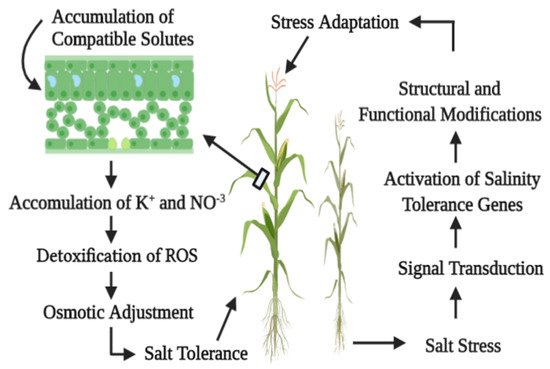
Plants have the ability to sustain their life under a saline environment through synthesis and accumulation of compatible solutes in the cytosol. These are soluble compounds with low molecular mass. These chemical compounds can maintain physiological and biochemical processes, without having interference in these processes. The chief components of compatible solutes are sugar alcohols (mannitol, sorbitol, ononitol), quaternary ammonia compounds (glycine betaine, proline betaine), proline, and tertiary sulfonium compounds. They act to scavenge the reactive oxygen species (ROS) and inhibit lipid peroxidation, hence preventing damage at the cellular level. These compatible solutes act in favor of osmotic adjustment and prevent ROS damage at the cellular level
[137][138][146,147]. These maintain macromolecular conformation in the cytosol, which may be changed due to the accumulation of charged ions, under saline conditions
[8][97][8,97]. These organic compounds are termed as compatible due to their consistency with the cell’s metabolism
[139][148], and their lowering of the water potential without altering cell water contents. These organic compounds are hydrophilic in nature, having the ability to replace water present on protein surfaces
[140][141][149,150], without interfering with their structure and function. These solutes play a key role in preventing the drastic effects of high ion concentration on enzymatic activities
[24][142][143][144][24,151,152,153]. Such important roles of compatible solutes lead to osmoregulation of plant cells under osmotic stress. In addition to osmoregulation, these organic compounds have a distinct role in protein stabilization, maintenance of membrane integrity, protection of OEC of PSII from dissociation
[145][154], and scavenging of reactive oxygen species (ROS). Mannitol, sorbitol, glycerol, proline, ononitol, and pinitol have been reported to scavenge ROS species
[146][155].
Mannitol (sugar alcohol) metabolism in higher plants is a superior attribute, contributing to salt and osmotic stress tolerance while playing a significant role as a compatible solute. It also improves plant responses under biotic stress as well, like under pathogen infestation
[147][156]. Mannitol is reported to be synthesized at the same time with either sucrose or raffinose saccharide. In salt-tolerant species, mannitol accumulation increases, indicating that high mannitol levels contribute to salt tolerance. Mannitol acts to scavenge the reactive oxygen species, thus protecting protein molecules
[148][149][157,158]. Pinitol and ononitol have been reported to accumulate under various stresses, predominantly drought and salt stress
[150][151][159,160]. Interestingly, polyols can be used as a potential biochemical marker for genetically engineered stress resistance plant genotypes
[152][153][161,162].
2.1. Salinity and Proline
Proline is an osmolyte, an amino acid, which is thought to play a significant role in inducing tolerance in plants against stressed conditions
[154][163]. Salt stress can result in elevation in proline levels
[74]. Ethephon, when used with sodium chloride in spinach, also increased proline levels
[155][164]. The importance of proline is highlighted by its existence in bacteria with a relationship to plants experiencing water or salinity stress. High proline levels can serve as a nitrogen source for plants during recovery
[156][165]. The precursor of proline synthesis is glutamate, involving pyrroline carboxylic acid synthetase and pyrroline carboxylic reductase
[157][166]. An increase was noted in the activity of pyrroline-5-carboxylate synthetase (P5CS) and a decline was recorded in proline dehydrogenase activity in potato seedlings under salt stress. These changes of enzymatic activity were more pronounced in salt-sensitive cultivars
[74][158][74,167]. However, an increase in proline contents of potato clones was recorded upon salt exposure
[50][159][50,168]. It serves to stabilize ultra-structural changes in cells, scavenge ROS (reactive oxygen species), and maintain cellular redox potential. Under stress conditions, a higher accumulation of proline is reported in cell cytosol, strengthening the ability of the cell to make ionic adjustments. Its accumulation is linearly related to stress tolerance in plants
[160][169]. Proline biosynthesis is reported to be mediated by Ca
[161][162][163][170,171,172] and abscisic acid
[8]. Previously, contrasting views about proline accumulation were also reported in plants under stress
[19][149][19,158], where it appeared as a salt stress injury symptom, e.g., rice
[164][173] and sorghum
[165][174].
Some plant genotypes do not respond to proline accumulation, but their salt tolerance potential can be enhanced through the exogenous application of proline
[31][166][31,175]. It may be helpful in counteracting the harmful effects through osmo-protection, resulting in a higher growth rate. Proline also increases the activities of antioxidant enzymes like SOD (superoxide dismutase) and POD (peroxidase)
[167][176]. Proline is not reported to scavenge ROS directly, but through enhanced antioxidant enzyme activity. It is reported to be more effective in mitigating the drastic effects of salinity than glycine betaine
[168][177]. Proline used at higher concentrations may prove to be lethal for the plant, causing ultra-structural damages leading to ROS generation
[169][178]. The effective dose of proline varies with genotype and plant developmental stage
[170][171][172][173][179,180,181,182]. Proline accumulation has been reported for drought sensitive and tolerant barley genotypes grown under saline conditions. Under salt stress, a considerable amount of proline was present, with relatively lower quantities in root tissues. Proline accumulation is reported to be more prominent in tolerant genotypes
[174][183].
2.2. Salinity and Polyamines
Polyamines are multivalent compounds consisting of two or more amino groups. In higher plants, these are identified as putrescine, spermidine, and spermine
[138][175][147,184]. These are involved in various physiological mechanisms including rhizogenesis, somatic embryogenesis, maintenance of cell pH and ionic balance
[29], pollen and flower formation, abscission, senescence, and dormancy. Endogenous polyamine synthesis can be stimulated by cytokinin
[176][177][185,186]. These compounds act to stabilize macromolecules like DNA and RNA. Moreover, polyamines have a significant role in numerous abiotic and biotic stresses
[142][178][151,187]. At the cellular level, polyamines contribute to regulating the plasma membrane potential, ionic homeostasis, and tolerance against salinity
[179][180][188,189]. Exogenously applied polyamine or ornithine caused a reduction in proline accumulation in plant tissues under salt stress. However, an alternate trend was observed in the case of non-stressed beans
[99][181][99,190]. Putrescine is characterized as a de-stressor agent and a nitrogen source under stressed conditions
[182][191]. Putrescine has been reported to reverse the biomass reduction in Indian mustard
[68][183][68,192]. Its production in plant cells follows two alternative pathways: conversion from ornithine or arginine. Putrescine is then converted to spermidine and subsequently to spermine by addition of an aminopropyl group. Spermine deficiency caused Ca ion imbalance in
Arabidopsis thaliana, thus indicating spermine as a maintainer of plant cell ionic homeostasis under salt stress
[184][193].
2.3. Salinity and Glycine-Betaine
Glycine-betaine (GB) is present in a wide range of organisms, from bacteria to higher plants and animals. In addition to being involved in osmoregulation, it maintains and regulates the performance of PSII protein complexes by protecting extrinsic regulatory protein against denaturation. It also stabilizes macromolecules, due to its ability to form strong bonds with water
[136]. It protects these macromolecules during drought and thermal stress, which is why it is sometimes called an “osmoprotectant”
[129][185][129,194]. Glycine-betaine accumulates in some crops under stress, like members of family
Poaceae and
Chenopodiaceae [186][195], and is absent entirely from other plants, like rice and tobacco. This directed the scientists to develop transgenic plants that have the ability to produce GB. In transgenic plants, the reproductive organs are capable of tolerating abiotic stresses if they can accumulate GB
[186][195]. The precursor for GB is choline, and the conversion is managed by enzymes like choline monooxygenase and betaine-aldehyde dehydrogenase
[142][187][151,196]. Choline supplementation to the growth media of the salt-stressed plant can act to restore the suppressed growth
[188][197]. GB is water soluble, is not harmful at higher concentrations, and accumulates mainly in plastids and chloroplasts. Exogenous application of GB promotes salinity tolerance in plant species which do not naturally produce GB. A plant can utilize exogenously applied GB via leaves
[189][198], as well as roots
[190][199]. After absorption, GB is translocated in phloem
[99][191][99,200]. GB is not directly involved in scavenging ROS species, but it alleviates the damaging effects of ROS by promoting enzymes responsible for the destruction or production suppression of ROS
[192][201].
The reproductive stage of any plant during GB application is considered critical to ensure maximum yield. In various studies, it was reported that the plant reproductive organs acquire higher levels of GB than the vegetative parts under stressed conditions. This indicates that high GB accumulation is more necessary for protecting the reproductive organs than it is for protecting the vegetative tissues from abiotic stresses, indicating that application timing is key
[172][193][181,202]. The natural GB accumulating species include sugar beets, spinach, wheat, barley, and sorghum. High GB concentration is linearly linked with increased tolerance. Osmotic adjustment is the major mechanism involved in increased tolerance to abiotic stresses, especially salt stress. GB is responsible for turgor maintenance through osmotic adjustment
[54][173][194][54,182,203]. However, this relationship is not satisfactory in some cases like
Triticum spp. and
Agropyron spp.
[195][204]. Thus, this relationship varies with genotype
[149][158]. The plant species which do not produce GB naturally can give a satisfactory yield and survival rate under salt stress conditions through the exogenous application of GB
[42][196][42,205]. Exogenous GB, once applied, is transported rapidly throughout the plant. Exogenous application of GB has been reported in many plant species, including tobacco, rice, soybean, barley, and wheat. In barley, GB application improved stress tolerance by lowering water potential, which improved survivability. GB plays a role in osmotic adjustment and ionic homeostasis by maintaining high K
+ concentration compared to Na
+ ions. Exogenous application of GB also increased the K
+/Na
+ ratio
[197][198][199][206,207,208]. GB also protects the photosynthetic apparatus. It enhances photosynthetic activity through increased stomatal conductance and reduced photorespiration
[42][200][201][42,209,210].
In contrast to its positive influence, some researchers have also suggested neutral or somewhat negative responses to exogenously applied GB in some plant genotypes. For example, it appeared to have a neutral influence on growth in cotton
[202][211], turnip, rapeseed, and tomato
[203][212]. For the commercial application of GB, the rate, duration, timing, and frequency should be considered
[149][204][158,213]. It can be used for seed treatment as well as foliar application. The application method is dependent on the plant material on which it will be applied, the timing of the application relative to plant developmental stage, and environmental conditions during the time of application
[205][214].
Exogenously applied GB improved salt tolerance in rice by improving relative water contents in the leaves and increasing antioxidant levels, including superoxide dismutase, ascorbate peroxidase, catalase, and glutathione reductase (GR)
[204][213]. Reduction in peroxidase activity was reported in a salt-tolerant rice genotype under salt stress. GB is also reported to reduce lipid peroxidation
[206][207][208][215,216,217]. GB can prevent membrane adulterations due to osmotic stress more efficiently than proline
[209][218]. Proline accumulation in leaves of salt-stressed plants is not reported to be correlated with exogenously applied glycine betaine
[54][171][54,180]. Sugar beet is identified as the foremost source of GB
[149][210][158,219]. It is appreciated as a valuable source of GB along with other beneficial compounds and is useful in inducing tolerance against salt stress in eggplant (
Solanum melongena L.) as compared to pure GB. It has a marked influence on the morphological (growth and yield) as well as physiological and biochemical (gas exchange, photosynthetic rate, transpiration, GB accumulation) attributes
[177][211][186,220].
3. Salinity and Phytohormones
Plant hormones are signaling molecules with the ability to alter the physiological mechanisms of the plant, even if present in very minute quantities
[212][248]. Common plant hormones are auxins, gibberellins, cytokinins, abscisic acid, and ethylene
[213][249]. Plants growing under salt stress experience imbalances in hormonal homeostasis. Stressed conditions drastically alter physiological mechanisms of the plant, creating massive changes in endogenous hormonal contents. Higher concentrations of toxic ions are negatively correlated with the levels of plant hormones like gibberellins, auxins, and cytokinin and are positively associated with the abscisic acid level
[214][215][250,251]. Exogenous plant hormone application on salt stressed plants was found to alleviate the negative effects of salinity on the morphological (leaf area, dry mass), physiological (chlorophyll content, stomatal conductance, photosynthetic rate), and yield characteristics of crops
[216][217][252,253].
Abscisic acid (ABA) and ethylene are involved in signaling under stress conditions. An increase in ABA concentration in plant cells has been reported under saline conditions
[218][254]. Carotenoids are the precursor for ABA synthesis, with roots and leaves the sites of synthesis
[219][255]. Water deficit in the root zone causes ABA generation in roots, and ABA transport to shoots is xylem mediated. The increase in pH of xylem sap increases the transport of ABA to the guard cells, where it regulates the stomatal opening and closing through the involvement of Ca ions
[220][256]. Alterations in ABA levels indirectly affect photosynthesis through disruption in stomatal opening and closing. The photosynthetic efficiency of the plant cell declines along with deregulation of translocation and assimilate partitioning of photosynthates
[8][221][8,257].
Exogenously applied plant growth regulators have been widely reported to enhance stress tolerance in numerous plant species
[222][258]. ABA is reported to be useful in alleviating plant salt stress under low water potential. ABA production in the plant cell is also related to ethylene synthesis under salt stress. The interaction between these two stress hormones is apparent from vegetative growth and seed germination under salt stress. During root inhibition by salt stress, ethylene regulates the ABA concentration. However, the reverse has been reported in the case of seed germination
[223][259]. Salicylic acid has displayed a defensive role in plants experiencing stress, signaling the plant to adapt to the stressful environment
[224][260].
Salinity and Growth Regulation
Brassinosteroids (BRs) are growth regulators which mitigate adverse growth patterns caused by salinity. It improves the germination of seeds experiencing salt stress. The improved germination rate has been reported for rice
[225][261] and tobacco. Application of BRs as a seed treatment enhanced the growth of rice seedlings under salt stress
[226][262]. It helps the plant to retain its green pigments and enhances nitrate reductase activity
[227][228][229][263,264,265] and nitrogen-fixing capability. Brassinosteroids (28-homoBL) increased dry matter accumulation and seed yield
[230][266]. Foliar application of brassinosteroids (24-epibrassinolide) on pepper plants grown with saline water greatly affected shoot growth parameters and leaf water contents as compared to roots. However, its effect on chlorophyll fluorescence was non-significant
[231][232][267,268]. Similar patterns of brassinosteroidal effects were observed in wheat grown under salt stress. 24-epibrassinolide application on salt-stressed wheat seedlings exhibited non-significant results in terms of plant biomass, chlorophyll content, photosynthesis rate, substomatal CO
2 concentration, and water use efficiency. The incremented water use efficiency can be related to higher transpiration rate shown by salt-stressed wheat seedlings, as a result of 24-epibrassinolide application
[233][234][269,270]. The efficiency of exogenously applied brassinosteroids to mitigate salinity effects varies with plant species, appropriate growth stage, dose, frequency, and method of brassinosteroidal application
[235][236][237][271,272,273]. The results of BR application also vary with climatic conditions—mainly temperature, light duration, and applied fertilizers
[238][274]. Brassinolide application to salt stressed
Vigna radiata caused enhancement in growth, photosynthetic rate, and maximum quantum yield of PSII. Generally, brassinolide has the potential to protect the photosynthetic apparatus under salt stress. It also contributed to improving the membrane stability index and leaf water potential. However, no significant results were recorded in the case of lipid peroxidation and electrolyte leakage. Brassinolide increases antioxidant enzyme and proline contents
[239][240][241][242][275,276,277,278]. Increases in proline level create a protective shield when the plant is under stress by acting as a source of carbon and nitrogen, a stabilizer of the plasma membrane, and an oxygen radical scavenger
[243][279]. Brassinosteroids also increase pigment levels in the plant
[244][280]. BRs also improve the nitrate and nitrite reductase activity in
Vigna radiata under salt stress. This effect can be attributed to the ability of BRs to modulate transcription and translation at the gene level, and to increase cell nitrate uptake. Increased stress tolerance caused by brassinolide application is observable as improved growth parameters such as shoot length, root length, and plant biomass
[106][245][106,281].