Taxol is one of the most effective anticancer drugs in the world that is widely used in the treatment of several cancers. The elucidation of the taxol biosynthetic pathway is the key to solve the problem of taxol supply. So far, the taxol biosynthetic pathway has been reported to require an estimated 20 steps of enzymatic reactions, and most of enzymes involved have been well characterized. In details, the source and formation of the taxane core and the process of the downstream synthetic pathway have been basically depicted, while the modification of the core taxane skeleton has not been fully reported, mainly concerning the developments from diol intermediates to 2-debenzoyltaxane.
- taxol
- biosynthetic pathway
- latest research progress
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
2. Taxol Biosynthetic Pathway
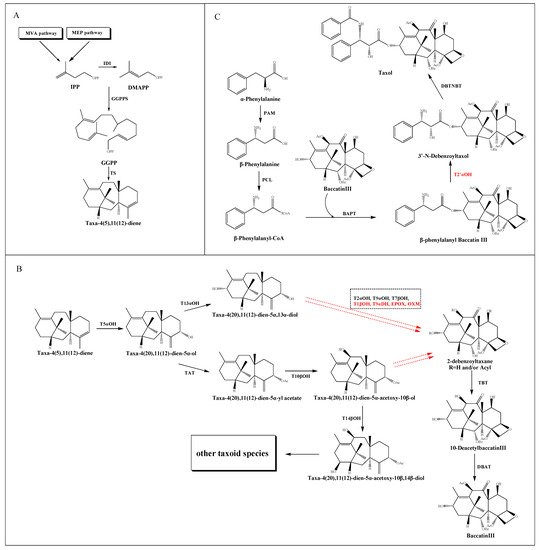
2.1. The Source and Formation of Taxane Core
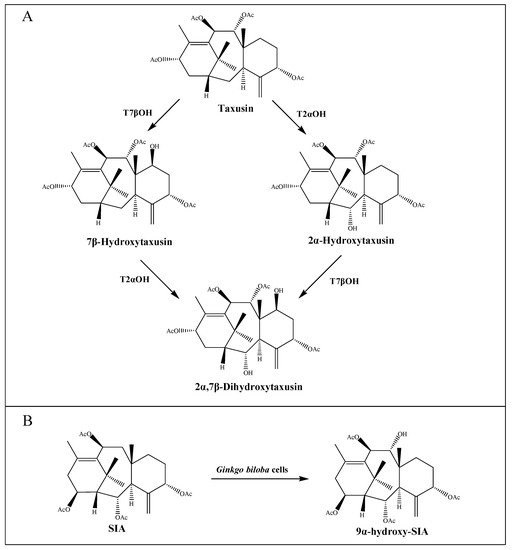
2.2. The Modification of the Core Taxane Skeleton
2.3. The Synthesis of β-Phenylalanyl-CoA Side Chain and the Assembly of Taxol
2.4. Key Enzymes Involved in the Taxol Pathway
| Enzyme Abbreviation | Size (kDa) | Probable Localization | GenBank Number |
|---|
| Geranylgeranyl diphosphate synthase GGPPS | 42 | Plastids | AF081514 | ||
| Taxadiene synthase TS | 98 | Plastids | AY364469 | ||
| Taxadiene-5α-hydroxylase T5αOH | 56 | Endoplasmic reticulum | AY289209 | ||
| Taxane-10β-hydroxylase T10βOH | 56 | Endoplasmic reticulum | AF318211 | ||
| Taxane-10β-hydroxylase | a | T10βOH | 55 | Endoplasmic reticulum | AY563635 |
| Taxadiene-13α-hydroxylase T13αOH | 54 | Endoplasmic reticulum | AY056019 | ||
| Taxane-2α-hydroxylase T2αOH | 55 | Endoplasmic reticulum | AY518383 | ||
| Taxane-9α-hydroxylase T9αOH | 55 | Endoplasmic reticulum | KF773141 | ||
| Taxane-7β-hydroxylase T7βOH | 56 | Endoplasmic reticulum | AY307951 | ||
| Taxadiene-5α-ol- | O | -acetyl transferase TAT | 49 | Cytosol | AF190130 |
| Taxane-2α- | O | -benzoyl transferase TBT | 50 | Cytosol | AF297618 |
| 10-deacetylbaccatin III-10- | O | acetyl transferase DBAT | 49 | Cytosol | AF193765 |
| Baccatin III: 3-amino, 13-phenylpropanoyltransferase BAPT | 50 | Cytosol | AY082804 | ||
| N | -benzoyl transferase DBTNBT | 49 | Cytosol | AF466397 | |
| Phenylalanine aminomutase PAM | 76 | Cytosol | AY582743 | ||
| β-phenylalanyl-CoA ligase | b | PCL | 59 | Cytosol | KM593667 |
References
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327.
- Wani, M.C.; Horwitz, S.B. Nature as a remarkable chemist: A personal story of the discovery and development of taxol. Anti-Cancer Drug. 2014, 25, 482–487.
- Howat, S.; Park, B.; Oh, I.S.; Jin, Y.; Lee, E.; Loake, G.J. Paclitaxel: Biosynthesis, production and future prospects. New Biotechnol. 2014, 31, 242–245.
- Priyadarshini, K.; Keerthi, A.U. Paclitaxel against cancer: A short review. Med. Chem. 2012, 2, 139–141.
- Holton, R.A.; Somoza, C.; Kim, H.B.; Liang, F.; Biediger, R.J.; Boatman, P.D.; Shindo, M.; Smith, C.C.; Kim, S. First total synthesis of taxol. 1. Functionalization of the B ring. J. Am. Chem. Soc. 1994, 116, 1597–1598.
- Nicolaou, K.C.; Yang, Z.; Liu, J.J.; Ueno, H.; Nantermet, P.G.; Guy, R.K.; Claiborne, C.F.; Renaud, J.; Couladouros, E.A.; Paulvannan, K.; et al. Total synthesis of taxol. Nature 1994, 367, 630–634.
- Danishefsky, S.J.; Masters, J.J.; Young, W.B.; Link, J.T.; Snyder, L.B.; Magee, T.V.; Jung, D.K.; Isaacs, R.C.A.; Bornmann, W.G.; Alaimo, C.A.; et al. Total synthesis of baccatin III and Taxol. J. Am. Chem. Soc. 1996, 118, 2843–2859.
- Fu, Y.; Li, S.; Zu, Y.; Yang, G.; Yang, Z.; Luo, M.; Jiang, S.; Wink, M.; Efferth, T. Medicinal chemistry of paclitaxel and its analogues. Curr. Med. Chem. 2009, 16, 3966–3985.
- Shao, F.; Wilson, I.; Qiu, D. The research progress of taxol in Taxus. Curr. Pharm. Biotechnol. 2021, 22, 360–366.
- Liu, W.C.; Gong, T.; Zhu, P. Advances in exploring alternative Taxol sources. RSC Adv. 2016, 6, 48800–48809.
- Wang, T.; Chen, Y.; Zhuang, W.; Zhang, F.; Shu, X.; Wang, Z.; Yang, Q. Transcriptome sequencing reveals regulatory mechanisms of taxol synthesis in Taxus wallichiana var. mairei. Int. J. Genom. 2019, 2019, 1596895.
- Gond, S.K.; Kharwar, R.N.; White, J.F., Jr. Will fungi be the new source of the blockbuster drug taxol? Fungal Biol. Rev. 2014, 28, 77–84.
- Ajikumar, P.K.; Xiao, W.H.; Tyo, K.E.J.; Wang, Y.; Simeon, F.; Leonard, E.; Mucha, O.; Phon, T.H.; Pfeifer, B.; Stephanopoulos, G. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 2010, 330, 70–74.
- Zhou, K.; Qiao, K.; Edgar, S.; Stephanopoulos, G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015, 33, 377–383.
- Besumbes, Ó.; Sauret-Güeto, S.; Phillips, M.A.; Imperial, S.; Rodríguez-Concepción, M.; Boronat, A. Metabolic engineering of isoprenoid biosynthesis in Arabidopsis for the production of taxadiene, the first committed precursor of Taxol. Biotechnol. Bioeng. 2004, 88, 168–175.
- Kovacs, K.; Zhang, L.; Linforth, R.T.; Whittaker, B.; Hayes, C.; Fray, R. Redirection of carotenoid metabolism for the efficient production of taxadiene [taxa-4(5),11(12)-diene] in transgenic tomato fruit. Transgen. Res. 2007, 16, 121–126.
- Anterola, A.; Shanle, E.; Perroud, P.; Quatrano, R. Production of taxa-4(5),11(12)-diene by transgenic Physcomitrella patens. Transgen. Res. 2009, 18, 655–660.
- Li, J.; Mutanda, I.; Wang, K.; Yang, L.; Wang, J.; Wang, Y. Chloroplastic metabolic engineering coupled with isoprenoid pool enhancement for committed taxanes biosynthesis in Nicotiana benthamiana. Nat. Commun. 2019, 10, 1–12.
- Wildung, M.R.; Croteau, R. A cDNA clone for taxadiene synthase, the diterpene cyclase that catalyzes the committed step of taxol biosynthesis. J. Biol. Chem. 1996, 271, 9201–9204.
- Hefner, J.; Ketchum, R.E.; Croteau, R. Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase from Taxus canadensis and assessment of the role of this prenyltransferase in cells induced for taxol production. Arch. Biochem. Biophys. 1998, 360, 62–74.
- Jarchow-Choy, S.K.; Koppisch, A.T.; Fox, D.T. Synthetic routes to methylerythritol phosphate pathway intermediates and downstream isoprenoids. Curr. Org. Chem. 2014, 18, 1050–1072.
- Roberts, S.C. Production and engineering of terpenoids in plant cell culture. Nat. Chem. Biol. 2007, 3, 387.
- Malik, S.; Cusidó, R.M.; Mirjalili, M.H.; Moyano, E.; Palazón, J.; Bonfill, M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process. Biochem. 2011, 46, 23.
- Kuang, X.J.; Sun, S.J.; Wei, J.H.; Li, Y.; Sun, C. Iso-Seq analysis of the Taxus cuspidata transcriptome reveals the complexity of Taxol biosynthesis. BMC Plant Biol. 2019, 19, 210.
- Jennewein, S.; Wildung, M.R.; Chau, M.; Walker, K.; Croteau, R. Random sequencing of an induced Taxus cell cDNA library for identification of clones involved in taxol biosynthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 9149–9154.
- Jennewein, S.; Rithner, C.D.; Williams, R.M.; Croteau, R.B. Taxol biosynthesis: Taxane 13 alpha-hydroxylase is a cytochrome p450-dependent monooxygenase. Proc. Natl. Acad. Sci. USA 2001, 98, 13595–13600.
- Walker, K.; Schoendorf, A.; Croteau, R. Molecular cloning of a taxa-4 (20), 11 (12)-dien-5α-ol-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Arch. Biochem. Biophys. 2000, 374, 371–380.
- Schoendorf, A.; Rithner, C.D.; Williams, R.M.; Croteau, R.B. Molecular cloning of a cytochrome P450 taxane 10 beta-hydroxylase cDNA from Taxus and functional expression in yeast. Proc. Natl. Acad. Sci. USA 2001, 98, 1501–1506.
- Jennewein, S.; Rithner, C.D.; Williams, R.M.; Croteau, R. Taxoid metabolism: Taxoid 14beta-hydroxylase is a cytochrome P450-dependent monooxygenase. Arch. Biochem. Biophys. 2003, 413, 262.
- Holton, R.A.; Kim, H.B.; Somoza, C.; Liang, F.; Biediger, R.J.; Boatman, P.D.; Shindo, M.; Smith, C.C.; Kim, S.C.; Nadizadeh, H.; et al. First total synthesis of taxol. 2. Completion of the C-ring and D-ring. J. Am. Chem. Soc. 1994, 116, 1599–1600.
- Vongpaseuth, K.; Roberts, S.C. Advancements in the understanding of paclitaxel metabolism in tissue culture. Curr. Pharm. Biotechnol. 2007, 8, 219–236.
- Walker, K.; Croteau, R. Taxol biosynthesis: Molecular cloning of a benzoyl-CoA: Taxane 2α-O-benzoyltransferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 13591–13596.
- Walker, K.; Croteau, R.M. Molecular cloning of a 10-deacetylbaccatin Ⅲ-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc. Natl. Acad. Sci. USA 2000, 97, 583–587.
- Walker, K.D.; Klettke, K.; Akiyama, T.; Croteau, R. Cloning, heterologous expression, and characterization of a phenylalanine aminomutase involved in Taxol biosynthesis. J. Biol. Chem. 2004, 279, 53947–53954.
- Ramírez-Estrada, K.; Altabella, T.; Onrubia, M.; Moyano, E.; Notredame, C.; Osuna, L.; VandenBossche, R.; Goossens, A.; Cusido, R.; Palazon, J. Transcript profiling of jasmonate-elicited Taxus cells reveals a β-phenylalanine-CoA ligase. Plant Biotechnol. J. 2016, 14, 85–96.
- Walker, K.; Fujisaki, S.; Long, R.; Croteau, R. Molecular cloning and heterologous expression of the C-13 phenylpropanoid side chain-CoA acyltransferase that functions in taxol biosynthesis. Proc. Natl. Acad. Sci. USA 2002, 99, 12715–12720.
- Walker, K.; Long, R.; Croteau, R. The final acylation step in taxol biosynthesis: Cloning of the taxoid C13-side-chain N-benzoyltransferase from Taxus. Proc. Natl. Acad. Sci. USA 2002, 99, 9166.
- Chau, M.; Croteau, R. Molecular cloning and characterization of a cytochrome P450 taxoid 2alpha-hydroxylase involved in Taxol biosynthesis. Arch. Biochem. Biophys. 2004, 427, 48–57.
- Chau, M.; Jennewein, S.; Walker, K.; Croteau, R. Taxol biosynthesis: Molecular cloning and characterization of a cytochrome P450 taxoid 7 β-hydroxylase. Chem. Biol. 2004, 11, 663–672.
- Zhang, N.; Han, Z.T.; Sun, G.L.; Hoffman, A.; Wilson, I.W.; Yang, Y.F.; Gao, Q.; Wu, J.Q.; Xie, D.; Dai, J.G.; et al. Molecular cloning and characterization of a cytochrome P450 taxoid 9α-hydroxylase in Ginkgo biloba cells. Biochem. Bioph. Res. Commun. 2014, 443, 938–943.
- McElroy, C.; Jennewein, S. Taxol biosynthesis and production: From forests to fermenters. In Biotechnology of Natural Products; Schwab, W., Lange, B., Wüst, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 145–185.
- Hao, D.C.; Ge, G.B.; Xiao, P.G.; Zhang, Y.Y.; Ling, Y.; Hans, E. The first insight into the tissue specific Taxus transcriptome via Illumina second generation sequencing. PLoS ONE 2011, 6, e21220.
- He, C.T.; Li, Z.L.; Zhou, Q.; Shen, C.; Huang, Y.Y.; Mubeen, S.; Yang, J.Z.; Yuan, J.G.; Yang, Z.Y. Transcriptome profiling reveals specific patterns of paclitaxel synthesis in a new Taxus yunnanensis cultivar. Plant Physiol. Biochem. 2018, 122, 10–18.
- Mubeen, S.; Li, Z.L.; Huang, Q.M.; He, C.T.; Yang, Z.Y. Comparative transcriptome analysis revealed the tissue-specific accumulations of taxanes among three experimental lines of Taxus yunnanensis. J. Agric. Food. Chem. 2018, 66, 10410–10420.
- Yu, C.N.; Guo, H.; Zhang, Y.Y.; Song, Y.B.; Pi, E.; Yu, C.L.; Zhang, L.; Dong, M.; Zheng, B.S.; Wang, H.Z.; et al. Identification of potential genes that contributed to the variation in the taxoid contents between two Taxus species (Taxus media and Taxus mairei). Tree Physiol. 2017, 37, 1659–1671.
- Zhou, T.; Luo, X.J.; Yu, C.N.; Zhang, C.C.; Zhang, L.; Song, Y.B.; Dong, M.; Shen, C.J. Transcriptome analyses provide insights into the expression pattern and sequence similarity of several taxol biosynthesis related genes in three Taxus species. BMC Plant Biol. 2019, 19, 33.
- Hao, J.; Guo, H.; Shi, X.A.; Wang, Y.; Wan, Q.H.; Song, Y.B.; Zhang, L.; Dong, M.; Shen, C.J. Comparative proteomic analyses of two Taxus species (Taxus × media and Taxus mairei) reveals variations in the metabolisms associated with paclitaxel and other metabolites. Plant Cell Physiol. 2017, 58, 1878–1890.
- Yu, C.N.; Luo, X.J.; Zhan, X.R.; Hao, J.; Zhang, L.; Song, Y.B.; Shen, C.J.; Dong, M. Comparative metabolomics reveals the metabolic variations between two endangered Taxus species (T. fuana and T. yunnanensis) in the Himalayas. BMC Plant Biol. 2018, 18, 197.
- Yu, C.N.; Zhang, C.C.; Xu, X.Y.; Huang, J.F.; Chen, Y.Y.; Luo, X.J.; Wang, H.Z.; Shen, C.J. Omic analysis of the endangered Taxaceae species Pseudotaxus chienii revealed the differences in taxol biosynthesis pathway between Pseudotaxus and Taxus yunnanensis trees. BMC Plant Biol. 2021, 21, 104.
- Li, S.T.; Zhang, P.; Zhang, M.; Fu, C.H.; Zhao, C.F.; Dong, Y.S.; Guo, A.Y.; Yu, L.J. Transcriptional profile of Taxus chinensis cells in response to methyl jasmonate. BMC Genom. 2012, 13, 295.
- Sun, G.; Yang, Y.; Xie, F.; Wen, J.-F.; Wu, J.; Wilson, I.W.; Liu, H.; Qiu, D. Deep sequencing reveals transcriptome re-programming of Taxus× media cells to the elicitation with methyl jasmonate. PLoS ONE 2013, 8, e62865.
- Jiang, L.Y.; Zhang, K.K.; Lü, X.; Yang, L.Y.; Wang, S.; Chen, D.F.; Yang, Y.F.; Qiu, D.Y. Characterization and expression analysis of genes encoding Taxol biosynthetic enzymes in Taxus spp. J. For. Res. 2021.
- Zhang, M.; Li, S.; Nie, L.; Chen, Q.; Xu, X.; Yu, L.; Fu, C. Two jasmonate-responsive factors, TcERF12 and TcERF15, respectively act as repressor and activator of tasy gene of taxol biosynthesis in Taxus chinensis. Plant Mol. Biol. 2015, 89, 463–473.
- Zhang, M.; Jin, X.; Chen, Y.; Wei, M.; Liao, W.; Zhao, S.; Fu, C.; Yu, L. TcMYC2a, a basic helix–loop–helix transcription factor, transduces JA-signals and regulates taxol biosynthesis in Taxus chinensis. Front. Plant Sci. 2018, 9, 863.
- Li, S.; Zhang, P.; Zhang, M.; Fu, C.; Yu, L. Functional analysis of a WRKY transcription factor involved in transcriptional activation of the DBAT gene in Taxus chinensis. Plant Biol. 2012, 15, 19–26.
- Yu, C.N.; Luo, X.J.; Zhang, C.C.; Xu, X.Y.; Huang, J.F.; Chen, Y.Y.; Feng, S.G.; Zhan, X.R.; Zhang, L.; Yuan, H.W.; et al. Tissue-specific study across the stem of Taxus media identifies a phloem-specific TmMYB3 involved in the transcriptional regulation of paclitaxel biosynthesis. Plant J. 2020, 103, 95–110.
- Hu, X.L.; Zhang, L.S.; Wilson, I.; Shao, F.J.; Qiu, D.Y. The R2R3-MYB transcription factor family in Taxus chinensis: Identification, characterization, expression profiling and posttranscriptional regulation analysis. PeerJ 2020, 8, e8473.
