Efficient water remediation methods towards the extraction of 137Cs are being studied. Prussian blue (PB) and its analogs have shown very high efficiencies in the capture of 137Cs+ ions.
- Prussian blue
- 137-Cesium
- water remediation
- magnetic extraction
- 137Cs+ selectivity
- radioactive contamination
1. Prussian Blue
1.1. Structure
Prussian blue (PB) is a dark blue pigment synthesized by ferrous ferrocyanide salts with chemical formula Fe
(CN)
. It has a porous structure with the capacity to adsorb the
Cs
ions into its pores and store them there. It is a metal organic framework (MOF) where the inorganic vertices, which donate electrons in the structure, are linked to each other via organic compounds. The complete chemical formula is Fe
[Fe
(CN)
]
·
H
O. The compound has a face-centered cubic structure (FCC) structure (
1) belonging to the Fm3̅m space group with a lattice parameter of 10.166 Å. Fe exists in two oxidation states within the structure: Fe
and Fe
. These ions form two different FCC lattices displaced by half a lattice parameter with respect to each other. However, the bi and tri-valent Fe are coordinated differently. Furthermore, they are linked to each other via cyanide groups (C ≡ N) i.e., C groups are linked to Fe
and N groups to Fe
with high and low spins respectively, in octahedral configurations. The Fe
and Fe
ratio of 3:4 implies that in order to obtain a charge neutrality within the structure a 25% vacancy of [Fe
(CN)
]
molecules is necessary [1]. Coordinated water molecules occupy the resulting octahedral cavities created by such vacancies; six water molecules are linked to Fe
. The other interstitial water molecules occupy the eight corners of the unit cell (¼, ¼, ¼) and are essential for the insertion of the
Cs
ions in the structure.
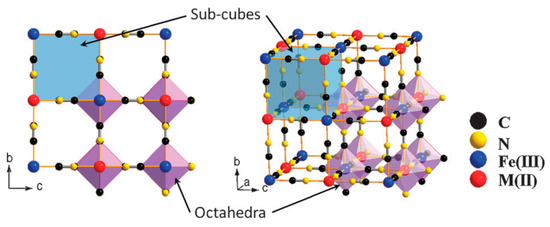
Framework of Prussian blue analogues. Adapted with permission from [2], Copyright RSC, 2012.
Fe
can be replaced by other transition metals with the same +2 oxidation states such as Ni, Mn, Cu and Co, coordinated exactly like Fe
in the structure and are called PB analogs. However, there are reports of Cd and Zn with slightly larger atomic radii also being incorporated into the structure owing to their +2 oxidation state [3]. The aim in including different species into the structure is to provoke a distortion of the PB lattice by producing vacancies of the high spin state molecule along with distortions in the vacant cages, in order to facilitate the capture and sequestration of the
Cs
[4].
1.2. 137Cs+ Ion Capture Mechanism in Prussian Blue
The compound is insoluble in water and the basic mechanism consists of ion exchange of
Cs
and H
with the former occupying hydrophilic vacancies [5]. PB analogs have very different mechanisms of ion exchange or capture depending upon the anionic and alkali metal cation concentrations. Since PB and its analogs contain large amounts of interstitial and coordinated water,
Cs
is captured by a defect created by a [Fe
(CN)
] vacancy, which creates a spherical cavity whose size is equivalent to the hydration radius of
Cs
. Nevertheless, recent calculations have demonstrated that a completely dehydrated
Cs
ion can be incorporated into the structure with the release of a water molecule from the interstitial sites [6]. This is similar to certain clays, where on dehydrating the interlayers the
Cs
selectivity increases [7]. On the other hand, water soluble analogs such as metal hexacyanoferrates (HCF) consisting of a alkali metal cation with a [Fe
(CN)
] anion, used for the extraction of
Cs
have shown less efficiency. In such compounds Na
or K
are incorporated during the synthesis of the MOF in order to render them water-soluble [8]. In addition to
Cs
capture mechanisms for non-soluble analogs; the water-soluble analogs mainly depend on the Na
or K
ion exchanges with Cs
. Takahashi et al., have studied the
Cs
uptake in KCuHCF PB analog in order to understand their lower adsorption capacity [8]. Three main mechanisms governed the
Cs
ion exchange according to them, with the
Cs
-K
ion exchanges being predominant, as also stipulated by other research groups. In case of low anionic vacancies, the percolation of
Cs
through the vacancies was prevalent. Finally, for low K
incorporation in the structure, proton exchange between
Cs
and K
ions was evidenced. Ayrault et al., report a degradation in the crystal structure of the KCuHF soluble compound after
Cs
adsorption which was not observed in the non-soluble counterpart [9].
1.3. Nanostructured Prussian Blue
Cs
adsorption in PB crystals is a very slow process. Fujita et al., have demonstrated that after two weeks of adsorption experiments, the depth of
Cs
adsorption was at most between 1–2 nm, irrespective of the crystal size [10]. This implies that most of the adsorption occurs on the surface of the crystallites. This low diffusion depth is mainly attributed to the blocking of the vacancies by captured
Cs
ions, which in turn hinders further
Cs
diffusion. Since the diffusion depth appears to be a constant, increasing the specific surface would therefore be a solution to increasing the
Cs
uptake. One way of augmenting the specific surface is by synthesizing nanoparticles of PB. The surface to volume ratio of crystallites increases as their size decreases; therefore nanoparticles have an extremely large surface to volume ratio. For example, a 3 nm nanoparticle will have 50% of its atoms on its surface. This would also imply that in the case of PB nanocrystals most of the vacancies and sites responsible for
Cs
adsorption would be available on the surface, thus enhancing its specific surface. To this end, different research groups have produced various PB analogs of type Metal(M)-Co, where the nature of M defines the efficiency of the uptake. Liu et al., have demonstrated that Zn assisted Fe-Co PB analogs present high
Cs
uptake efficiency [11]. They also observed that the size of the PB analog particle reduced with the reduction of Fe in the structure; for pure Zn-Co analogs, a crystallite size of ~73 nm was calculated, displaying the highest
Cs
adsorption as depicted in
2.
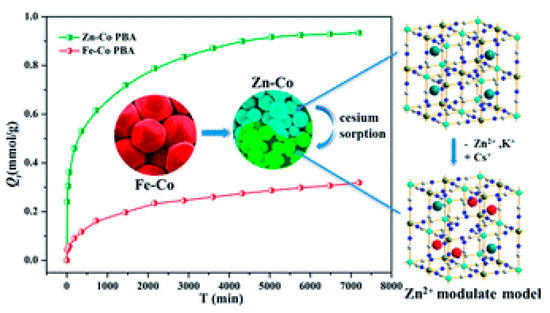
Adsorption efficiency of Fe-Co and Zn-Co prussian blue (PB) analogs as a function of time. The Zn-Co PB analog exhibits a higher Q
(Q
is the adsorption capacity per unit gram of the sorbent at a given time t) owing to the reduction in size of the nanoparticles. Adapted from [11] under the Creative Commons agreement from RSC, 2017.
Considering that the
Cs
adsorption depth is only about 1–2 nm, hollow PB nanoparticles may offer many more advantages. They not only have a very active surface area due to their large surface to volume ratio but their hollow interior is also capable of capturing and storing
Cs
[12]. A surfactant polyvinylpyrrolidone (PVP) was used to stabilize the nanoparticle and increase their dispersion in aqueous solutions.
3 compares the efficiency of solid and hollow PB cubes of ~200 nm, in the capture of
Cs
. The elemental mapping of
3B depicts a higher concentration of captured
Cs
ions within the hollow structures than the filled ones in
3A. Nevertheless,
Cs
diffusion depth greater than 2 nm would require higher activation energy at room temperature. Other methods are required to determine the exact diffusion depth in such structures, as these results are mainly qualitative. Besides, it is well known that the use of a surfactant shields the active sites and prevents the capture of
Cs
. In order to avoid such shielding effects, PB could be coated onto support materials instead through hydroxyl bonds that anchor the PB to the support material. Carboxylic groups also tend to immobilize the PB particles in a sturdier manner. Wi et al., used a polyvinyl support surface functionalized with acrylic acid. This allowed converting the OH groups to COOH and providing a better adhesion of the PB [13]. The PB nanoparticles were immobilized on the PVP sponge and an increase in
Cs
uptake efficiency by five times was reported, compared to the hydroxyl bond functionalization.
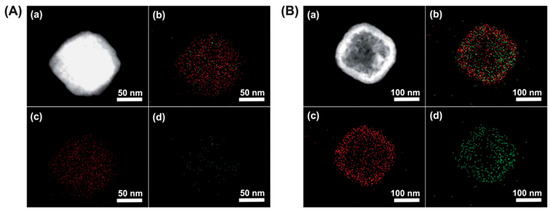
Elemental mapping images of solid (
) and hollow PB (
) nanoparticles of 190 nm in diameter. (
) Dark-field TEM image, (
) elemental mapping of both Fe and Cs (
) elemental mapping of Fe, and (
) elemental mapping of Cs. Adapted with permission from [12], Copyright RSC, 2012.
2. Magnetic Extraction Using Prussian Blue
There are reports of photo-induced magnetism where an electromagnetic radiation induces a residual magnetization even after the excitation is turned off, [14] due to low and high spin combinations of the transition metals in PB analogs. On their own, PB and its analogs exhibit ferromagnetism at a Curie temperature of 11 K with a saturation magnetization of 3.4 emu/g as obtained by Tokoro et al., for Mn-Rb-Fe PB analogs [15]. The presence of Mn in the structure creates a Jahn-Teller distortion [16] by changing the M–CN–M bond angle and deviating it from 180°, whereupon inducing ferromagnetism. Among the various PB analogs, Mn based ones have shown the highest saturation magnetization [17]. One method of decreasing the Curie temperature of PB is by synthesizing nanoparticles of PB. Uemura et al., have demonstrated a decrease in Tc from 5.5 K in bulk PB to 4 K for PB nanoparticles protected by PVP [18]. Nevertheless, finding practical applications involving magnetic extraction would require having a Tc at around room temperature. Also, humidity increases the Curie temperature for Co-Cr PB analogs, [15] thus making it difficult for their direct application in aqueous media. This implies that most methods using PB for
Cs
extraction, do not prescribe any efficient approach to recover the exhausted adsorbent.
Literature on nanostructured PB alone is very scarce as they are generally combined with magnetic nanomaterials like superparamagnetic iron oxide nanoparticles (SPIONs) i.e., Fe
O
or γ-Fe
O
nanoparticles. In the past, there have been reviews briefly describing
Cs
adsorption employing magnetic PB-Fe
O
nanoparticles [19]. In nanostructures, physical properties such as magnetic moment as well as adsorption vary as a function of the nanoparticle size and further depend upon the surfactants used to stabilize them during synthesis. In the paragraph that follows, the efficiency of magnetic PB nanoparticles and their combination with magnetic nanoparticles is assessed.
Core-shell structures with the magnetite constituting the core and the PB active layer constituting the shell have been employed. Jang et al., have reported that the poly(diallyldimethylammoniumchloride) (PDDA)@Iron oxide nanoparticles can act as nucleation sites for the precipitated PB, resulting in the coating of a negatively charged PB on the PDDA@Iron oxide nanoparticle surface [20]. Furthermore, they have also studied the magnetic properties of Fe
O
and have observed a decrease in the saturation magnetization from 56 emu/g for pure Fe
O
to 12 emu/g for the PB-Fe
O
nanocomposite. The reduction was mostly due to the shielding of the superparamagnetism of Fe
O
by the PB capping. Nevertheless, successful magnetic extraction was achieved with the nanocomposite [21]. PB analog compounds tend to show degradation in various applications after successive cycles of reuse. Chang et al.,
4A, have synthesized Fe
O
(shell)-PB (core) nanocomposites with sizes between 20–40 nm; [22] two different concentrations of FeCl
were used during the synthesis. The higher Fe concentrations produced Fe
O
cores with a higher magnetic saturation moment (
4B), which conversely had an adverse effect on the
Cs
adsorption due to the higher shielding of the active sites in the PB core as depicted in
4C.
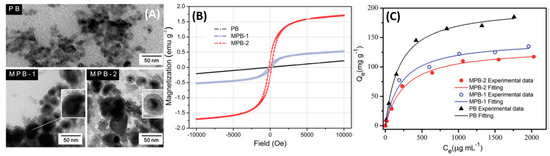
(
) TEM images of PB and magnetic PB (MPB) nanoparticles. (
) Field-dependent magnetization of PB and magnetic PB nanoparticles. (
) Adsorption capacity of samples under different Cs
concentration.
= 25 °C; contact time = 6 h; [Cs
]
= 50–2500 μg/mL;
/
= 4 μg/mL. Adapted with permission from [22], Copyright RSC, 2016.
Some researchers have also used different ferrimagnetic nanoparticles like CoFe
O
combined with PB for the extraction of cesium and have indicated very high efficiencies compared to pure CoFe
O
, which has some interesting
Cs
adsorption capacity by itself [23]. CoFe
O
possesses the spinel structure and tends to be physically more robust but is a hard magnetic compound compared to Fe
O
. The latter is a soft magnet with a very small residual magnetization and can therefore be used in successive cycles of extraction.
References
- Kumar, A.; Yusuf, S.M.; Keller, L. Structural and magnetic properties of Fe[Fe(CN)6]∙4H2O. Phys. Rev. B 2005, 71, 054414.
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546.
- Nie, P.; Shen, L.; Luo, H.; Ding, B.; Xu, G.; Wang, J.; Zhang, X. Prussian blue analogues: A new class of anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 5852–5857.
- Matsuda, T.; Kim, J.; Moritomo, Y. Control of the alkali cation alignment in Prussian blue framework. Dalton Trans. 2012, 41, 7620–7623.
- Ishizaki, M.; Akiba, S.; Ohtani, A.; Hoshi, Y.; Ono, K.; Matsuba, M.; Togashi, T.; Kananizuka, K.; Sakamoto, M.; Takahashi, A.; et al. Proton-exchange mechanism of specific Cs+ adsorption via lattice defect sites of Prussian blue filled with coordination and crystallization water molecules. Dalton Trans. 2013, 42, 16049–16055.
- Ruankaew, N.; Yoshida, N.; Watanabe, Y.; Nakano, H.; Phongphanphanee, S. Size-dependent adsorption sites in a Prussian blue nanoparticle: A 3D-RISM study. Chem. Phys. Lett. 2017, 684, 117–125.
- Eberl, D.D. Alkali Cation Selectivity and Fixation by Clay Minerals. Clays Clay Miner. 1980, 28, 161–172.
- Takahashi, A.; Tanaka, H.; Minami, K.; Noda, K.; Ishizaki, M.; Kurihara, M.; Ogawa, H.; Kawamoto, T. Unveiling Cs-adsorption mechanism of Prussian blue analogs: Cs+-percolation via vacancies to complete dehydrated state. RSC Adv. 2018, 8, 34808–34816.
- Ayrault, S.; Jimenez, B.; Garnier, E.; Fedoroff, M.; Jones, D.J.; Loos-Neskovic, C. Sorption Mechanisms of Cesium on CuII2FeII(CN)6 and CuII3[FeIII(CN)6]2 Hexacyanoferrates and Their Relation to the Crystalline Structure. J. Solid State Chem. 1998, 141, 475–485.
- Fujita, H.; Miyajima, R.; Sakoda, A.J.A. Limitation of adsorptive penetration of cesium into Prussian blue crystallite. Adsorption 2015, 21, 195–204.
- Liu, J.; Li, X.; Rykov, A.I.; Fan, Q.; Xu, W.; Cong, W.; Jin, C.; Tang, H.; Zhu, K.; Ganeshraja, A.S.; et al. Zinc-modulated Fe–Co Prussian blue analogues with well-controlled morphologies for the efficient sorption of cesium. J. Mater. Chem. A 2017, 5, 3284–3292.
- Torad, N.L.; Hu, M.; Imura, M.; Naito, M.; Yamauchi, Y. Large Cs adsorption capability of nanostructured Prussian Blue particles with high accessible surface areas. J. Mater. Chem. 2012, 22, 18261–18267.
- Wi, H.; Kang, S.-W.; Hwang, Y. Immobilization of Prussian blue nanoparticles in acrylic acid-surface functionalized poly(vinyl alcohol) sponges for cesium adsorption. Environ. Eng. Res. 2019, 24, 173–179.
- Pajerowski, D.M.; Gardner, J.E.; Frye, F.A.; Andrus, M.J.; Dumont, M.F.; Knowles, E.S.; Meisel, M.W.; Talham, D.R. Photoinduced Magnetism in a Series of Prussian Blue Analogue Heterostructures. Chem. Mater. 2011, 23, 3045–3053.
- Tokoro, H.; Ohkoshi, S.-I. Novel magnetic functionalities of Prussian blue analogs. Dalton Trans. 2011, 40, 6825–6833.
- Buzin, E.R.; Prellier, W.; Mercey, B.; Simon, C.; Raveau, B. Relations between structural distortions and transport properties in Nd0.5Ca0.5MnO3 strained thin films. J. Phys. Condens. Matter 2002, 14, 3951–3958.
- Nakotte, H.; Shrestha, M.; Adak, S.; Boergert, M.; Zapf, V.S.; Harrison, N.; King, G.; Daemen, L.L. Magnetic properties of some transition-metal Prussian Blue Analogs with composition M3[M′(C,N)6]2·xH2O. J. Sci. Adv. Mater. Devices 2016, 1, 113–120.
- Uemura, T.; Kitagawa, S. Prussian Blue Nanoparticles Protected by Poly(vinylpyrrolidone). J. Am. Chem. Soc. 2003, 125, 7814–7815.
- Liu, X.; Chen, G.-R.; Lee, D.-J.; Kawamoto, T.; Tanaka, H.; Chen, M.-L.; Luo, Y.-K. Adsorption removal of cesium from drinking waters: A mini review on use of biosorbents and other adsorbents. Bioresour. Technol. 2014, 160, 142–149.
- Jang, S.-C.; Kang, S.-M.; Kim, G.Y.; Rethinasabapathy, M.; Haldorai, Y.; Lee, I.; Han, Y.-K.; Renshaw, J.C.; Roh, C.; Huh, Y.S. Versatile Poly(Diallyl Dimethyl Ammonium Chloride)-Layered Nanocomposites for Removal of Cesium in Water Purification. Materials 2018, 11, 998.
- Jang, J.; Lee, D.S. Magnetic Prussian Blue Nanocomposites for Effective Cesium Removal from Aqueous Solution. Ind. Eng. Chem. Res. 2016, 55, 3852–3860.
- Chang, L.; Chang, S.; Chen, W.; Han, W.; Li, Z.; Zhang, Z.; Dai, Y.; Chen, D. Facile one-pot synthesis of magnetic Prussian blue core/shell nanoparticles for radioactive cesium removal. RSC Adv. 2016, 6, 96223–96228.
- Hassan, M.R.; Aly, M.I. Adsorptive removal of cesium ions from aqueous solutions using synthesized Prussian blue/magnetic cobalt ferrite nanoparticles. Part. Sci. Technol. 2019, 1–11.
