, 532–542.
- Barnawal, D.; Bharti, N.; Tripathi, A.; Pandey, S.S.; Chanotiya, C.S.; Kalra, A. ACC-deaminase-producing endophyte Brachybacterium paraconglomeratum strain SMR20 ameliorates Chlorophytum salinity stress via altering phytohormone generation. J. Plant Growth Reg. 2016, 35, 553–564.
- Beaudoin, N.; Serizet, C.; Gosti, F.; Giraudat, J. Interaction between abscisic acid and ethylene signalling cascades. Plant Cell. 2000, 2, 1103–1115.
- Khan, N.; Bano, A.; Rahman, M.A.; Rathinasabapathi, B.; Babar, M.A. UPLC-HRMS-based untargeted metabolic profiling reveals changes in chickpea (Cicer arietinum) metabolome following long-term drought stress. Plant Cell Environ. 2019, 42, 115–132.
- Anuradha, S.; Rao, S.S.R. Effect of brassinosteroids on salinity stress induced inhibition of seed germination and seedling growth of rice (Oryza sativa L.). Plant Growth Regul. 2001, 33, 151–153.
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26.
- Anuradha, S.; Rao, S.S.R. Application of brassinosteroids to rice seeds (Oryza sativa L.) reduced the impact of salt stress on growth, prevented photosynthetic pigments loss and increased nitrate reductase activity. Plant Growth Regul. 2003, 40, 29–32.
- Shahid, M.A.; Balal, R.M.; Khan, N.; Simón-Grao, S.; Alfosea-Simón, M.; Cámara-Zapata, J.M.; Mattson, N.S.; Garcia-Sanchez, F. Rootstocks influence the salt tolerance of Kinnow mandarin trees by altering the antioxidant defense system, osmolyte concentration, and toxic ion accumulation. Sci. Hortic. 2019, 10, 1.
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernández-Zapata, J.C.; Nicolás, J.J.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 30, 588–599.
- Houimli, S.I.M.; Denden, M.; El-Hadj, S.B. Induction of salt tolerance in pepper (Capsicum annum) by 24-epibrassinolide. Eurasian J. Biosci. 2008, 2, 83–90.
- Thussagunpanit, J.; Jutamanee, K.; Sonjaroon, W.; Kaveeta, L.; Chai-Arree, W.; Pankean, P.; Suksamrarn, A. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynth 2015, 1, 312–320.
- Qayyum, B.; Shabbaz, M.; Akram, N.A. Interachive effect of foliar application of 24—Epibrassinolide and root zone salinity on morpho – physiological attributes of wheat (triticum aestivum L.). Int. J. Agric. Biol. 2007, 9, 584–589.
- Hairat, S.; Khurana, P. Improving Photosynthetic Responses during Recovery from Heat Treatments with Brassinosteroid and Calcium Chloride in Indian Bread Wheat Cultivars. Am. J. Plant Sci. 2015, 6, 1827–1849.
- Amzallag, G.N. Brassinosteroids as metahormones evidences from specific influence during critical period in sorghum development. Plant Biol. 2002, 4, 656–663.
- Gruszka, D.; Janeczko, A.; Dziurka, M.; Pociecha, E.; Oklestkova, J.; Szarejko, I. Barley Brassinosteroid Mutants Provide an Insight into Phytohormonal Homeostasis in Plant Reaction to Drought Stress. Front. Plant Sci. 2016, 7, 1824.
- Kamuro, Y.; Takatsuto, S. Practical application of brassinosteroids in agricultural fields. In Brassinosteroids: Steroidal Plant Hormones; Sakurai, A., Yokota, T., Clouse, S.D., Eds.; Springer: Tokyo, Japan, 1999; pp. 223–241.
- Ali, B.; Hasan, S.A.; Hayat, S.; Hayat, Q.; Yadav, S.; Fariduddin, Q.; Ahmad, A. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environ. Exp. Bot. 2008, 62, 153–159.
- Hasan, S.A.; Hayat, S.; Ali, B.B.; Ahmad, A. 28-Homobrassinolide protects chickpea (Cicer arietinum) from cadmium toxicity by stimulating antioxidants. Environ. Pollut. 2008, 151, 60–66.
- Rady, M.M.; Osman, A.S. Response of growth and antioxidant system of heavymetal-contaminated tomato plants to 24-epibrassinolide. Afr. J. Agric. Res. 2012, 7, 3249–3254.
- Jain, M.; Mathur, G.; Koul, S.; Sarin, N. Ameliorative effects of proline on salt induced lipid peroxidation in Cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep. 2001, 20, 463–468.
- Abdullahi, B.A.; Gu, X.; Gan, Q.; Yang, Y. Brassinolide amelioration of aluminum toxicity in mung bean seedling growth. J. Plant Nutr. 2003, 26, 1725–1734.
- Martínez-Cuenca, M.R.; Primo-Capella, A.; Forner-Giner, M.A. Influence of Rootstock on Citrus Tree Growth: Effects on Photosynthesis and Carbohydrate Distribution, Plant Size, Yield, Fruit Quality, and Dwarfing Genotypes. Plant Growth 2016, 16, 107.
- Khelil, A.; Menu, T.; Ricard, B. Adaptive response to salt involving carbohydrate metabolism in leaves of a salt-sensitive tomato cultivars. Plant Physiol. Biochem. 2007, 45, 551–559.
- Bartels, D.; Sunkar, R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005, 24, 23–58.
- Hoekstra, F.A.; Golovina, E.A.; Buitkink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438.
- Pierik, R.; Christa, T. The Art of Being Flexible: How to Escape from Shade, Salt, and Drought. Top. Rev. Plast. 2014, 166, 5–22.
- Libal-Wekseler, J.; Fernak, B.; Michael, S.T.; Zhu, J.K. Carbohydrate metabolism is affected in response to salt stress. Horticulture 1997, 20, 1043–1045.
- Khan, N.; Bano, A. Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int. J. Phytorem. 2016, 18, 211–221.
- Schreiber, L. Transport barriers made of cutin, suberin and associated waxes. Trends Plant Sci. 2010, 15, 546–553.
- Lux, A.; Šottníková, A.; Opatrná, J.; Greger, M. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol. Plant. 2004, 120, 537–545.
- Shabala, S. Learning from halophytes: Physiological basis and strategies to improve abiotic stress tolerance in crops. Ann. Bot. 2013, 112, 1209–1221.
- White, P.J.; Broadley, M.R. Chloride in soil and its uptake and movement within the plant. A review. Ann. Bot. 2001, 88, 967–988.
- Grattan, S.R.; Grieve, C.M. Mineral nutrient acquisition and response by plants grown in saline environments. In Hand Book of Plant and Crop Stress; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 203–299.
