Exosomes, nanovesicles of ≈30–150 nm in diameter, can be isolated from the bodily fluids of dairy cows (e.g., blood plasma, milk, and follicular fluid), and present a unique opportunity to studying the molecular cues that underlie poor reproductive performance.
- exosome
- mass-spectrometry
- proteomics
- SWATH
1. Introduction
Dairy cow fertility has been in decline for the past 20 years [1][2][3]. Selective breeding for milk production traits, negative energy balance (NEB), poor health or infection during the transition period (3 weeks before and the 3 weeks after calving), and early pregnancy loss have all been attributed to this decline [3][4][5]. These factors are thought to be linked but the underlying biological mechanisms responsible for these perturbations to reproductive performance have not yet been fully established.
Although it is widely accepted that increased metabolic pressure due to increased milk production is associated with poor reproductive outcomes, average producing cows may also experience reproductive challenges [6]. There are reports that discuss the lesser significance of increased milk production on fertility, and instead highlight genetic potential, nutritional intake, health status and farm management as major contributing factors to fertility status of the cow [7]. However, reliable predictors of future reproductive performance remain to be determined.Body condition scoring (BCS), and more recently BCS linked to timing of pubertal onset, is one of the few key indicators used by dairy farmers to manage and predict herd profitability [8].
Heifers can be separated into high- and low-fertility groups based on their genetic merit and other measurable physical traits [9]. However, this model has been found to be substandard when trying to address underlying causes of subfertility, and newer models expressing the extremes of the fertility spectrum have been developed in order to better explore the mechanisms responsible for the decline in calving rates over the past two decades. Although these newer models have allowed for improved sampling and study of the physiological stresses leading to poor reproductive performance, the biological mechanisms driving the disease process resulting in subfertility remain to be elucidated.
Exosomes, nanovesicles of ≈30–150 nm in diameter, can be isolated from the bodily fluids of dairy cows (e.g., blood plasma, milk, and follicular fluid), and present a unique opportunity to studying the molecular cues that underlie poor reproductive performance [10]. Exosomes are most commonly formed by the inward budding of multivesicular bodies (MVB) in the cell and begin as intraluminal vesicles (ILVs), and play a critical role in cell–cell signaling [11][12]. The molecular contents of circulating exosomes derived from the blood plasma and milk of dairy cows have been characterized to some extent, and contain, for example, proteins, mRNA, micro(mi)RNAs, and lipids [10][13]. It is possible that miRNA contained in the blood plasma exosomes of dairy cows serve as an epigenetic regulator of biological signaling pathways, including inflammation, which in turn may affect reproduction and development of the fetus during pregnancy [14]. Additionally, qualitative differences in proteomic exosomal cargo have been previously established in milk and plasma samples between high- and low-fertility dairy cows, and between cattle with and without uterine infection [15][16][17]. Quantitative differences in exosomal proteins between these high- and low-fertility groups are yet to be fully elucidated and may hold the key to identifying potential biomarkers for fertility. Exosomes contained in the blood plasma, for instance, can provide a systemic snapshot of valuable information about the health-status of the animal, which may be directly or indirectly related to reproductive status.
2. Exosomes
Formation and Function
Within the cell there is a complex protein synthesis and sorting pathway, whereby protein folding and glycosylation begin in the endoplasmic reticulum (ER). Mature and proproteins are further modified as they pass through the Golgi apparatus, and following this are transported via transport vesicles to early endosomes (see Figure 1, next page) [18]. Early endosomes mature further into late endosomes, whereby they are transported to the cell surface and exocytosed via direct fusion with the plasma membrane [19]. Endocytosed materials may also be transferred to late endosomes and transported to lysosomes, or recycled back to the cell surface [20]. Late endosomes contain nucleic acids, proteins, lipids, and trans-Golgi Network (TGN)-derived transport vesicles; hence they are also termed multivesicular bodies (MVBs) [21]. ILVs within MVBs are released as extracellular vesicles (EVs), a subpopulation of which are termed exosomes [18][22]. Proteins involved in MVB formation and cargo sorting (endosomal sorting complexes required for transport (ESCRT) pathway) and its accessory proteins are also typically found in exosomes [22][23]. Therefore, ESCRT proteins such as Tumor Suppressor Gene 101 (TSG101) are used experimentally as positive exosomal markers, as are members of the tetraspanin family (CD9, CD63, CD81); the latter of which have recently been implicated as important mediators in mammalian reproduction [22][24][25].
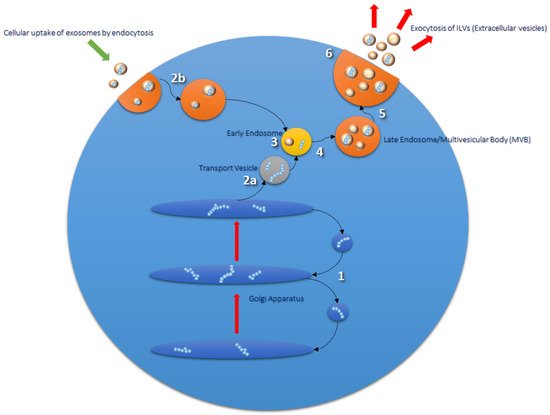
Figure 1. Routes of exosomal formation and release from the cell. The Golgi apparatus (1) transports and modifies proteins received from the endoplasmic reticulum (ER). Mature proteins and proproteins are transferred from the Golgi to endosomes via transport vesicles (2a and 3). Early endosomes go on to form late endosomes/multivesicular bodies (MVBs) (4 and 5), which are composed of intraluminal vesicles (ILVs) formed from the inward budding of the endosomal membrane during the maturation process. Endosomal sorting complex required for transport (ESCRT) proteins are involved in this process and are found in ILV cargo. MVBs fuse with the plasma membrane of the cell to release their contents into the extracellular milieu; extracellular vesicles (EVs) (6). EVs are taken up by the cell via endocytosis or phagocytosis (2b) and transported to endosomal compartments and lysosomes for processing [26].
Exosomal molecular cargo can be endocytosed by target cells via a number of different mechanisms; direct receptor–ligand interaction, through cell surface adhesion molecules such as integrins or cadherins that initiate endocytosis, or by the opsonization of exosomes inducing phagocytosis in the recipient cell [27][28]. It has been suggested that the uptake of exosomes may also depend on the recipient-cell type, as a study involving exosomes isolated from various cancer cell lines demonstrated differences in uptake by recipient cells regardless of the cell type of exosomal origin [29]. This suggests that exosomes can interact with any cell type, independent of the cell from which they themselves are derived, albeit by different mechanisms of endocytosis. Interestingly, Sung et al. (2020) confirmed pathfinding behaviour of cells as they migrate towards exosomal tracks in 2D and 3D models, and created a double reporter system to follow the release, uptake, and acidification of exosomal deposits in internalized compartments containing exosomes [30]. The results of these studies present promising directions for future research when considering the use of exosomes for targeted therapeutics.
Whereas exosomes were historically thought to contain cellular waste, more recent exosomal profiling has resulted in the understanding that they are intrinsic to cell maintenance, cell–cell signaling, immune modulation, and progression of tumor-derived cells and metastasis [22]. This has led to research into their ability to carry biomarkers of disease in easily attainable biological fluids such as blood, saliva, and urine [31][32][33][34], and their potential as therapeutic targets and delivery vehicles [27][31][35]. Currently, researchers have begun to establish EV profiles that will assist in determining the proportions of the various EV subtypes in any given biological sample, with the aim to better understand heterogenous populations of EVs and their distinct functions [36][37].
3. Bovine Reproduction
The reproductive health of dairy cows has been associated with a number of physiological factors and environmental factors. Heat stress has been implicated as an epigenetic modifier than may negatively impact upon the reproductive status of offspring [38][39], while NEB has been linked to poor transition around the time of calving and metabolic stress [40][41]. Importantly, non-esterified fatty acid (NEFA) surplus as a result of NEB has been shown to result in poor immune function and increased likelihood of uterine infection [40]. Inflammatory mediators from the prostaglandin (PG) family are known to play a part in reproductive processes in cattle, and as such have been the subject of investigations surrounding impaired fertility in dairy herds [42]. Qin and colleagues (2020) examined the effects of high NEFA concentrations on PG production in bovine endometrial (BEND) cells and observed decreased levels of prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) in cell culture media supernatant compared to controls [43]. Similarly, cows with metritis were found to have a differential abundance of common uterine bacteria compared with healthy cows [44]. Researchers have therefore attempted to establish ways to better manage cattle during times of physiological and metabolic challenge in hopes of improving reproductive health. For example, micronutrient supplementation during the transition period improved outcomes without altering the methylation state of the cows [45]. Thus, factors affecting reproductive performance of dairy herds are various and complex, and ways of determining intervention at an earlier stage may improve outcomes at a minimal cost to farmers and herds.
Exosomes have been the focus of bovine studies examining effects on implantation and embryo development. Two separate studies confirmed that exosomes derived from the bovine uterus increased gene and protein expression of the pregnancy-recognition-associated protein interferon-tau (IFN-τ) when cocultured with bovine embryos in vitro [46][47]. Another study implicated a role in exosome secretion from both conceptus and endometrium in facilitating crosstalk during the attachment period, while exosomes derived from follicular fluid have been shown to improve oocyte competence and resistance to environmental stressors such as heat shock [48][49]. Collectively, these studies suggest that exosomes are widely involved in bovine reproduction, thus supporting further evaluation of their contents and function.
While the protein cargo of exosomes has been somewhat characterized qualitatively, larger scale in-depth studies of quantitative differences between high- and low-fertility groups have not been conducted [13][17][50][51]. Dysregulation of the immune system, metabolic perturbations around the time of calving, and impaired embryonic-maternal crosstalk during implantation have all been associated with poor reproductive outcomes, and all of which exosomes are known to play a part [2][6][13][17][47][52]. Quantitative differences in exosomal protein cargo may have a significant impact on the overall health of dairy cows, upon which fertility may be directly or indirectly impacted. Differences may also serve as a valuable tool for predicting reproductive outcomes early on in the life of the cow and warrants further investigation.
3.1. The Immune System
Successful reproduction in dairy cows relies on a competent immune system, especially during the periparturient period. Compromised immunity is associated with poor transition during the calving period and significant physiological stress, resulting in increased risk of postpartum uterine infection, mastitis, and an extended postpartum anestrous interval (PPAI). Studies have focused on various aspects of the immune system to better understand reproductive failings around early embryonic loss, postpartum uterine infection, and associated poor reproductive outcomes. Exosomes carry lipid mediators derived from arachidonic acid (AA), and enzymes involved in their synthesis, including inflammatory mediators associated with reproduction [53][54][55]. For example, PGs are small lipid compounds classed as eicosanoids, which among a diverse number of actions can behave as inflammatory mediators that are not only upregulated during infection and inflammation, but also play a critical role in establishment and maintenance of pregnancy in cattle [42][56][57]. PGE2 and PGF2α are responsible for establishing or inhibiting bovine pregnancy, respectively [42]. Upregulation of inflammatory pathways during critical time points in the reproductive cycle of dairy cows could therefore have a severe impact on their reproductive health (see Figure 2). In an in vitro model of uterine inflammation, PGE2 and PGF2α were found to be differentially expressed by bovine endometrial epithelial (bEEL) and stromal (bCSC) cells when exposed to inflammatory stimuli [58]. In further experiments, bEEL expression of PGF2α was increased when coincubated with plasma exosomes derived from dairy cows with uterine infection [51]. Fatty acid cyclooxygenase-2 (COX2), which is upstream of the proinflammatory PGE2, has been highlighted as a potential target for therapies including the use of nonsteroidal anti-inflammatory drugs (NSAIDs) (see Figure 2) [2][40], although NSAIDS have previously been found to be ineffectual on Cox2 mRNA levels [59]. Interestingly, NSAIDS were successful in inhibiting lipopolysaccharide (LPS)-induced PGE2 and tumor necrosis factor-alpha (TNFα) mRNA production, indicating a mechanism of action separate to Cox2 activity [59]. A recent meta-analysis aimed to compare antibiotic with non-antibiotic methods (e.g., NSAIDs) of treatment for acute puerperal metritis (APM) in postpartum cattle [60]. Unfortunately, due to a shortage of comparable studies, the researchers were unable to perform the analysis for non-antibiotic methods, therefore the use of NSAIDs to treat postpartum uterine infection in cattle remains largely unverified.
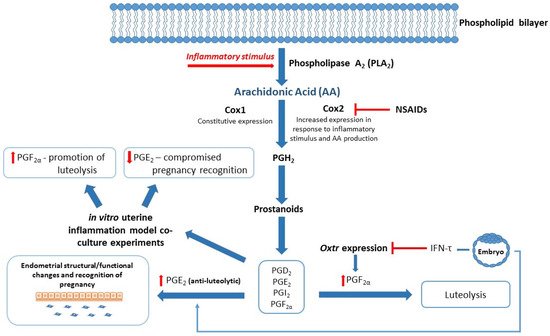
Figure 2. Blended model of reproduction and inflammation: Arachidonic Acid (AA)/Eicosanoid Pathway. Fatty acid cyclooxygenase 1/2 (Cox 1/2) converts AA to downstream effector molecules (Prostanoids and Prostaglandins (PGs)) following inflammatory stimuli. Interferon-tau (IFN-τ) produced by the conceptus inhibits Oxytocin receptor (Oxtr) expression and prevents luteolysis of luteinized granulosa cells to maintain progesterone secretion. IFN-τ stimulates PGE2 production in the endometrium, resulting in structural and functional changes required for pregnancy recognition. In vitro studies show altered expression of PGF2α and PGE2 when exposed to inflammatory stimuli, which in turn may compromise events leading to successful establishment of pregnancy. Nonsteroidal anti-inflammatory drugs (NSAIDs) target the PG inflammatory cascade by inhibiting Cox2 expression and reducing production of PGH2 and associated inflammatory mediators.
3.2. The Transition Period
The transition period is a demanding phase in the life of dairy cows and challenging from the farm management perspective. It is typically defined as the period ranging from 3 weeks before and after calving [61] and represents a time of metabolic stress for the dairy cow, as the animal undergoes immense physiological changes in preparation for and during early lactation. Dairy cows that have been selectively bred for milk production traits experience greater metabolic pressure associated with increased milk production. Subsequently, this results in a greater incidence of postpartum uterine infection and mastitis, leading to ongoing health issues and negative implications for further reproduction [1][2][41][62]. Markers of metabolic distress such as β-hydroxybutyrate (BHB), triacylglycerols (TAG) and fatty acids (FA) were found to be altered in the blood plasma [2][8][61]. In addition to this, hypocalcemia resulting in ‘milk fever’ can occur, which results in the death of approximately 1 in 20 affected cows, reduces both the productive lifespan and milk production with each milk fever episode, and comes with associated costs of treatment and prevention [1][41][63]. The impact of metabolic distress during the transition period on future calving is of interest to reproductive studies. Increased metabolic pressure around the time of calving leads to lengthened PPAI and pre- and postovulatory dysfunction, which can significantly delay return to estrous and time to mating and is therefore of major concern to dairy farmers who operate under a seasonal-calving pasture-based system [2][8].
Numerous studies have focused on the link between BCS, NEB, and feed-intake during the transition period as a method of immunomodulation, in hopes of improving management of the transition dairy cow [64][65][66][67]. The use of exosomes as a potential source of biomarkers for low- versus high-risk populations of dairy cows has been investigated, with promising, although inconclusive, results [13]. Exosomes derived from the blood plasma of healthy versus dairy cows with cytological endometritis have been found to differ in protein composition when analyzed by liquid chromatography–mass spectrometry (LC-MS), which included proteins associated with innate immunity, acute immune response, and immune regulation [68]. Similarly, an in vitro study applied blood plasma exosomes isolated from dairy cows with and without uterine infection to endometrial cell lines to study their effects on PG production and found a decrease in luteolytic promoter PGF2α produced by cells treated with exosomes derived from the infected cows [51]. This suggests the involvement of PGF2α in disrupting normal reproductive processes and offers a potential target for improving outcomes in these animals. Despite this, the transition period still proves to be a challenging time for dairy farmers and their herds, and further research is required to better identify at-risk cows in hopes of preventing postpartum infection and maintaining reproductive efficiency.
Thus far, partly due to the ethical nature of conducting in vivo experiments, studies have steered towards in vitro modeling of bovine uterine infection. However, this may not be representative of the full spectrum of physiological mechanisms involved in, and leading to, high- or low-fertility and susceptibility to reproductive disruption in early life and during the transition or postpartum period. Bodily fluid samples obtained from cattle with and without disease may already be compromised regarding differences in molecular content, as it would be expected that inflammatory/disease markers would be present in affected animals at the time of disease occurrence. A more useful and predictive method of testing for differences would require sampling at the baseline stage, long before cattle experience reproductive and immune challenges. For example, sampling may occur around the time of puberty or earlier in order to establish a predictive model of reproductive performance and predisposition for disease in the early stages of reproductive life. Currently, Fertility Breeding Value (FBV) and BCS are the only tools available to dairy farmers to assist in the herd selection process, which does not consider the individual genetics or physiology of animals, but merely relies on physical attributes and genetic lineage as predictors [9][69]. Early biomarkers of fertility would aim to provide the dairy industry with reliable data that can assist in herd selection and lessen the burden of operational costs associated with poor reproductive performance. While lipid and inflammatory mediators transported by exosomes have been linked to reproduction in cattle, differences in protein cargo may give a better understanding of cattle fertility and the mechanisms that underlie perturbations to healthy reproduction.
References
- Mitchell, M.D.; Crookenden, M.A.; Vaswani, K.; Roche, J.R.; Peiris, H.N. The frontiers of biomedical science and its application to animal science in addressing the major challenges facing Australasian dairy farming. Anim. Prod. Sci. 2020, 60.
- Roche, J.R.; Burke, C.R.; Crookenden, M.A.; Heiser, A.; Loor, J.L.; Meier, S.; Mitchell, M.D.; Phyn, C.V.C.; Turner, S.-A. Fertility and the transition dairy cow. Reprod. Fertil. Dev. 2018, 30, 85.
- Berry, D.P.; Friggens, N.C.; Lucy, M.C.; Roche, J.R. Milk Production and Fertility in Cattle. Annu. Rev. Anim. Biosci. 2016, 4, 269–290.
- Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Almughlliq, F.B.; Meier, S.; Burke, C.R.; Roche, J.R.; Reed, C.B.; Arachchige, B.J.; Reed, S.; et al. Proteome profiling of exosomes derived from plasma of heifers with divergent genetic merit for fertility. J. Dairy Sci. 2018, 101, 6462–6473.
- Garnsworthy, P.C.; Sinclair, K.D.; Webb, R. Integration of Physiological Mechanisms That Influence Fertility in Dairy Cows. Animal 2008, 2, 1144–1152.
- Formigoni, A.; Trevisi, E. Transition Cow: Interaction with Fertility. Vet. Res. Commun. 2003, 27, 143–152.
- Lucy, M.C. Reproductive Loss in High-Producing Dairy Cattle: Where Will It End? J. Dairy Sci. 2001, 84, 1277–1293.
- Roche, J.F.; Mackey, D.; Diskin, M.D. Reproductive management of postpartum cows. Anim. Reprod. Sci. 2000, 60–61, 703–712.
- Bowley, F.; Green, R.; Amer, P.; Meier, S. Novel approaches to genetic analysis of fertility traits in New Zealand dairy cattle. J. Dairy Sci. 2015, 98, 2005–2012.
- Mitchell, M.; Scholz-Romero, K.; Reed, S.; Peiris, H.; Koh, Y.; Meier, S.; Walker, C.; Burke, C.; Roche, J.; Rice, G.; et al. Plasma exosome profiles from dairy cows with divergent fertility phenotypes. J. Dairy Sci. 2016, 99, 7590–7601.
- Raimondo, F.; Morosi, L.; Chinello, C.; Magni, F.; Pitto, M. Advances in membranous vesicle and exosome proteomics improving biological understanding and biomarker discovery. Proteomics 2011, 11, 709–720.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Crookenden, M.; Walker, C.; Peiris, H.; Koh, Y.; Heiser, A.; Loor, J.; Moyes, K.; Murray, A.; Dukkipati, V.; Kay, J.; et al. Short communication: Proteins from circulating exosomes represent metabolic state in transition dairy cows. J. Dairy Sci. 2016, 99, 7661–7668.
- Sohel, M.M.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K.; et al. Exosomal and Non-Exosomal Transport of Extra-Cellular Micrornas in Follicular Fluid: Implications for Bovine Oocyte Developmental Competence. PLoS ONE 2013, 8, e78505.
- Crookenden, M.A.; Walker, C.G.; Peiris, H.; Koh, Y.; Almughlliq, F.; Vaswani, K.; Reed, S.; Heiser, A.; Loor, J.J.; Kay, J.K.; et al. Effect of Circulating Exosomes from Transition Cows on Madin-Darby Bovine Kidney Cell Function. J. Dairy Sci. 2017, 100, 5687–5700.
- Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Meier, S.; Burke, C.R.; Macdonald, K.A.; Roche, J.R.; Almughlliq, F.; Arachchige, B.J.; Reed, S.; et al. Characterization of Exosomes from Body Fluids of Dairy Cows. J. Anim. Sci. 2017, 95, 3893–3904.
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; McDougall, S.; Graham, E.M.; Burke, C.R.; Arachchige, B.J.; Reed, S.; Mitchell, M.D. Proteomic content of circulating exosomes in dairy cows with or without uterine infection. Theriogenology 2018, 114, 173–179.
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208.
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632.
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19.
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The Biogenesis and Functions of Exosomes. Traffic 2002, 3, 321–330.
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727.
- Simons, M.; Raposo, G. Exosomes—Vesicular Carriers for Intercellular Communication. Curr. Opin. Cell Biol. 2009, 21, 575–581.
- Jankovičová, J.; Neuerová, Z.; Sečová, P.; Bartóková, M.; Bubeníčková, F.; Komrsková, K.; Postlerová, P.; Antalíková, J. Tetraspanins in Mammalian Reproduction: Spermatozoa, Oocytes and Embryos. Med. Microbiol. Immunol. 2020, 209, 407–425.
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT Pathway. Dev. Cell 2011, 21, 77–91.
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, Rna Cargo Selection, Content, Release, and Uptake; Springer: New York, NY, USA, 2016; pp. 301–312.
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Librelotto, D.R.N.; Gasparri, M.L.; Bishayee, A.; Nabavi, S.M.; Curti, V.; Daglia, M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018, 36, 328–334.
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223.
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 2018, 18, 1–9.
- Sung, B.H.; Von Lersner, A.; Guerrero, J.; Krystofiak, E.S.; Inman, D.; Pelletier, R.; Zijlstra, A.; Ponik, S.M.; Weaver, A.M. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat. Commun. 2020, 11, 1–15.
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538.
- Han, Y.; Jia, L.; Zheng, Y.; Li, W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018, 14, 633–643.
- Meng, X.; Pan, J.; Sun, S.; Gong, Z. Circulating exosomes and their cargos in blood as novel biomarkers for cancer. Transl. Cancer Res. 2018, 7, S226–S242.
- Panfoli, I. Cancer exosomes in urine: A promising biomarker source. Transl. Cancer Res. 2017, 6, S1389–S1393.
- Nazimek, K.; Bryniarski, K.; Santocki, M.; Ptak, W. Exosomes as mediators of intercellular communication: Clinical implications. Pol. Arch. Intern. Med. 2015, 125, 370–380.
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977.
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17.
- Huber, E.; Notaro, U.; Recce, S.; Rodríguez, F.; Ortega, H.; Salvetti, N.; Rey, F. Fetal programming in dairy cows: Effect of heat stress on progeny fertility and associations with the hypothalamic-pituitary-adrenal axis functions. Anim. Reprod. Sci. 2020, 216, 106348.
- Lee, J.; Lee, S.; Son, J.; Lim, H.; Kim, E.; Kim, D.; Ha, S.; Hur, T.; Lee, S.; Choi, I. Analysis of Circulating-Microrna Expression in Lactating Holstein Cows under Summer Heat Stress. PLoS ONE 2020, 15, e0231125.
- Bradford, B.; Yuan, K.; Farney, J.; Mamedova, L.; Carpenter, A. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. Dairy Sci. 2015, 98, 6631–6650.
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71.
- Arosh, J.A.; Banu, S.K.; Kimmins, S.; Chapdelaine, P.; MacLaren, L.A.; Fortier, M.A. Effect of Interferon-Tau on Prostaglandin Biosynthesis, Transport, and Signaling at the Time of Maternal Recognition of Pregnancy in Cattle: Evidence of Polycrine Actions of Prostaglandin E2. Endocrinology 2004, 145, 5280–5293.
- Qin, X.; Yang, S.; Zhang, Y.; Li, L.; Li, P.; Long, M.; Guo, Y. Effects of non-esterified fatty acids on relative abundance of prostaglandin E2 and F2α synthesis-related mRNA transcripts and protein in endometrial cells of cattle in vitro. Anim. Reprod. Sci. 2020, 221, 106549.
- Chen, H.; Fu, K.; Pang, B.; Wang, J.; Li, H.; Jiang, Z.; Feng, Y.; Tian, W.; Cao, R. Determination of uterine bacterial community in postpartum dairy cows with metritis based on 16S rDNA sequencing. Vet. Anim. Sci. 2020, 10, 100102.
- Gasselin, M.; Boutinaud, M.; Prézelin, A.; Debournoux, P.; Fargetton, M.; Mariani, E.; Zawadzki, J.; Kiefer, H.; Jammes, H. Effects of micronutrient supplementation on performance and epigenetic status in dairy cows. Animal 2020, 14, 2326–2335.
- Qiao, F.; Ge, H.; Ma, X.; Zhang, Y.; Zuo, Z.; Wang, M.; Wang, Y. Bovine uterus-derived exosomes improve developmental competence of somatic cell nuclear transfer embryos. Theriogenology 2018, 114, 199–205.
- Kusama, K.; Nakamura, K.; Bai, R.; Nagaoka, K.; Sakurai, T.; Imakawa, K. Intrauterine Exosomes Are Required for Bovine Conceptus Implantation. Biochem. Biophys. Res. Commun. 2018, 495, 1370–1375.
- Rodrigues, T.A.; Tuna, K.M.; Alli, A.A.; Tribulo, P.; Hansen, P.J.; Koh, J.; Paula-Lopes, F.F. Follicular fluid exosomes act on the bovine oocyte to improve oocyte competence to support development and survival to heat shock. Reprod. Fertil. Dev. 2019, 31, 888.
- Nakamura, K.; Kusama, K.; Bai, R.; Sakurai, T.; Isuzugawa, K.; Godkin, J.D.; Suda, Y.; Imakawa, K. Induction of IFNT-Stimulated Genes by Conceptus-Derived Exosomes during the Attachment Period. PLoS ONE 2016, 11, e0158278.
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Holland, O.; Meier, S.; Roche, J.R.; Burke, C.R.; Crookenden, M.A.; Arachchige, B.J.; et al. Circulating Exosomes May Identify Biomarkers for Cows at Risk for Metabolic Dysfunction. Sci. Rep. 2019, 9, 1–12.
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; McDougall, S.; Graham, E.M.; Burke, C.R.; Mitchell, M.D. Effect of exosomes from plasma of dairy cows with or without an infected uterus on prostaglandin production by endometrial cell lines. J. Dairy Sci. 2017, 100, 9143–9152.
- Saeed-Zidane, M.; Linden, L.; Salilew-Wondim, D.; Held, E.; Neuhoff, C.; Tholen, E.; Hoelker, M.; Schellander, K.; Tesfaye, D. Cellular and exosome mediated molecular defense mechanism in bovine granulosa cells exposed to oxidative stress. PLoS ONE 2017, 12, e0187569.
- Giller, K.; Drews, B.; Bérard, J.; Kienberger, H.; Schmicke, M.; Frank, J.; Spanier, B.; Daniel, H.; Geisslinger, G.; Ulbrich, S.E. Bovine embryo elongation is altered due to maternal fatty acid supplementation. Biol. Reprod. 2018, 99, 600–610.
- Zhang, N.; Wang, L.; Luo, G.; Tang, X.; Ma, L.; Zheng, Y.; Liu, S.; Price, C.A.; Jiang, Z. Arachidonic Acid Regulation of Intracellular Signaling Pathways and Target Gene Expression in Bovine Ovarian Granulosa Cells. Animals 2019, 9, 374.
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; de Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes Account for Vesicle-Mediated Transcellular Transport of Activatable Phospholipases and Prostaglandins. J. Lipid Res. 2010, 51, 2105–2120.
- Banu, S.K.; Arosh, J.A.; Chapdelaine, P.; Fortier, M.A. Expression of Prostaglandin Transporter in the Bovine Uterus and Fetal Membranes During Pregnancy1. Biol. Reprod. 2005, 73, 230–236.
- Ledgard, A.M.; Meier, S.; Peterson, A.J. Evaluation of the uterine environment early in pregnancy establishment to characterise cows with a potentially superior ability to support conceptus survival. Reprod. Fertil. Dev. 2011, 23, 737–747.
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Arachchige, B.J.; Reed, S.; Mitchell, M.D. Eicosanoid Pathway Expression in Bovine Endometrial Epithelial and Stromal Cells in Response to Lipopolysaccharide, Interleukin 1 Beta, and Tumor Necrosis Factor Alpha. Reprod. Biol. 2018, 18, 390–396.
- Myers, M.J.; Scott, M.L.; Deaver, C.M.; Farrell, D.E.; Yancy, H.F. Biomarkers of inflammation in cattle determining the effectiveness of anti-inflammatory drugs. J. Vet. Pharmacol. Ther. 2010, 33, 1–8.
- Haimerl, P.; Arlt, S.; Borchardt, S.; Heuwieser, W. Antibiotic treatment of metritis in dairy cows—A meta-analysis. J. Dairy Sci. 2017, 100, 3783–3795.
- Drackley, J.K. Biology of Dairy Cows During the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273.
- Trevisi, E.; Minuti, A. Assessment of the innate immune response in the periparturient cow. Res. Vet. Sci. 2018, 116, 47–54.
- Champness, D. Milk Fever (Hypocalcaemia) in Cows. 2007. Available online: (accessed on 8 February 2021).
- Vailati-Riboni, M.; Kanwal, M.; Bulgari, O.; Meier, S.; Priest, N.; Burke, C.; Kay, J.; McDougall, S.; Mitchell, M.; Walker, C.; et al. Body condition score and plane of nutrition prepartum affect adipose tissue transcriptome regulators of metabolism and inflammation in grazing dairy cows during the transition period. J. Dairy Sci. 2016, 99, 758–770.
- Vailati-Riboni, M.; Farina, G.; Batistel, F.; Heiser, A.; Mitchell, M.; Crookenden, M.; Walker, C.; Kay, J.; Meier, S.; Roche, J.; et al. Far-off and close-up dry matter intake modulate indicators of immunometabolic adaptations to lactation in subcutaneous adipose tissue of pasture-based transition dairy cows. J. Dairy Sci. 2017, 100, 2334–2350.
- Roche, J.R.; Heiser, A.; Mitchell, M.D.; Crookenden, M.A.; Walker, C.G.; Kay, J.K.; Riboni, M.V.; Loor, J.J.; Meier, S. Strategies to Gain Body Condition Score in Pasture-Based Dairy Cows During Late Lactation and the Far-Off Nonlactating Period and Their Interaction with Close-up Dry Matter Intake. J. Dairy Sci. 2017, 100, 1720–1738.
- Crookenden, M.; Walker, C.; Heiser, A.; Murray, A.; Dukkipati, V.; Kay, J.; Meier, S.; Moyes, K.; Mitchell, M.; Loor, J.; et al. Effects of precalving body condition and prepartum feeding level on gene expression in circulating neutrophils. J. Dairy Sci. 2017, 100, 2310–2322.
- Miller, B.A.; Brewer, A.; Nanni, P.; Lim, J.J.; Callanan, J.J.; Grossmann, J.; Kunz, L.; De Almeida, A.M.; Meade, K.G.; Chapwanya, A. Characterization of circulating plasma proteins in dairy cows with cytological endometritis. J. Proteom. 2019, 205, 103421.
- Meier, S.; Fisher, B.; Eketone, K.; McNaughton, L.R.; Amer, P.R.; Beatson, P.; Bryant, J.R.; Dodds, K.G.; Spelman, R.; Roche, J.R.; et al. Calf and Heifer Development and the Onset of Puberty in Dairy Cows with Divergent Genetic Merit for Fertility. N. Z. Soc. Anim. Prod. Proc. 2017, 77, 205–210.
