Intrinsic apoptosis, the response to intracellular cell death stimuli, is regulated by the interplay of the B-cell lymphoma 2 (Bcl-2) family and their membrane interactions. Bcl-2 proteins mediate a number of processes including development, homeostasis, autophagy, and innate and adaptive immune responses and their dysregulation underpins a host of diseases including cancer. The Bcl-2 family is characterized by the presence of conserved sequence motifs called Bcl-2 homology (BH) motifs, as well as a transmembrane region, which form the interaction sites and intracellular location mechanism, respectively. Bcl-2 proteins have been recognized in the earliest metazoans including Porifera (sponges), Placozoans, and Cnidarians (e.g., Hydra). A number of viruses have gained Bcl-2 homologs and subvert innate immunity and cellular apoptosis for their replication, but they frequently have very different sequences to their host Bcl-2 analogs. Though most mechanisms of apoptosis initiation converge on activation of caspases that destroy the cell from within, the numerous gene insertions, deletions, and duplications during evolution have led to a divergence in mechanisms of intrinsic apoptosis. Currently, the action of the Bcl-2 family is best understood in vertebrates and nematodes but new insights are emerging from evolutionarily earlier organisms.
- apoptosis
- Bcl-2
- evolution
- mechanism
- structure analysis
1. Introduction
| Name(s) | Species | Functions | References |
|---|---|---|---|
| Bcl-2 | H. sapiens | Prosurvival | [13][14][10,11] |
| Bcl-w | H. sapiens | Prosurvival | [15][16][17][12,13,14] |
| Bcl-xL | H. sapiens | Prosurvival | [18][19][15,16] |
| Mcl-1 | H. sapiens | Prosurvival | [20][21][17,18] |
| Bfl-1/A1 | H. sapiens | Prosurvival | [22][23][19,20] |
| Bcl-b | H. sapiens | Prosurvival | [24][25][21,22] |
| Boo/Diva | M. musculus | Prosurvival | [24][26][21,23] |
| NRZ | D. rerio | Prosurvival | [27][28][24,25] |
| Bak | H. sapiens | Proapoptotic | [29][30][26,27] |
| Bax | H. sapiens | Proapoptotic | [31][32][28,29] |
| Bok | H. sapiens | Proapoptotic | [33][30] |
| Bad | H. sapiens | Proapoptotic | [34][31] |
| Bid | H. sapiens | Proapoptotic | [35][32] |
| Bik | H. sapiens | Proapoptotic | [36][33] |
| Bim | H. sapiens | Proapoptotic | [37][34] |
| Bmf | H. sapiens | Proapoptotic | [38][35] |
| Hrk | H. sapiens | Proapoptotic | [39][36] |
| Noxa | H. sapiens | Proapoptotic | [40][37] |
| Puma | H. sapiens | Proapoptotic | [41][38] |
| Beclin | H. sapiens | Proautophagic | [42][39] |
| Bcl-wav | D. rerio | Proapoptotic | [43][44][40,41] |
| Buffy | D. melanogaster | Proapoptotic | [45][42] |
| DeBcl | D. melanogaster | Prosurvival | [46][43] |
| BHRF1 | Epstein–Barr virus | Prosurvival | [47][48][44,45] |
| KsBcl-2 | Kaposi Sarcoma herpesvirus | Prosurvival | [49][46] |
| E1B19K | Human adenovirus | Prosurvival | [50][47] |
| M11 | mγ68 herpesvirus | Prosurvival | [51][52][48,49] |
| A179L | African swine fever virus | Prosurvival | [53][54][50,51] |
| F1L | Vaccina virus, variola virus | Prosurvival | [55][56][52,53] |
| DPV022 | Deer poxvirus | Prosurvival | [57][58][54,55] |
| M11L | Myxomavirus | Prosurvival | [59][60][56,57] |
| FPV039 | Fowl poxvirus | Prosurvival | [61][62][58,59] |
| CNP058 | Canary poxvirus | Prosurvival | [63][64][60,61] |
| SPPV14 | Sheep poxvirus | Prosurvival | [65][62][661] |
| TANV16L | Tanapoxvirus | Prosurvival | [672] |
| ORFV125 | Orf virus | Prosurvival | [68][69][63] |
| GIV66 | Grouper iridovirus | Prosurvival | [70][71][64,65] |
| N1 | Vaccinia virus | Prosurvival, NF-κb | [72][73][66,67] |
| A46 | Vaccinia virus | NF-κb | [74][75][68,69] |
| A49 | Vaccinia virus | NF-κb | [76][77][70,71] |
| A52 | Vaccinia virus | NF-κb | [78][72] |
| B14 | Vaccinia virus | NF-κb | [78][72[79],73] |
| K7 | Vaccinia virus | NF-κb, IFN signaling | [80][74[81],75] |
| LB-Bcl-2 | L. baicalensis | Prosurvival | [82][76] |
| LB-Bak-2 | L. baicalensis | Proapoptotic | [7][82][6,76] |
| BHP1 | G. cydonium | Prosurvival | [83][77] |
| BHP2 | G. cydonium | Prosurvival | [7][83][6,77] |
| trBcl-2L1 | T. adherens | Prosurvival | [843] |
| trBcl-2L2 | T. adherens | Prosurvival | [843] |
| trBcl-2L3/trBax | T. adherens | Proapoptotic | [843] |
| trBcl-2L4/trBak | T. adherens | Proapoptotic | [843] |
| Hy-Bcl-2-4 | H. vulgaris | Proapoptotic | [854] |
| Hy-BH3-only-2 | H. vulgaris | Proapoptotic | [854] |
| Hy-Bak1 | H. vulgaris | Proapoptotic | [854] |
| Hy-Bax | H. vulgaris | Proapoptotic | [854] |
| EGL-1 | C. elegans | Proapoptotic | [86][87][79,80] |
| CED-9 | C. elegans | Prosurvival | [88][89][81,82] |
| vMIA | Cytomegalovirus | Prosurvival | [90][91][83,84] |
2. Virus-Encoded Bcl-2 Homologs
2.1. Bcl-2 Homologs Encoded by Herpesviridae
2.2. Poxvirus Bcl-2 Homologs
3. The Nonmammalian Bcl-2 Family
4. The Role of Mitochondrial Membrane Interactions
5. Conclusions
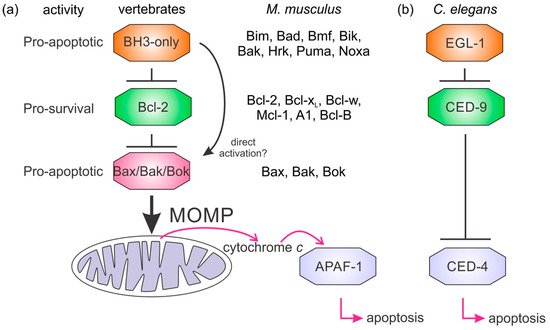
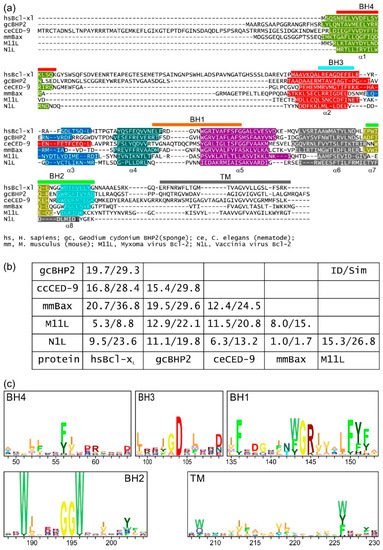
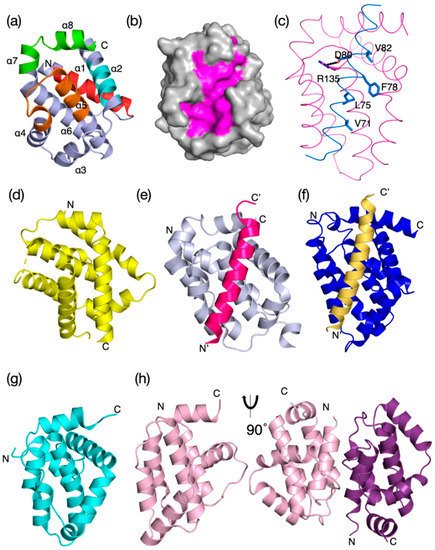
It is not clear how the Bcl-2 family arose; one hypothesis is that it occurred through horizontal gene transfer from a symbiont [179][172], but multiple Bcl-2 genes occurred early in metazoan evolution. Even in the sponges, a phylum considered to be the sister group of all metazoans [142][135], multiple Bcl-2 fold proteins have been identified in genomes, such as that of A. queenslandica [144][137] where seven such proteins were recognized. In contrast to the early appearance of Bcl-2 fold proteins, BH3-only proteins have not yet been identified in Porifera or Placozoa. Ctenophores and some ecdysozoans [65][141] appear to have lost the genes required for Bcl-2-regulated apoptosis altogether (Figure 4a). Emerging results from biophysical and biochemical measurements performed on the nonmammalian Bcl-2 family including those from sponges [7][6], placozoans [843] and cnidarians [854] indicate that the basic architecture of intrinsic apoptosis is maintained for these basal metazoans. Structural studies have shown that the molecular details of interactions have been conserved from sponges to man [7][6] and viruses have assimilated Bcl-2 proteins [116][111]. A key difference between sponges, placozoans, and cnidarians is an apparent absence of BH3-only proteins in sponges and placozoans. The essential role of the BH3-only proteins, at least in mammals, is to neutralize the prosurvival proteins to allow the MOM to activate the proapoptotic Bcl-2 proteins [160][153]. Based on these results, a hypothesis for a simple model for intrinsic apoptosis in the absence of BH3-ony proteins could be envisaged as the prosurvival proteins keeping the proapoptotic proteins in check. Alternately, as recently proposed, the Bak-like protein may partially fulfill the role of BH3-only proteins [843] (Figure 4b). The investigation of the more evolutionary distant members of the Bcl-2 family has exposed the substantial complexity in Bcl-2-mediated signaling at the foundation of metazoan evolution and underscores the pivotal role these proteins play in biology. Functional and mechanistic studies to date have only just begun to unravel the role Bcl-2 has played during the early stages of metazoan life, and future studies are likely to discover new twists to Bcl-2 signaling.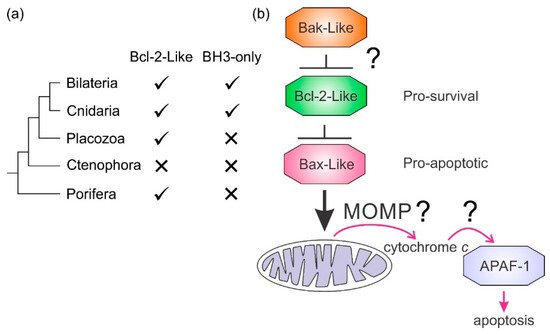
References
- Koonin, E.V.; Aravind, L. Origin and evolution of eukaryotic apoptosis: The bacterial connection. Cell Death Differ. 2002, 9, 394–404.Chathura D. Suraweera; Denis R. Burton; Mark G. Hinds; Marc Kvansakul; Crystal structures of the sheeppox virus encoded inhibitor of apoptosis SPPV14 bound to the proapoptotic BH3 peptides Hrk and Bax. FEBS Letters 2020, 594, 2016-2026, 10.1002/1873-3468.13807.
- Huettenbrenner, S.; Maier, S.; Leisser, C.; Polgar, D.; Strasser, S.; Grusch, M.; Krupitza, G. The evolution of cell death programs as prerequisites of multicellularity. Mutat. Res. 2003, 543, 235–249.Chathura D. Suraweera; Mohd Ishtiaq Anasir; Srishti Chugh; Airah Javorsky; Rachael E. Impey; Mohammad Hasan Zadeh; Tatiana P. Soares da Costa; Mark G. Hinds; Marc Kvansakul; Structural insight into tanapoxvirus‐mediated inhibition of apoptosis. The FEBS Journal 2020, 287, 3733-3750, 10.1111/febs.15365.
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193.Nikolay Popgeorgiev; Jaison D Sa; Lea Jabbour; Suresh Banjara; Trang Thi Minh Nguyen; Aida Akhavan-E-Sabet; Rudy Gadet; Nikola Ralchev; Stéphen Manon; Mark G. Hinds; et al.Hans-Jürgen OsigusBernd SchierwaterPatrick O. HumbertRuth RimokhGermain GilletMarc Kvansakul Ancient and conserved functional interplay between Bcl-2 family proteins in the mitochondrial pathway of apoptosis. Science Advances 2020, 6, eabc4149, 10.1126/sciadv.abc4149.
- Strasser, A.; Vaux, D.L. Viewing BCL2 and cell death control from an evolutionary perspective. Cell Death Differ. 2018, 25, 13–20.Suresh Banjara; Jaison D Sa; Mark G. Hinds; Marc Kvansakul; The structural basis of Bcl-2 mediated cell death regulation in hydra. Biochemical Journal 2020, 477, 3287-3297, 10.1042/bcj20200556.
- Suresh Banjara; Chathura Suraweera; Mark Hinds; Marc Kvansakul; The Bcl-2 Family: Ancient Origins, Conserved Structures, and Divergent Mechanisms. Neil D. Young; Tiffany J. Harris; Marco Evangelista; Sharon Tran; Merridee A. Wouters; Tatiana P. Soares Da Costa; Nadia J. Kershaw; Robin B. Gasser; Brian J. Smith; Erinna F. Lee; et al.W. Douglas Fairlie Diversity in the intrinsic apoptosis pathway of nematodes. Communications Biomlolecules gy 2020, 10, 128, 10.3390/biom10010128., 3, 1-12, 10.1038/s42003-020-01208-5.
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5.
- Caria, S.; Hinds, M.G.; Kvansakul, M. Structural insight into an evolutionarily ancient programmed cell death regulator—The crystal structure of marine sponge BHP2 bound to LB-Bak-2. Cell Death Dis. 2017, 8, e2543.
- Kvansakul, M.; Hinds, M.G. The Bcl-2 family: Structures, interactions and targets for drug discovery. Apoptosis 2015, 20, 136–150.
- Kvansakul, M.; Caria, S.; Hinds, M.G. The Bcl-2 Family in Host-Virus Interactions. Viruses 2017, 9, 290.
- Kvansakul, M.; Hinds, M.G. The structural biology of BH3-only proteins. Methods Enzymol. 2014, 544, 49–74.
- Holm, L.; Laakso, L.M. Dali server update. Nucleic Acids Res. 2016, 44, W351–W355.
- Wheeler, T.J.; Clements, J.; Finn, R.D. Skylign: A tool for creating informative, interactive logos representing sequence alignments and profile hidden Markov models. BMC Bioinform. 2014, 15, 7.
- Petros, A.M.; Medek, A.; Nettesheim, D.G.; Kim, D.H.; Yoon, H.S.; Swift, K.; Matayoshi, E.D.; Oltersdorf, T.; Fesik, S.W. Solution structure of the antiapoptotic protein bcl-2. Proc. Natl. Acad. Sci. USA 2001, 98, 3012–3017.
- Vaux, D.L.; Cory, S.; Adams, J.M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 1988, 335, 440–442.
- Denisov, A.Y.; Madiraju, M.S.; Chen, G.; Khadir, A.; Beauparlant, P.; Attardo, G.; Shore, G.C.; Gehring, K. Solution structure of human BCL-w: Modulation of ligand binding by the C-terminal helix. J. Biol. Chem. 2003, 278, 21124–21128.
- Gibson, L.; Holmgreen, S.P.; Huang, D.C.; Bernard, O.; Copeland, N.G.; Jenkins, N.A.; Sutherland, G.R.; Baker, E.; Adams, J.M.; Cory, S. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene 1996, 13, 665–675.
- Hinds, M.G.; Lackmann, M.; Skea, G.L.; Harrison, P.J.; Huang, D.C.; Day, C.L. The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. EMBO J. 2003, 22, 1497–1507.
- Boise, L.H.; Gonzalez-Garcia, M.; Postema, C.E.; Ding, L.; Lindsten, T.; Turka, L.A.; Mao, X.; Nunez, G.; Thompson, C.B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993, 74, 597–608.
- Muchmore, S.W.; Sattler, M.; Liang, H.; Meadows, R.P.; Harlan, J.E.; Yoon, H.S.; Nettesheim, D.; Chang, B.S.; Thompson, C.B.; Wong, S.L.; et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 1996, 381, 335–341.
- Rinkenberger, J.L.; Horning, S.; Klocke, B.; Roth, K.; Korsmeyer, S.J. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000, 14, 23–27.
- Day, C.L.; Chen, L.; Richardson, S.J.; Harrison, P.J.; Huang, D.C.; Hinds, M.G. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J. Biol. Chem. 2005, 280, 4738–4744.
- Smits, C.; Czabotar, P.E.; Hinds, M.G.; Day, C.L. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure 2008, 16, 818–829.
- Karsan, A.; Yee, E.; Kaushansky, K.; Harlan, J.M. Cloning of human Bcl-2 homologue: Inflammatory cytokines induce human A1 in cultured endothelial cells. Blood 1996, 87, 3089–3096.
- Ke, N.; Godzik, A.; Reed, J.C. Bcl-B, a novel Bcl-2 family member that differentially binds and regulates Bax and Bak. J. Biol. Chem. 2001, 276, 12481–12484.
- Rautureau, G.J.; Yabal, M.; Yang, H.; Huang, D.C.; Kvansakul, M.; Hinds, M.G. The restricted binding repertoire of Bcl-B leaves Bim as the universal BH3-only prosurvival Bcl-2 protein antagonist. Cell Death Dis. 2012, 3, e443.
- Rautureau, G.J.; Day, C.L.; Hinds, M.G. The structure of Boo/Diva reveals a divergent Bcl-2 protein. Proteins 2010, 78, 2181–2186.
- Arnaud, E.; Ferri, K.F.; Thibaut, J.; Haftek-Terreau, Z.; Aouacheria, A.; Le Guellec, D.; Lorca, T.; Gillet, G. The zebrafish bcl-2 homologue Nrz controls development during somitogenesis and gastrulation via apoptosis-dependent and -independent mechanisms. Cell Death Differ. 2006, 13, 1128–1137.
- Suraweera, C.D.; Caria, S.; Jarva, M.; Hinds, M.G.; Kvansakul, M. A structural investigation of NRZ mediated apoptosis regulation in zebrafish. Cell Death Dis. 2018, 9, 967.
- Chittenden, T.; Flemington, C.; Houghton, A.B.; Ebb, R.G.; Gallo, G.J.; Elangovan, B.; Chinnadurai, G.; Lutz, R.J. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995, 14, 5589–5596.
- Moldoveanu, T.; Liu, Q.; Tocilj, A.; Watson, M.; Shore, G.; Gehring, K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol. Cell 2006, 24, 677–688.
- Oltvai, Z.N.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74, 609–619.
- Suzuki, M.; Youle, R.J.; Tjandra, N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell 2000, 103, 645–654.
- Hsu, S.Y.; Kaipia, A.; McGee, E.; Lomeli, M.; Hsueh, A.J. Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc. Natl. Acad. Sci. USA 1997, 94, 12401–12406.
- Yang, E.; Zha, J.; Jockel, J.; Boise, L.H.; Thompson, C.B.; Korsmeyer, S.J. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell 1995, 80, 285–291.
- Wang, K.; Yin, X.M.; Chao, D.T.; Milliman, C.L.; Korsmeyer, S.J. BID: A novel BH3 domain-only death agonist. Genes Dev. 1996, 10, 2859–2869.
- Han, J.; Sabbatini, P.; White, E. Induction of apoptosis by human Nbk/Bik, a BH3-containing protein that interacts with E1B 19K. Mol. Cell Biol. 1996, 16, 5857–5864.
- O’Connor, L.; Strasser, A.; O’Reilly, L.A.; Hausmann, G.; Adams, J.M.; Cory, S.; Huang, D.C. Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998, 17, 384–395.
- Puthalakath, H.; Villunger, A.; O’Reilly, L.A.; Beaumont, J.G.; Coultas, L.; Cheney, R.E.; Huang, D.C.; Strasser, A. Bmf: A proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 2001, 293, 1829–1832.
- Inohara, N.; Ding, L.; Chen, S.; Nunez, G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L). EMBO J. 1997, 16, 1686–1694.
- Hijikata, M.; Kato, N.; Sato, T.; Kagami, Y.; Shimotohno, K. Molecular cloning and characterization of a cDNA for a novel phorbol-12-myristate-13-acetate-responsive gene that is highly expressed in an adult T-cell leukemia cell line. J. Virol. 1990, 64, 4632–4639.
- Nakano, K.; Vousden, K.H. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell 2001, 7, 683–694.
- Liang, X.H.; Kleeman, L.K.; Jiang, H.H.; Gordon, G.; Goldman, J.E.; Berry, G.; Herman, B.; Levine, B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J. Virol. 1998, 72, 8586–8596.
- Prudent, J.; Gillet, G.; Popgeorgiev, N. Nrz but not zBcl-xL antagonizes Bcl-wav pro-apoptotic activity in zebrafish. Commun. Integr. Biol. 2014, 7, e28008.
- Prudent, J.; Popgeorgiev, N.; Bonneau, B.; Thibaut, J.; Gadet, R.; Lopez, J.; Gonzalo, P.; Rimokh, R.; Manon, S.; Houart, C.; et al. Bcl-wav and the mitochondrial calcium uniporter drive gastrula morphogenesis in zebrafish. Nat. Commun. 2013, 4, 2330.
- Quinn, L.; Coombe, M.; Mills, K.; Daish, T.; Colussi, P.; Kumar, S.; Richardson, H. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. EMBO J. 2003, 22, 3568–3579.
- Colussi, P.A.; Quinn, L.M.; Huang, D.C.; Coombe, M.; Read, S.H.; Richardson, H.; Kumar, S. Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery. J. Cell Biol. 2000, 148, 703–714.
- Henderson, S.; Huen, D.; Rowe, M.; Dawson, C.; Johnson, G.; Rickinson, A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc. Natl. Acad. Sci. USA 1993, 90, 8479–8483.
- Huang, Q.; Petros, A.M.; Virgin, H.W.; Fesik, S.W.; Olejniczak, E.T. Solution structure of the BHRF1 protein from Epstein-Barr virus, a homolog of human Bcl-2. J. Mol. Biol. 2003, 332, 1123–1130.
- Sarid, R.; Sato, T.; Bohenzky, R.A.; Russo, J.J.; Chang, Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional Bcl-2 homologue. Nat. Med. 1997, 3, 293–298.
- White, E.; Sabbatini, P.; Debbas, M.; Wold, W.S.; Kusher, D.I.; Gooding, L.R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol. Cell Biol. 1992, 12, 2570–2580.
- Wang, G.H.; Garvey, T.L.; Cohen, J.I. The murine gammaherpesvirus-68 M11 protein inhibits Fas- and TNF-induced apoptosis. J. Gen. Virol. 1999, 80 Pt 10, 2737–2740.
- Sinha, S.; Colbert, C.L.; Becker, N.; Wei, Y.; Levine, B. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy 2008, 4, 989–997.
- Brun, A.; Rivas, C.; Esteban, M.; Escribano, J.M.; Alonso, C. African swine fever virus gene A179L, a viral homologue of Bcl-2, protects cells from programmed cell death. Virology 1996, 225, 227–230.
- Banjara, S.; Caria, S.; Dixon, L.K.; Hinds, M.G.; Kvansakul, M. Structural Insight into African Swine Fever Virus A179L-Mediated Inhibition of Apoptosis. J. Virol. 2017, 91, e02228-16.
- Stewart, T.L.; Wasilenko, S.T.; Barry, M. Vaccinia virus F1L protein is a tail-anchored protein that functions at the mitochondria to inhibit apoptosis. J. Virol. 2005, 79, 1084–1098.
- Kvansakul, M.; Yang, H.; Fairlie, W.D.; Czabotar, P.E.; Fischer, S.F.; Perugini, M.A.; Huang, D.C.; Colman, P.M. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008, 15, 1564–1571.
- Burton, D.R.; Caria, S.; Marshall, B.; Barry, M.; Kvansakul, M. Structural basis of Deerpox virus-mediated inhibition of apoptosis. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 1593–1603.
- Banadyga, L.; Lam, S.C.; Okamoto, T.; Kvansakul, M.; Huang, D.C.; Barry, M. Deerpox virus encodes an inhibitor of apoptosis that regulates Bak and Bax. J. Virol. 2011, 85, 1922–1934.
- Opgenorth, A.; Graham, K.; Nation, N.; Strayer, D.; McFadden, G. Deletion analysis of two tandemly arranged virulence genes in myxoma virus, M11L and myxoma growth factor. J. Virol. 1992, 66, 4720–4731.
- Kvansakul, M.; van Delft, M.F.; Lee, E.F.; Gulbis, J.M.; Fairlie, W.D.; Huang, D.C.; Colman, P.M. A structural viral mimic of prosurvival Bcl-2: A pivotal role for sequestering proapoptotic Bax and Bak. Mol. Cell 2007, 25, 933–942.
- Banadyga, L.; Gerig, J.; Stewart, T.; Barry, M. Fowlpox virus encodes a Bcl-2 homologue that protects cells from apoptotic death through interaction with the proapoptotic protein Bak. J. Virol. 2007, 81, 11032–11045.
- Anasir, M.I.; Caria, S.; Skinner, M.A.; Kvansakul, M. Structural basis of apoptosis inhibition by the fowlpox virus protein FPV039. J. Biol. Chem. 2017, 292, 9010–9021.
- Tulman, E.R.; Afonso, C.L.; Lu, Z.; Zsak, L.; Kutish, G.F.; Rock, D.L. The genome of canarypox virus. J. Virol. 2004, 78, 353–366.
- Anasir, M.I.; Baxter, A.A.; Poon, I.K.H.; Hulett, M.D.; Kvansakul, M. Structural and Functional Insight into Canarypox Virus CNP058 Mediated Regulation of Apoptosis. Viruses 2017, 9, 305.
- Okamoto, T.; Campbell, S.; Mehta, N.; Thibault, J.; Colman, P.M.; Barry, M.; Huang, D.C.; Kvansakul, M. Sheeppox Virus SPPV14 Encodes a Bcl-2-like Cell Death Inhibitor that Counters a Distinct Set of Mammalian Pro-apoptotic Proteins. J. Virol. 2012, 15, 11501–11511.
- Chathura D. Suraweera; Denis R. Burton; Mark G. Hinds; Marc Kvansakul; Crystal structures of the sheeppox virus encoded inhibitor of apoptosis SPPV14 bound to the proapoptotic BH3 peptides Hrk and Bax. FEBS Letters 2020, 594, 2016-2026, 10.1002/1873-3468.13807.
- Chathura D. Suraweera; Mohd Ishtiaq Anasir; Srishti Chugh; Airah Javorsky; Rachael E. Impey; Mohammad Hasan Zadeh; Tatiana P. Soares da Costa; Mark G. Hinds; Marc Kvansakul; Structural insight into tanapoxvirus‐mediated inhibition of apoptosis. The FEBS Journal 2020, 287, 3733-3750, 10.1111/febs.15365.
- Westphal, D.; Ledgerwood, E.C.; Tyndall, J.D.; Hibma, M.H.; Ueda, N.; Fleming, S.B.; Mercer, A.A. The orf virus inhibitor of apoptosis functions in a Bcl-2-like manner, binding and neutralizing a set of BH3-only proteins and active Bax. Apoptosis 2009, 14, 1317–1330.
- Chathura D. Suraweera; Mark G. Hinds; Marc Kvansakul; Crystal structures of ORFV125 provide insight into orf virus-mediated inhibition of apoptosis. Biochemical Journal 2020, 477, 4527-4541, 10.1042/bcj20200776.
- Banjara, S.; Mao, J.; Ryan, T.M.; Caria, S.; Kvansakul, M. Grouper iridovirus GIV66 is a Bcl-2 protein that inhibits apoptosis by exclusively sequestering Bim. J. Biol. Chem. 2018, 293, 5464–5477.
- Lin, P.W.; Huang, Y.J.; John, J.A.; Chang, Y.N.; Yuan, C.H.; Chen, W.Y.; Yeh, C.H.; Shen, S.T.; Lin, F.P.; Tsui, W.H.; et al. Iridovirus Bcl-2 protein inhibits apoptosis in the early stage of viral infection. Apoptosis 2008, 13, 165–176.
- Bartlett, N.; Symons, J.A.; Tscharke, D.C.; Smith, G.L. The vaccinia virus N1L protein is an intracellular homodimer that promotes virulence. J. Gen. Virol. 2002, 83, 1965–1976.
- Cooray, S.; Bahar, M.W.; Abrescia, N.G.; McVey, C.E.; Bartlett, N.W.; Chen, R.A.; Stuart, D.I.; Grimes, J.M.; Smith, G.L. Functional and structural studies of the vaccinia virus virulence factor N1 reveal a Bcl-2-like anti-apoptotic protein. J. Gen. Virol. 2007, 88, 1656–1666.
- Fedosyuk, S.; Grishkovskaya, I.; de Almeida Ribeiro, E., Jr.; Skern, T. Characterization and structure of the vaccinia virus NF-kappaB antagonist A46. J. Biol.Chem. 2014, 289, 3749–3762.
- Gonzalez, J.M.; Esteban, M. A poxvirus Bcl-2-like gene family involved in regulation of host immune response: Sequence similarity and evolutionary history. Virol. J. 2010, 7, 59.
- Neidel, S.; Maluquer de Motes, C.; Mansur, D.S.; Strnadova, P.; Smith, G.L.; Graham, S.C. Vaccinia virus protein A49 is an unexpected member of the B-cell Lymphoma (Bcl)-2 protein family. J. Biol. Chem. 2015, 290, 5991–6002.
- Mansur, D.S.; Maluquer de Motes, C.; Unterholzner, L.; Sumner, R.P.; Ferguson, B.J.; Ren, H.; Strnadova, P.; Bowie, A.G.; Smith, G.L. Poxvirus targeting of E3 ligase beta-TrCP by molecular mimicry: A mechanism to inhibit NF-kappaB activation and promote immune evasion and virulence. PLoS Pathog. 2013, 9, e1003183.
- Graham, S.C.; Bahar, M.W.; Cooray, S.; Chen, R.A.; Whalen, D.M.; Abrescia, N.G.; Alderton, D.; Owens, R.J.; Stuart, D.I.; Smith, G.L.; et al. Vaccinia virus proteins A52 and B14 Share a Bcl-2-like fold but have evolved to inhibit NF-kappaB rather than apoptosis. PLoS Pathog. 2008, 4, e1000128.
- Chen, R.A.; Jacobs, N.; Smith, G.L. Vaccinia virus strain Western Reserve protein B14 is an intracellular virulence factor. J. Gen. Virol. 2006, 87, 1451–1458.
- Schroder, M.; Baran, M.; Bowie, A.G. Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J. 2008, 27, 2147–2157.
- Kalverda, A.P.; Thompson, G.S.; Vogel, A.; Schroder, M.; Bowie, A.G.; Khan, A.R.; Homans, S.W. Poxvirus K7 protein adopts a Bcl-2 fold: Biochemical mapping of its interactions with human DEAD box RNA helicase DDX3. J. Mol. Biol. 2009, 385, 843–853.
- Wiens, M.; Belikov, S.I.; Kaluzhnaya, O.V.; Schroder, H.C.; Hamer, B.; Perovic-Ottstadt, S.; Borejko, A.; Luthringer, B.; Muller, I.M.; Muller, W.E. Axial (apical-basal) expression of pro-apoptotic and pro-survival genes in the lake baikal demosponge Lubomirskia baicalensis. DNA Cell Biol. 2006, 25, 152–164.
- Wiens, M.; Diehl-Seifert, B.; Muller, W.E. Sponge Bcl-2 homologous protein (BHP2-GC) confers distinct stress resistance to human HEK-293 cells. Cell Death Differ. 2001, 8, 887–898.
- Nikolay Popgeorgiev; Jaison D Sa; Lea Jabbour; Suresh Banjara; Trang Thi Minh Nguyen; Aida Akhavan-E-Sabet; Rudy Gadet; Nikola Ralchev; Stéphen Manon; Mark G. Hinds; et al.Hans-Jürgen OsigusBernd SchierwaterPatrick O. HumbertRuth RimokhGermain GilletMarc Kvansakul Ancient and conserved functional interplay between Bcl-2 family proteins in the mitochondrial pathway of apoptosis. Science Advances 2020, 6, eabc4149, 10.1126/sciadv.abc4149.
- Suresh Banjara; Jaison D Sa; Mark G. Hinds; Marc Kvansakul; The structural basis of Bcl-2 mediated cell death regulation in hydra. Biochemical Journal 2020, 477, 3287-3297, 10.1042/bcj20200556.
- Yan, N.; Gu, L.; Kokel, D.; Chai, J.; Li, W.; Han, A.; Chen, L.; Xue, D.; Shi, Y. Structural, biochemical, and functional analyses of CED-9 recognition by the proapoptotic proteins EGL-1 and CED-4. Mol. Cell 2004, 15, 999–1006.
- Conradt, B.; Horvitz, H.R. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 1998, 93, 519–529.
- Hengartner, M.O.; Horvitz, H.R. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell 1994, 76, 665–676.
- Woo, J.S.; Jung, J.S.; Ha, N.C.; Shin, J.; Kim, K.H.; Lee, W.; Oh, B.H. Unique structural features of a BCL-2 family protein CED-9 and biophysical characterization of CED-9/EGL-1 interactions. Cell Death Differ. 2003, 10, 1310–1319.
- Goldmacher, V.S.; Bartle, L.M.; Skaletskaya, A.; Dionne, C.A.; Kedersha, N.L.; Vater, C.A.; Han, J.W.; Lutz, R.J.; Watanabe, S.; Cahir McFarland, E.D.; et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 1999, 96, 12536–12541.
- Ma, J.; Edlich, F.; Bermejo, G.A.; Norris, K.L.; Youle, R.J.; Tjandra, N. Structural mechanism of Bax inhibition by cytomegalovirus protein vMIA. Proc. Natl. Acad. Sci. USA 2012, 109, 20901–20906.
- Gavathiotis, E.; Suzuki, M.; Davis, M.L.; Pitter, K.; Bird, G.H.; Katz, S.G.; Tu, H.C.; Kim, H.; Cheng, E.H.; Tjandra, N.; et al. BAX activation is initiated at a novel interaction site. Nature 2008, 455, 1076–1081.
- Lalle, P.; Aouacheria, A.; Dumont-Miscopein, A.; Jambon, M.; Venet, S.; Bobichon, H.; Colas, P.; Deleage, G.; Geourjon, C.; Gillet, G. Evidence for crucial electrostatic interactions between Bcl-2 homology domains BH3 and BH4 in the anti-apoptotic Nr-13 protein. Biochem. J. 2002, 368, 213–221.
- De Motes, C.M.; Cooray, S.; Ren, H.; Almeida, G.M.F.; McGourty, K.; Bahar, M.W.; Stuart, D.I.; Grimes, J.M.; Graham, S.C.; Smith, G.L. Inhibition of Apoptosis and NF-kB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence. PLoS Pathog. 2011, 7, e1002430.
- Huang, D.C.; Strasser, A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell 2000, 103, 839–842.
- Popgeorgiev, N.; Jabbour, L.; Gillet, G. Subcellular Localization and Dynamics of the Bcl-2 Family of Proteins. Front. Cell Dev. Biol. 2018, 6, 13.
- Aouacheria, A.; Brunet, F.; Gouy, M. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators. Mol. Biol. Evol. 2005, 22, 2395–2416.
- Day, C.L.; Smits, C.; Fan, F.C.; Lee, E.F.; Fairlie, W.D.; Hinds, M.G. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J. Mol. Biol. 2008, 380, 958–971.
- Bouillet, P.; Strasser, A. BH3-only protein—Sevolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J. Cell Sci. 2002, 115, 1567–1574.
- Huang, K.; O’Neill, K.L.; Li, J.; Zhou, W.; Han, N.; Pang, X.; Wu, W.; Struble, L.; Borgstahl, G.; Liu, Z.; et al. BH3-only proteins target BCL-xL/MCL-1, not BAX/BAK, to initiate apoptosis. Cell Res. 2019.
- Metzstein, M.M.; Stanfield, G.M.; Horvitz, H.R. Genetics of programmed cell death in C. elegans: Past, present and future. Trends Genet. 1998, 14, 410–416.
- Prudent, J.; Popgeorgiev, N.; Bonneau, B.; Gillet, G. Bcl-2 proteins, cell migration and embryonic development: Lessons from zebrafish. Cell Death Dis. 2015, 6, e1910.
- Hardwick, J.M.; Chen, Y.B.; Jonas, E.A. Multipolar functions of BCL-2 proteins link energetics to apoptosis. Trends Cell Biol. 2012, 22, 318–328.
- Aouacheria, A.; Baghdiguian, S.; Lamb, H.M.; Huska, J.D.; Pineda, F.J.; Hardwick, J.M. Connecting mitochondrial dynamics and life-or-death events via Bcl-2 family proteins. Neurochem. Int. 2017, 109, 141–161.
- Oberstein, A.; Jeffrey, P.D.; Shi, Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J. Biol. Chem. 2007, 282, 13123–13132.
- Ku, B.; Woo, J.S.; Liang, C.; Lee, K.H.; Hong, H.S.; E, X.; Kim, K.S.; Jung, J.U.; Oh, B.H. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog. 2008, 4, e25.
- Lee, E.F.; Perugini, M.A.; Pettikiriarachchi, A.; Evangelista, M.; Keizer, D.W.; Yao, S.; Fairlie, W.D. The BECN1 N-terminal domain is intrinsically disordered. Autophagy 2016, 12, 460–471.
- Huang, W.; Choi, W.; Hu, W.; Mi, N.; Guo, Q.; Ma, M.; Liu, M.; Tian, Y.; Lu, P.; Wang, F.L.; et al. Crystal structure and biochemical analyses reveal Beclin 1 as a novel membrane binding protein. Cell Res. 2012, 22, 473–489.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Aktipis, C.A.; Boddy, A.M.; Jansen, G.; Hibner, U.; Hochberg, M.E.; Maley, C.C.; Wilkinson, G.S. Cancer across the tree of life: Cooperation and cheating in multicellularity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370.
- Domazet-Loso, T.; Klimovich, A.; Anokhin, B.; Anton-Erxleben, F.; Hamm, M.J.; Lange, C.; Bosch, T.C. Naturally occurring tumours in the basal metazoan Hydra. Nat. Commun. 2014, 5, 4222.
- Peters, E.C.; Halas, J.C.; McCarty, H.B. Calicoblastic neoplasms in Acropora palmata, with a review of reports on anomalies of growth and form in corals. J. Natl. Cancer Inst. 1986, 76, 895–912.
- Hesselman, D.M.; Blake, N.J.; Peters, E.C. Gonadal neoplasms in hard shell clams Mercenaria spp., from the Indian River, Florida: Occurrence, prevalence, and histopathology. J. Invertebr. Pathol. 1988, 52, 436–446.
- Albuquerque, T.A.F.; Drummond do Val, L.; Doherty, A.; de Magalhaes, J.P. From humans to hydra: Patterns of cancer across the tree of life. Biol. Rev. Camb. Philos. Soc. 2018, 93, 1715–1734.
- Lasi, M.; Pauly, B.; Schmidt, N.; Cikala, M.; Stiening, B.; Kasbauer, T.; Zenner, G.; Popp, T.; Wagner, A.; Knapp, R.T.; et al. The molecular cell death machinery in the simple cnidarian Hydra includes an expanded caspase family and pro- and anti-apoptotic Bcl-2 proteins. Cell Res. 2010, 20, 812–825.
- Kvansakul, M.; Hinds, M.G. Structural biology of the Bcl-2 family and its mimicry by viral proteins. Cell Death Dis. 2013, 4, e909.
- Chiou, S.K.; Tseng, C.C.; Rao, L.; White, E. Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells. J. Virol. 1994, 68, 6553–6566.
- Zhang, T.Y.; Chen, H.Y.; Cao, J.L.; Xiong, H.L.; Mo, X.B.; Li, T.L.; Kang, X.Z.; Zhao, J.H.; Yin, B.; Zhao, X.; et al. Structural and functional analyses of hepatitis B virus X protein BH3-like domain and Bcl-xL interaction. Nat. Commun. 2019, 10, 3192.
- Kvansakul, M.; Wei, A.H.; Fletcher, J.I.; Willis, S.N.; Chen, L.; Roberts, A.W.; Huang, D.C.; Colman, P.M. Structural basis for apoptosis inhibition by Epstein-Barr virus BHRF1. PLoS Pathog. 2010, 6, e1001236.
- Fitzsimmons, L.; Cartlidge, R.; Chang, C.; Sejic, N.; Galbraith, L.C.A.; Suraweera, C.D.; Croom-Carter, D.; Dewson, G.; Tierney, R.J.; Bell, A.I.; et al. EBV BCL-2 homologue BHRF1 drives chemoresistance and lymphomagenesis by inhibiting multiple cellular pro-apoptotic proteins. Cell Death Differ. 2019.
- Desbien, A.L.; Kappler, J.W.; Marrack, P. The Epstein-Barr virus Bcl-2 homolog, BHRF1, blocks apoptosis by binding to a limited amount of Bim. Proc. Natl. Acad. Sci. USA 2009, 106, 5663–5668.
- Virgin, H.W.T.; Latreille, P.; Wamsley, P.; Hallsworth, K.; Weck, K.E.; Dal Canto, A.J.; Speck, S.H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997, 71, 5894–5904.
- Piya, S.; White, E.J.; Klein, S.R.; Jiang, H.; McDonnell, T.J.; Gomez-Manzano, C.; Fueyo, J. The E1B19K oncoprotein complexes with Beclin 1 to regulate autophagy in adenovirus-infected cells. PLoS ONE 2011, 6, e29467.
- Chathura D. Suraweera; Mark G. Hinds; Marc Kvansakul; Poxviral Strategies to Overcome Host Cell Apoptosis. Pathogens 2020, 10, 6, 10.3390/pathogens10010006.
- Wasilenko, S.T.; Stewart, T.L.; Meyers, A.F.; Barry, M. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 14345–14350.
- Fischer, S.F.; Ludwig, H.; Holzapfel, J.; Kvansakul, M.; Chen, L.; Huang, D.C.; Sutter, G.; Knese, M.; Hacker, G. Modified vaccinia virus Ankara protein F1L is a novel BH3-domain-binding protein and acts together with the early viral protein E3L to block virus-associated apoptosis. Cell Death Differ. 2006, 13, 109–118.
- Campbell, S.; Thibault, J.; Mehta, N.; Colman, P.M.; Barry, M.; Kvansakul, M. Structural insight into BH3 domain binding of vaccinia virus antiapoptotic F1L. J. Virol. 2014, 88, 8667–8677.
- Czabotar, P.E.; Westphal, D.; Dewson, G.; Ma, S.; Hockings, C.; Fairlie, W.D.; Lee, E.F.; Yao, S.; Robin, A.Y.; Smith, B.J.; et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell 2013, 152, 519–531.
- Campbell, S.; Hazes, B.; Kvansakul, M.; Colman, P.; Barry, M. Vaccinia virus F1L interacts with Bak using highly divergent Bcl-2 homology domains and replaces the function of Mcl-1. J. Biol. Chem. 2010, 285, 4695–4708.
- Caria, S.; Marshall, B.; Burton, R.L.; Campbell, S.; Pantaki-Eimany, D.; Hawkins, C.J.; Barry, M.; Kvansakul, M. The N Terminus of the Vaccinia Virus Protein F1L Is an Intrinsically Unstructured Region That Is Not Involved in Apoptosis Regulation. J. Biol. Chem. 2016, 291, 14600–14608.
- Yu, E.; Zhai, D.; Jin, C.; Gerlic, M.; Reed, J.C.; Liddington, R. Structural determinants of caspase-9 inhibition by the vaccinia virus protein, F1L. J. Biol. Chem. 2011, 286, 30748–30758.
- Zhai, D.; Yu, E.; Jin, C.; Welsh, K.; Shiau, C.W.; Chen, L.; Salvesen, G.S.; Liddington, R.; Reed, J.C. Vaccinia virus protein F1L is a caspase-9 inhibitor. J. Biol. Chem. 2010, 285, 5569–5580.
- Marshall, B.; Puthalakath, H.; Caria, S.; Chugh, S.; Doerflinger, M.; Colman, P.M.; Kvansakul, M. Variola virus F1L is a Bcl-2-like protein that unlike its vaccinia virus counterpart inhibits apoptosis independent of Bim. Cell Death Dis. 2015, 6, e1680.
- Everett, H.; Barry, M.; Lee, S.F.; Sun, X.; Graham, K.; Stone, J.; Bleackley, R.C.; McFadden, G. M11L: A novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med. 2000, 191, 1487–1498.
- Douglas, A.E.; Corbett, K.D.; Berger, J.M.; McFadden, G.; Handel, T.M. Structure of M11L: A myxoma virus structural homolog of the apoptosis inhibitor, Bcl-2. Protein Sci. 2007, 16, 695–703.
- Neilan, J.G.; Lu, Z.; Afonso, C.L.; Kutish, G.F.; Sussman, M.D.; Rock, D.L. An African swine fever virus gene with similarity to the proto-oncogene bcl-2 and the Epstein-Barr virus gene BHRF1. J. Virol. 1993, 67, 4391–4394.
- Banjara, S.; Shimmon, G.L.; Dixon, L.K.; Netherton, C.L.; Hinds, M.G.; Kvansakul, M. Crystal Structure of African Swine Fever Virus A179L with the Autophagy Regulator Beclin. Viruses 2019, 11, 789.
- Hernaez, B.; Cabezas, M.; Munoz-Moreno, R.; Galindo, I.; Cuesta-Geijo, M.A.; Alonso, C. A179L, a new viral Bcl2 homolog targeting Beclin 1 autophagy related protein. Curr. Mol. Med. 2013, 13, 305–316.
- Aoyagi, M.; Zhai, D.; Jin, C.; Aleshin, A.E.; Stec, B.; Reed, J.C.; Liddington, R.C. Vaccinia virus N1L protein resembles a B cell lymphoma-2 (Bcl-2) family protein. Protein Sci. 2007, 16, 118–124.
- Kim, Y.; Lee, H.; Heo, L.; Seok, C.; Choe, J. Structure of vaccinia virus A46, an inhibitor of TLR4 signaling pathway, shows the conformation of VIPER motif. Protein Sci. 2014, 23, 906–914.
- Neil D. Young; Tiffany J. Harris; Marco Evangelista; Sharon Tran; Merridee A. Wouters; Tatiana P. Soares Da Costa; Nadia J. Kershaw; Robin B. Gasser; Brian J. Smith; Erinna F. Lee; et al.W. Douglas Fairlie Diversity in the intrinsic apoptosis pathway of nematodes. Communications Biology 2020, 3, 1-12, 10.1038/s42003-020-01208-5.
- Simion, P.; Philippe, H.; Baurain, D.; Jager, M.; Richter, D.J.; Di Franco, A.; Roure, B.; Satoh, N.; Queinnec, E.; Ereskovsky, A.; et al. A Large and Consistent Phylogenomic Dataset Supports Sponges as the Sister Group to All Other Animals. Curr. Biol. 2017, 27, 958–967.
- Feuda, R.; Dohrmann, M.; Pett, W.; Philippe, H.; Rota-Stabelli, O.; Lartillot, N.; Worheide, G.; Pisani, D. Improved Modeling of Compositional Heterogeneity Supports Sponges as Sister to All Other Animals. Curr. Biol. 2017, 27, 3864–3870.
- Srivastava, M.; Simakov, O.; Chapman, J.; Fahey, B.; Gauthier, M.E.; Mitros, T.; Richards, G.S.; Conaco, C.; Dacre, M.; Hellsten, U.; et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature 2010, 466, 720–726.
- Srivastava, M.; Begovic, E.; Chapman, J.; Putnam, N.H.; Hellsten, U.; Kawashima, T.; Kuo, A.; Mitros, T.; Salamov, A.; Carpenter, M.L.; et al. The Trichoplax genome and the nature of placozoans. Nature 2008, 454, 955–960.
- Lasi, M.; David, C.N.; Bottger, A. Apoptosis in pre-Bilaterians: Hydra as a model. Apoptosis 2010, 15, 269–278.
- Chapman, J.A.; Kirkness, E.F.; Simakov, O.; Hampson, S.E.; Mitros, T.; Weinmaier, T.; Rattei, T.; Balasubramanian, P.G.; Borman, J.; Busam, D.; et al. The dynamic genome of Hydra. Nature 2010, 464, 592–596.
- Aouacheria, A.; Combet, C.; Tompa, P.; Hardwick, J.M. Redefining the BH3 Death Domain as a ‘Short Linear Motif’. Trends Biochem. Sci. 2015, 40, 736–748.
- Yan, N.; Chai, J.; Lee, E.S.; Gu, L.; Liu, Q.; He, J.; Wu, J.W.; Kokel, D.; Li, H.; Hao, Q.; et al. Structure of the CED-4-CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nature 2005, 437, 831–837.
- Lee, E.F.; Clarke, O.B.; Evangelista, M.; Feng, Z.; Speed, T.P.; Tchoubrieva, E.B.; Strasser, A.; Kalinna, B.H.; Colman, P.M.; Fairlie, W.D. Discovery and molecular characterization of a Bcl-2-regulated cell death pathway in schistosomes. Proc. Natl. Acad. Sci. USA 2011, 108, 6999–7003.
- Bender, C.E.; Fitzgerald, P.; Tait, S.W.; Llambi, F.; McStay, G.P.; Tupper, D.O.; Pellettieri, J.; Sanchez Alvarado, A.; Salvesen, G.S.; Green, D.R. Mitochondrial pathway of apoptosis is ancestral in metazoans. Proc. Natl. Acad. Sci. USA 2012, 109, 4904–4909.
- Tamura, R.; Takada, M.; Sakaue, M.; Yoshida, A.; Ohi, S.; Hirano, K.; Hayakawa, T.; Hirohashi, N.; Yura, K.; Chiba, K. Starfish Apaf-1 activates effector caspase-3/9 upon apoptosis of aged eggs. Sci. Rep. 2018, 8, 1611.
- Eimon, P.M.; Ashkenazi, A. The zebrafish as a model organism for the study of apoptosis. Apoptosis 2010, 15, 331–349.
- Kratz, E.; Eimon, P.M.; Mukhyala, K.; Stern, H.; Zha, J.; Strasser, A.; Hart, R.; Ashkenazi, A. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ. 2006, 13, 1631–1640.
- Glasauer, S.M.; Neuhauss, S.C. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genom. 2014, 289, 1045–1060.
- Kuwana, T.; Mackey, M.R.; Perkins, G.; Ellisman, M.H.; Latterich, M.; Schneiter, R.; Green, D.R.; Newmeyer, D.D. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 2002, 111, 331–342.
- Wilfling, F.; Weber, A.; Potthoff, S.; Vogtle, F.N.; Meisinger, C.; Paschen, S.A.; Hacker, G. BH3-only proteins are tail-anchored in the outer mitochondrial membrane and can initiate the activation of Bax. Cell Death Differ. 2012, 19, 1328–1336.
- Wilson-Annan, J.; O’Reilly, L.A.; Crawford, S.A.; Hausmann, G.; Beaumont, J.G.; Parma, L.P.; Chen, L.; Lackmann, M.; Lithgow, T.; Hinds, M.G.; et al. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J. Cell Biol. 2003, 162, 877–887.
- Wattenberg, B.W.; Clark, D.; Brock, S. An artificial mitochondrial tail signal/anchor sequence confirms a requirement for moderate hydrophobicity for targeting. Biosci. Rep. 2007, 27, 385–401.
- O’Neill, K.L.; Huang, K.; Zhang, J.; Chen, Y.; Luo, X. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 2016, 30, 973–988.
- Gomez-Fernandez, J.C. Functions of the C-terminal domains of apoptosis-related proteins of the Bcl-2 family. Chem Phys. Lipids 2014, 183, 77–90.
- Chin, H.S.; Li, M.X.; Tan, I.K.L.; Ninnis, R.L.; Reljic, B.; Scicluna, K.; Dagley, L.F.; Sandow, J.J.; Kelly, G.L.; Samson, A.L.; et al. VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nat. Commun. 2018, 9, 4976.
- Ke, F.F.S.; Vanyai, H.K.; Cowan, A.D.; Delbridge, A.R.D.; Whitehead, L.; Grabow, S.; Czabotar, P.E.; Voss, A.K.; Strasser, A. Embryogenesis and Adult Life in the Absence of Intrinsic Apoptosis Effectors BAX, BAK, and BOK. Cell 2018, 173, 1217–1230.
- Zheng, J.H.; Grace, C.R.; Guibao, C.D.; McNamara, D.E.; Llambi, F.; Wang, Y.M.; Chen, T.; Moldoveanu, T. Intrinsic Instability of BOK Enables Membrane Permeabilization in Apoptosis. Cell Rep. 2018, 23, 2083–2094.
- Hsu, Y.T.; Youle, R.J. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 1998, 273, 10777–10783.
- Edlich, F.; Banerjee, S.; Suzuki, M.; Cleland, M.M.; Arnoult, D.; Wang, C.; Neutzner, A.; Tjandra, N.; Youle, R.J. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 2011, 145, 104–116.
- Wolter, K.G.; Hsu, Y.T.; Smith, C.L.; Nechushtan, A.; Xi, X.G.; Youle, R.J. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997, 139, 1281–1292.
- Hsu, Y.T.; Wolter, K.G.; Youle, R.J. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 3668–3672.
- Nechushtan, A.; Smith, C.L.; Hsu, Y.T.; Youle, R.J. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999, 18, 2330–2341.
- Echeverry, N.; Bachmann, D.; Ke, F.; Strasser, A.; Simon, H.U.; Kaufmann, T. Intracellular localization of the BCL-2 family member BOK and functional implications. Cell Death Differ. 2013, 20, 785–799.
- Todt, F.; Cakir, Z.; Reichenbach, F.; Youle, R.J.; Edlich, F. The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria. Cell Death Differ. 2013, 20, 333–342.
- Cuconati, A.; White, E. Viral homologs of BCL-2: Role of apoptosis in the regulation of virus infection. Genes Dev. 2002, 16, 2465–2478.
- Wang, G.; Barrett, J.W.; Nazarian, S.H.; Everett, H.; Gao, X.; Bleackley, C.; Colwill, K.; Moran, M.F.; McFadden, G. Myxoma virus M11L prevents apoptosis through constitutive interaction with Bak. J. Virol. 2004, 78, 7097–7111.
- Poncet, D.; Larochette, N.; Pauleau, A.L.; Boya, P.; Jalil, A.A.; Cartron, P.F.; Vallette, F.; Schnebelen, C.; Bartle, L.M.; Skaletskaya, A.; et al. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 2004, 279, 22605–22614.
- Kortschak, R.D.; Samuel, G.; Saint, R.; Miller, D.J. EST analysis of the cnidarian Acropora millepora reveals extensive gene loss and rapid sequence divergence in the model invertebrates. Curr. Biol. 2003, 13, 2190–2195.
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758.
- Clavier, A.; Rincheval-Arnold, A.; Colin, J.; Mignotte, B.; Guenal, I. Apoptosis in Drosophila: Which role for mitochondria? Apoptosis 2016, 21, 239–251.
- Doumanis, J.; Dorstyn, L.; Kumar, S. Molecular determinants of the subcellular localization of the Drosophila Bcl-2 homologues DEBCL and BUFFY. Cell Death Differ. 2007, 14, 907–915.
- Kroemer, G. Mitochondrial implication in apoptosis. Towards an endosymbiont hypothesis of apoptosis evolution. Cell Death Differ. 1997, 4, 443–456.
