Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Junior Corneille Fingu Mabola and Version 2 by Peter Tang.
Aphids are responsible for the spread of more than half of the known phytovirus species. Virus transmission within the plant–aphid–phytovirus pathosystem depends on vector mobility which allows the aphid to reach its host plant and on vector efficiency in terms of ability to transmit phytoviruses.
- host selection
- plant–aphid–virus pathosystem
- vector activity
- vector-born virus
- vectorial transmission efficiency
1. Introduction
Phytoviruses are an important group of phytopathogenic agents. They are responsible for major crop yield losses estimated at around USD 60 billion annually worldwide [1]. They are obligate parasites, mainly composed of genetic material (nucleic acid: RNA or DNA) within a protein shell (capsid). Due to this minimalist constitution, phytoviruses are unable to reach new hosts. However, they must switch from one plant to another before the previous one dies in order to survive [2][3][2,3]. So, phytoviruses have developed various dispersal strategies, most importantly relying on vectors [2]. Several groups of fungi, nematodes, mites and insects play this role. Herbivorous insects are known to be vectors of most phytoviruses due to their mobility and behaviour allowing them to circumvent plant immobility in order to spread [2][4][2,4]. Various insect orders are recognised as phytoviruses vectors, such as Coleoptera, Orthoptera, Lepidoptera, Dermaptera, Diptera, Thysanoptera [5], but especially Hemiptera [6]. Aphids (Hemiptera: Aphidididae) are by far the most important phytoviruses vector group. They are involved in spreading more than half of the known phytovirus species (275 species within 19 genera) [5]. Consequently, this review will focus especially on aphids.
The classification of phytoviruses is based on their mode of transmission. It takes into account three events: (1) acquisition during the insect meal on an infected plant; (2) retention or circulation within the insect vector organism; and (3) inoculation during a new insect meal on a healthy plant [1][7][1,7]. These three events occur in different ways, resulting in several classifications of phytoviruses discussed in [5]. In this review, we have chosen the classification taking into account the site and retention time of phytoviruses in insect vector organisms. Then, we distinguish phytoviruses transmitted by non-circulative and circulative modes. (1) Non-circulatively transmitted phytoviruses are limited to the tips of the stylets (non-persistent or stylet-borne viruses) and foregut (semipersistent or foregut-borne viruses) of the vector [8]. These phytoviruses are acquired and transmitted during short probing punctures by the insect vector in plant epidermal and mesophyll cells. After acquisition, the vector becomes directly infectious within minutes to a few hours or during many hours for non-persistent and semipersistent phytoviruses, respectively [5]. (2) Circulatively transmitted phytoviruses require a long-lasting sap ingestions phase, followed by an incubation or latent period. During this period, the phytovirus is ingested and circulates in its vector’s organism until it reaches its salivary gland (persistent or salivary gland-borne). Then, the vector becomes able to transmit the phytovirus for at least several days, or even its whole life-cycle [2][5][2,5].
The phytovirus transmission process by insect vector rely on two mains steps: firstly, the vector activity, comprising host-seeking, feeding and shifting or dispersal behaviours; secondly, the vector efficiency depending on their intrinsic ability to transmit phytoviruses [9]. Furthermore, several factors related to the phytovirus and/or the host plant, but also the insect–plant–phytovirus interactions can influence the phytoviruses transmission process and have significant epidemiological consequences [6]. Phytoviruses have been demonstrated to use different mechanisms to improve their spread, including manipulating their vector’s activity and transmission efficiency directly or through their shared host plants [9]. Life history traits can provide indications of how the vector benefits from the relationship between the components in the interaction [3]. There are also external factors from the pathosystem components that can influence the epidemiology of viral diseases. These include biotic and abiotic factors, used for experimental purpose or resulting from crop protection strategies.
Given the potential impact of the above on the epidemiology of insect-vector-borne phytovirus diseases, a better understanding of insect vector activity, transmission efficiency and factors responsible for their alterations may allow the development of more effective crop protection strategies.
2. Vector Activity in Aphids
Vector activity is related to a set of behaviours on which the phytovirus relies on reaching new hosts. It includes host-seeking behaviour (HSB), probing and feeding behaviour (FB) and dispersal (or shifting) behaviour (DB). Except for FB, vector activity is closely linked to the displacement of the insect towards its host plant. Then, knowing how they move themselves will provide a better understanding of how they reach their host plant to spread phytoviruses.
Aphid life cycles are known to be diversified with several morphological forms associated to different host plants according to the season and environmental conditions (see the review in [10] and Figure 1). Depending on their mobility, adults are either winged or wingless. Winged morphs, especially spring and summer migrants, are known to be important in conquering new hosts, establishing new colonies and, at the same time, responsible for spreading phytoviruses over long distances. Spring migrants transmit phytoviruses acquired from alternate hosts in early spring to crop plants while summer migrants transmit phytoviruses within the field from one plot to another, more or less distant, during plant-to-plant movements over several generations [11]. As for wingless, they spread the infection by walking from one plant to another in the vicinity of the initially colonised plant [12]. Finally, fall migrants are involved in the transmission of new phytoviruses which were acquired in the field from distant sources to the alternate hosts (reservoir plants) [11]. Therefore, the emergence of winged forms would be favourable to the further phytoviruses spread.
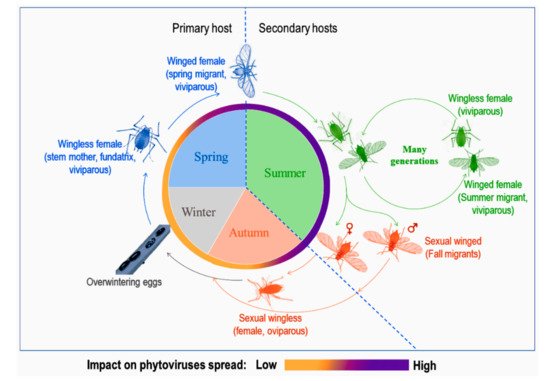
Figure 1. A representation of a dieocic aphid life cycle with at least two plant hosts and its impact on phytoviruses spread, adapted from [11][13][11,13].
3. Vectorial Transmission Efficiency
Vectorial transmission efficiency (VTE) is the probability of obtaining plants infected by a given phytovirus using a given vector under well-defined environmental conditions [14][66]. It relies on two main components: (1) vector efficiency, which is defined as the intrinsic capacity of a vector to transmit one or more phytoviruses, and (2) interference of factors related to experimental methods and biological materials when assessing VTE.
3.1. Vector Efficiency
Vector efficiency (VE) refers to the interaction between a phytovirus and its vector. This interaction is characterised by a given level of specificity of transmissibility such that a phytovirus recognises its vector, or especially, the virion or a viral protein motif recognises the site of retention upon acquisition by its vector. This specificity level in different pathosystems can be exclusive, i.e., one vector transmits only one phytovirus species and this virus has a single vector (the case of grapevine fan leaf virus (GFLV), transmitted by the nematode Xiphinema index), or very broadly: one genus or species of phytovirus has several vectors or one vector is involved in several pathosystems [15][67]. For example, on the one hand, the whitefly Bemisia tabaci transmits several phytoviruses of different genera and families and, on the other hand, the Closteroviruses are transmitted by several groups of insects including mealybugs, whiteflies and aphids. In aphids, Potyviruses are transmitted by more than thirty species [15][67]. Myzus persicae is a vector of more than hundred phytoviruses belonging to different genera and families [13]. It should be noted that, when a phytovirus is transmitted by several vectors, one is recognised as the most efficient vector, making it a reference vector for transmitting this phytovirus. This is the case of M. persicae which serves as a reference vector for PVY, on the basis of which the relative efficiency factors (REFs) of the remaining vectors of this phytovirus are evaluated [9][16][9,59].
The specificity in the vector–phytovirus relationship described above is regulated by determinants which at the same time regulates the transmissibility of each phytovirus by its vector. These determinants depend on the mode of transmission (circulative or non-circulative), the site of retention (stylet-born or foregut-born or salivary gland-born phytoviruses) and the strategy adopted by the phytovirus [17][68]. For stylet-born phytoviruses (non-circulative, non-persistent), there is either the strategy of direct attachment and retention of the virion on the putative receptors on the stylets of the vector; or the phytovirus uses helper components serving as intermediates between the virion and the vector’s receptors on its stylets (Blanc et al., 2014). The latter strategy is used by Potyviruses and Caulimoviruses where the helper component is designated as HC-Pro and P2, respectively [18][69]. As for the foregut-born (non-circulative, semi-persistent) phytoviruses, this is called a capsid binding strategy using the minor capsid protein (CPm). This is the case for Criniviruses [19][70]. Finally, the salivary gland-born (circulative, persistent) phytoviruses bind to the receptors of their vectors mainly through their coat protein [17][68].
3.2. Interference Factors of Biological Materials and Experimental Methods
When performing bioassays evaluating VTE in a given pathosystem, the result interpretation must take into account the biological material used., i.e., the use of the reference vector or the one whose REF is closer to one compared to phytovirus under study will allow to attainment of higher infection rates compared to a less efficient vector [9]. For example, M. euphorbiae, often considered as the most efficient potato-colonising aphid vector of PVY, will provide infection rates close to those that would be obtained with M. persicae, the reference vector in this pathosystem [9][20][9,71]. However, the study aiming to establish REFs of seventeen aphid species with three different biotypes each for the transmission of three PVY strains (PVYNTN, PVYNO, and PVYN-Wi) reveals that difference in VTE can also occur depending on the variability of biotypes within the same insect species and also of strains within the same phytovirus species [21][72]. For plants, VTE varies according to plant species status as main or alternative host of phytovirus and the associated vector [20][71], to resistant/susceptible character to phytovirus [22][73], but also according to plant phenology [20][71].
Several factors related to the protocol for monitoring the transmission process of a phytovirus by a vector on a plant can constitute sources of variation when performing VTE experiments under controlled conditions. In addition to above mentioned factors concerning the vector, its rearing conditions, growth stage and the number of individuals used to implement the test also influence the VTE results [9][21][23][9,72,74]. Moreover, it has been well established that individuals that previously underwent a pre-acquisition fasting period transmitted more efficiently the phytovirus compared to non-starved individuals [24][25][26][75,76,77]. Within the source plant, the phytoviruses concentration is not uniform [9][21][9,72]. It would be more concentrated on the plant younger top leaves, compared to older ones [23][74]. Finally, the AAP/IAP ratio can influence the VTE as reported by Sadeghi and colleagues [27][78] who found that a short AAP (6 h) followed by a long IAP (120 h) and a long AAP (48 h) followed by a short IAP (6 h) were the only factors to differ to the VTE of twenty R. padi clones transmitting BYDV-PAV isolate.
4. Life History Traits
Life history traits (LHT) are biological parameters that reveal the insect performance in a given environment. Several parameters are used to evaluate aphid performance, including reproduction rate, body growth, development time, survival rate, longevity, colony growth. In plant–aphid–phytovirus pathosystems, the aphid LHTs are indicative of the host plant–phytovirus relationship.
Circulative phytoviruses are known to encourage their vectors to colonise plants. The performances of aphids settled on plants infected with circulative phytoviruses is improved compared to those on healthy plants. This is the case of Micromyzus kalimpongensis Basu which had higher fecundity, faster growth rate during nymphal instars and longer adult longevity on cardamom bushy dwarf virus (CBDV)-infected compared to noninfected plants enhancing colony growth [28][57]. Moreover, dos Santos and colleagues [29][20] had recorded 25% of R. padi population increase on BYDV-infected wheat than non-infected plants. Additionally, non-circulative phytoviruses discourage the vector settlement on infected plants, resulting in the deterioration of their LHT. For example, measurements of growing parameters carried out on M. persicae settling on a PVY-infected tobacco plant revealed a significant decrease in body and head width, body and cornicle length, and the gap between compound eyes [30][79]. Similarly, delayed body growth and prolonged development duration were reported for A. gossypii and M. persicae, respectively on CMV infected plants [3][31][3,62]. Reproduction rate and population growth rate of A. idaei were also negatively impacted on R. idaeus infected by RLMV or BRNV [32][80]. However, there are a few exceptional cases where vector settling performance on circulative and non-circulative phytovirus-infected plants has been reduced and improved compared to healthy plants, respectively. These were the cases of A. gossypii, vector of papaya ringspot virus (PRSV) on C. pepo [33][25] and M. persicae, vector of turnip yellows virus (TuYV) on Camelina sp. [34][44].
Aphid performance as vectors of phytoviruses on their respective shared host plants was generally related to plant quality, which can be categorised into the following two characteristics: (1) the nutrient content, especially sugar and amino acids; and (2) the presence/absence of toxins and feeding deterrents. The performance level of phytovirus vectors is often linked to the high nutrient content of host plant. For example, Gadhave and colleagues [33][25] demonstrated the positive effect of free essential (lysine, arginine and threonine) and non-essential (homocysteine and glycine) amino acids and soluble carbohydrates (cellobiose, raffinose and galactose) increase on A. gossypii performances. Nevertheless, the accumulation of certain amino acids can produce negative effects on some aphids. For example, weak performance of A. idaei presented in the previous paragraph would be due to the accumulation of glutamate on BRNV and RLMV infected plants. This would be a strategy used by these phytoviruses to induce vector dispersal [32][80]. Another strategy used by non-circulative phytoviruses is the biosynthesis of toxins and feeding deterrents. In Section 2.3, we discussed the case of 4MI3M, one aphid feeding-deterrent synthesised by CMV to discourage prolonged sap ingestion by vectors [31][62]. This resulted in a reduction in the M. persicae growth rate. When aphids were transferred from infected to healthy plants, there was no significant difference in growth rates between individuals from infected and control plants [31][62].
5. Factors Due to External Components from Plant–Insect–Phytovirus Interaction
Integrating additional components which are external to the plant–insect–phytovirus relationship may have an effect on different aspects of vector life that may influence the phytovirus spread. Biological, biochemical, chemical, and physical factors have been incorporated as a fourth level in the plant–insect–phytovirus relationship on an experimental basis or as a developing or already widely used plant protection tool in agriculture.
5.1. Biological Components
Various beneficial organisms in agriculture have been studied for their integration into crop protection strategies. These include macro-organisms such as parasitoids and predators, but also micro-organisms such as bacteria, fungi and viruses. In phytovirus pathosystems, the impact of the presence of some of these organisms has also been studied.
5.2. Biochemical Components
Some substances biosynthesised by insects or plants in a specific context can be isolated or synthesised for research purposes. For example, (E)-β-farnesene (EβF) is a VOC that serves as an alarm pheromone in numerous aphid species. It is released in response to a predator attack, inducing dispersal behaviour in conspecifics [35][85]. Lin and colleagues [12] demonstrated that EβF release induced the dispersal of M. persicae and M. euphorbiae influencing the spread of phytoviruses under laboratory conditions.
5.3. Chemical and Physical Components
Physico-chemical factors, sometimes related to environmental characteristics, are of great importance. Impacts on plant growth, but also on relationships in phytovirus pathosystems, are dependent on soil factors such as salinity which negatively influenced the aphid population size, altered the soybean VOC and reduced the relative level of soybean mosaic virus (SMV) in aphid-infested plants. Incidence of SMV was dependent on salt-stressed fields [36][86]. Similarly, water stress (including drought and flooding) conditions altered the vector FB and the accumulation of phytoviruses in vectors’ organisms by modulating plant quality (phloem amino acid availability and defence expression). This resulted in the disruption of the vector performance and phytovirus transmission efficiency in TuYV- [1], cauliflower mosaic virus (CaMV)-, turnip mosaic virus (TuMV) [37][58] and SMV- [38][87] pathosystems.
In the current context of climate change, del Toro and colleagues [39][88] recently provided insight into the potential impact of elevated temperature and CO2 concentration in the atmosphere on the spread of phytoviruses. The probability of transmission of phytovirus by a vector reduced as the virus titers in the donor leaves under elevated temperature and CO2 concentration conditions. Finally, chemical insecticides used against phytovirus vectors, notably synthetic chemicals, but also essential oils, would have consequences on the propagation of phytoviruses by acting directly on insect behaviour, or indirectly as an elicitor of the plant’s immune defence. For example, flonicamid and sulfoxaflor, both systemic insecticides, reduced the percentage of probing time spent in the E2 phase of M. persicae exposed to treated Physallis floridana Rydb., but induced a higher percentage of probing time spent in the C and F phases compared to the control [40][48]. Additionally, Vazyl-Y mineral oil spray elicited potato’s immune defence system and significantly reduced the infection rate on treated leaves compared to control [41][89].
