Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract that consists of Crohn’s disease (CD) and ulcerative colitis (UC). Cytokines are thought to be key mediators of inflammation-mediated pathological processes of IBD. These cytokines play a crucial role through the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) signaling pathways. Several small molecules inhibiting JAK have been used in clinical trials, and one of them has been approved for IBD treatment. Many anti-inflammatory phytochemicals have been shown to have potential as new drugs for IBD treatment.
- inflammatory bowel disease (IBD)
- janus kinase (JAK)
- phytochemicals
1. Background
Plants’ phytochemicals have been used as a source of traditional medicine for millennia [1]. A large portion of current drugs for disease treatment have originated from plants, even though new drugs have been developed using synthetic chemistry [2]. Recently, the significance of phytochemicals has been emphasized for therapeutic applications with fewer side effects in various inflammation-related diseases, including cancer, diabetes, rheumatoid arthritis, and inflammatory bowel disease (IBD) [3][4]. Phytochemicals are good sources of new anti-inflammatory drugs that regulate various inflammatory responses against inflammatory diseases [5].
Inflammatory bowel disease is a typical chronic inflammation-mediated disease of the gastrointestinal (GI) tract [6]. IBD is typically classified into two major forms of chronic and relapsing-remitting inflammation: Crohn’s disease (CD) and ulcerative colitis (UC), which is increasing in incidence and prevalence worldwide [7]. Although both diseases have common clinical features, such as diarrhea and abdominal pain, they differ in various aspects. Crohn’s disease is characterized by discontinuous inflammation at any location in the digestive tract, from the mouth to the anus, which can spread into the deeper layers of the bowel [8]. On the other hand, ulcerative colitis is a long-standing chronic inflammation of the colonic mucosa, which affects certain parts or the entire colon, and is most commonly limited to the mucosal surface [9]. Despite the fact that CD and UC are distinct entities, the precise causes of disease pathogenesis remain largely unknown.
The significance of treatment and prevention of IBD has been steadily increasing [10]. Although many drugs have been developed to treat IBD, these drugs have adverse effects on the GI tract [11]. In addition to conventional drugs, there have been important advances in IBD therapy targeting cytokines that have been well documented to play a key role in both the chronic and relapsing phases of IBD [12]. For example, tumor necrosis factor (TNF) inhibitors have been progressively used as therapies for IBD. However, the development of new drugs has been emphasized because unresponsive patients or patients who have lost response to anti-TNF therapy are growing, and a wide array of cytokines besides TNF are involved in the pathogenesis of IBD [13][14][15]. Currently, inhibitors of JAK and STAT that prevent multiple pro-inflammatory cytokine signaling pathways in IBD have been considered as new therapeutic approaches [16][17]. Most cytokines in IBD play crucial roles in chronic inflammatory responses by activating the JAK–STAT pathways (
) [18]. Binding of cytokines to their respective transmembrane receptors promotes the activation of JAK, which allows the translocation of STATs to the nucleus. Eventually, they regulate the transcription of specific target genes [19]. Accumulating evidence supports the idea that therapeutic intervention of the JAK–STAT pathway can efficiently modulate the complex inflammation driven by various cytokines in IBD [20]. In particular, tofacitinib, a JAK inhibitor, has already been approved and is clinically used for UC patients, and many other inhibitors are now being studied in preclinical and clinical trial phases [17]. JAK inhibitors are small molecules that block JAK activity, which transduces signals from cytokine-receptors to STAT [21]. In addition, direct inhibitors of STAT have been studied in preclinical models of IBD, even though no clinical trials have been undertaken for IBD patients [22]. Compared to conventional treatments, targeting the JAK–STAT pathway is considered a new therapeutic strategy for IBD patients [20].
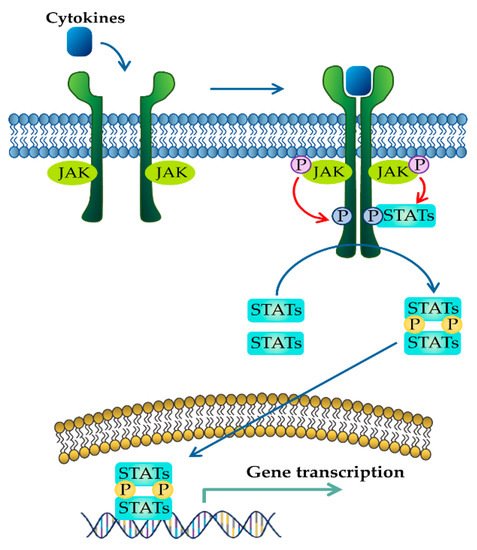
JAK–STAT signaling pathway activated in response to cytokines. Binding of cytokines to their cognate receptors triggers the phosphorylation of JAK and its receptors. After that, recruited STAT is phosphorylated and translocated as homo- or heterodimers to the nucleus, where they upregulate the transcription of cytokine-responsive genes.
Anti-inflammatory phytochemicals are known to have anti-IBD activity by modulating the production of inflammatory cytokines [23]. To investigate the function of phytochemicals targeting JAK–STAT pathways in IBD, we searched for articles in PubMed with three main key words (phytochemical, IBD, and anti-inflammation). In addition, we limited phytochemicals that regulated JAK–STAT pathways in in vivo animal model systems. Numerous studies have shown that phytochemicals, including phenolic compounds, terpenoids, alkaloids, and organosulfur compounds, could be used as therapeutic agents as they modulate cellular inflammatory mechanisms associated with IBD [24]. In particular, some phytochemicals have been reported to inhibit activation of the JAK–STAT pathway [25]. Considering the advantages of phytochemicals with few side effects, phytochemicals targeting the JAK–STAT pathways would be a good source of new drugs for the treatment of IBD [26].
2. JAK–STAT Signaling Pathway in IBD
The JAK–STAT pathway is a well-conserved signaling pathway and is involved in many cellular processes, including cell division, cell death, and regulatory immune function [27]. The JAK–STAT pathway plays a pathogenic role in many diseases, and its hyperactivation is associated with inflammatory and autoimmune diseases such as rheumatoid arthritis, IBD, systemic lupus erythematosus, and psoriasis [28]. Although the etiology of IBD remains unknown, mucosal immune and non-immune cells in the inflamed gut of IBD patients spontaneously release pro-inflammatory cytokines such as TNF-α, interferon gamma (IFN-γ), interleukin (IL) 1 beta (IL-1β), IL-6, IL-8, and IL-12, which play a central pathologic role in IBD [29]. These extracellular cytokines in IBD modulate inflammatory responses by activating the JAK–STAT pathway. The binding of cytokines to their cognate receptors triggers the conformational change of the receptors that alter the position of the associated JAK, resulting in phosphorylation of JAK and tyrosine residues on cytokine receptors [30]. Phosphorylated tyrosine residues on cytokine receptors serve as binding sites for STATs, and the recruitment of STAT to the receptor induces the phosphorylation of STAT by JAK [31]. Ultimately, phosphorylated STATs dissociate from their receptor docking sites and form homo- or heterodimers. After that, they translocate from the cytoplasm into the nucleus, where they regulate the transcription of cytokine-responsive genes (
) [19]. The JAK–STAT pathway is a significant intracellular downstream signaling mediator used by various inflammatory cytokines that are increased in IBD. Thus, inhibitors targeting the JAK–STAT pathway have the advantage of suppressing multiple cytokine pathways in the treatment of IBD.
2.1. JAK Family of Proteins and JAK Inhibitors
The Janus kinase (JAK) protein family is a non-receptor protein tyrosine kinase family that includes four proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2 (TYK2). JAK3 is predominantly found in hematopoietic cells, whereas the expression of JAK1, JAK2, and TYK2 is not restricted to specific tissues [32]. JAK is a critical intracellular signaling mediator that transduces signals from cell surface cytokine receptors to the nucleus in IBD [33]. JAK dysregulation can result in pathological processes in IBD [34]. In addition, various cytokines have been widely accepted as crucial inflammatory mediators leading to immunological events in IBD patients [16]. In this regard, we focus on specific cytokines that have been reported to play a key role in IBD pathogenesis and discuss the JAKs activated by these cytokines.
JAK differentially associates with diverse cytokine receptors activated by various cytokines and activates different types of STAT members [19]. In other words, the JAK protein functions as a transmitter between cytokine receptors and STATs in multiple combinations, which allows the generation of specific responses to many different cytokines [35]. Each JAK protein associates with different subunits of cytokine receptors facilitating multiple combinations with different JAK proteins, which exhibit intracellular complexity of IBD [33][36]. Depending on the activated signaling from specific cytokines to their cognate receptors, the pairing of JAK is determined (
). Binding of IL-2 family cytokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21) to the type I receptor common γ-chain (γc) activates JAK1 and JAK3 [37][38]. Granulocyte-macrophage colony-stimulating factor (GM-CSF), which has been reported to be increased in the serum of CD patients, binds to the type I receptor β-chain and is mediated through JAK2 [39][40]. IL-6 has been reported to have a direct correlation with disease activity in IBD [41]. Binding of IL-6 to type I receptor common glycoprotein 130 (gp130) primarily activates JAK1 and TYK2, followed by JAK2 and TYK2 [42][43]. IL-12 and IL-23 signal through the IL-12 receptor leads to the activation of JAK2 and TYK2 [44][45]. IL-10 and IL-22 bind to type II cytokine receptors, which activate JAK1 and TYK2 [28]. Signaling between IFN-γ and IFN-γ receptor requires JAK1 and JAK2 [46].
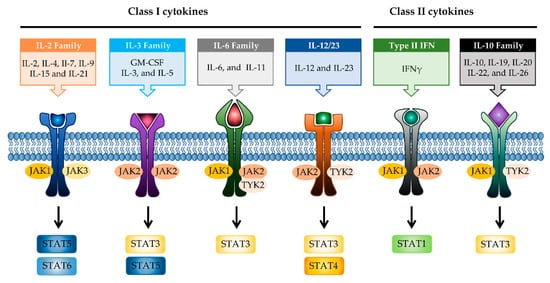
JAK-STAT signaling pathways in IBD. Multiple combinations with JAK proteins and STAT proteins are determined depending on the cytokines and their cognate receptors. Each cytokine family playing a key role in IBD pathogenesis is divided into two classes. JAK, janus kinase; STAT, signal transducer activator and activation of transcription; IL, interleukin; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon.
Various inflammatory cytokines are linked to the pathological effects of IBD through JAK proteins [47]. Thus, therapy inhibiting JAK may block multiple proinflammatory cytokine signaling pathways in IBD, unlike therapy targeting cytokines or cytokine receptors. Currently, several JAK inhibitors are being evaluated for the treatment of IBD patients, and one of them, tofacitinib, has already been approved for active UC patients [48] (
). Tofacitinib is a strong selective inhibitor of JAK3 and JAK1 and has modest selectivity for JAK2 and TYK2 [49][50]. It can mainly block proinflammatory cytokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21) by inhibiting JAK1/3 and modulating other cytokines that use JAK2 and TYK2. Other JAK inhibitors are being developed in clinical trials, including peficitinib and TD-1473 as pan-JAK inhibitors [51][52], and filgotinib and upadacitinib with selectivity for JAK1 [53][54]. Although these JAK inhibitors target specific JAKs, higher doses could lead to off-target binding or immunosuppressive adverse effects [55]. Therefore, we could alternatively consider phytochemicals, which can modulate JAK pathways in IBD, with fewer side effects than synthetic chemical drugs for the management of adverse processes related to JAK inhibition.
2.2. STAT Family of Proteins and STAT Inhibitors
The signal transducer and activator of transcription (STAT) family, which is a critical transcription factor that mediates cytokine-driven signaling, has been actively investigated in IBD pathology [56]. The STAT protein family is composed of seven proteins: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 [57]. Increased expression and activation of STAT1 has been reported in active IBD patients [56][58]. However, the function of STAT1 depends on the cell type in IBD; it is pro-inflammatory in lymphocytes and anti-inflammatory in macrophages/intestinal epithelial cells [59][60]. The phosphorylation of STAT1 is mediated by JAK1/JAK2 or JAK1/TYK2 and has fundamental relevance to signaling via the IFN-γ and related family of receptors [46][61]. STAT1 is also known to be activated by gp130 and γC family receptors [62].
STAT2 is mostly involved in type I interferon (IFN-α and IFN-β) [63]. Although STAT2 has been less studied in IBD pathology compared to other STAT proteins, STAT2 has been suggested to be downregulated in IBD [56].
STAT3 has been well studied to have a fundamental role in IBD. STAT3 is phosphorylated by JAK1, JAK2, or TYK2 activated via signaling of the gp130 family of cytokines (IL-6 and IL-11) or IL-10 family members such as IL-10 and IL-22 [64]. Several studies have reported that the expression and phosphorylation of STAT3 are increased in IBD [65][66]. In addition, downregulation of STAT3 has been shown to improve disease severity in a murine model of colitis [67][68]. The IL-6-STAT3 signaling is involved in the proliferation of lamina propria T cells and blocking of these attenuates chronic intestinal inflammation in experimental colitis [69]. However, similar to STAT1, STAT3 is known to play a role in both pro- and anti-inflammatory effects. STAT3, which is activated by cytokines such as IL-22 and IL-10, plays a protective role in IBD. IL-22 has been reported to induce wound healing, resulting in epithelial regeneration [70][71][72]. STAT3 phosphorylation by IL-10, which is produced in a wide range of innate leukocytes (macrophages, neutrophils, and dendritic cells), might play a role in preventing the disease in experimental colitis models [73][74]. Taken together, STAT3 promotes pro-inflammatory signals in acquired immune cells in IBD, whereas its role in innate immune cells is the suppression of colitis by enhancing mucosal protection.
STAT4 is phosphorylated by JAK2 and TYK2 in response to IL-12- or IL-23-dependent signaling [44][75]. STAT4 is thought to be linked to IBD based on its essential role in the function of T helper type 1 (Th1) cells, which are thought to be important for CD pathogenesis [76]. STAT4 signaling in response to IL-12 is involved in promoting inflammatory reactions by inducing the expression of the Th1-secreted cytokine, IFN-γ [77]. Increased expression of STAT4 in IBD patients has been shown to be involved in chronic inflammation [78][79]. Indeed, STAT4 knock out mice showed protective effects against experimental colitis [80][81]. Thus, targeting STAT4 may have therapeutic potential against IBD.
STAT5 is predominantly activated through JAK1 and JAK3 in response to the γC family of receptors by IL-2, -7, -9, -15, and -21, and is also activated by JAK2 in response to the type I receptor β-chain by the IL-3 family [38][82]. Several studies have shown that STAT5 plays a protective role in colitis. As a protective mechanism, STAT5 has been shown to be essential for the proliferation of intestinal epithelial stem cells, leading to the regeneration of crypt epithelium [83]. STAT5 also plays a crucial role in IL-2 dependent forkhead box P3 (FOXP3) induction in Treg cells that can prevent intestinal inflammation in experimental colitis [84][85]. Thus, STAT5 may not be an appropriate therapeutic target for the treatment of IBD.
STAT6 phosphorylation arises from JAK1 and JAK3, similar to STAT5; however, it is only induced by the γC family of receptors such as IL-4R and IL-13R [62]. STAT6 has been shown to be involved in T helper cell type 2 (Th2)-dependent IBD pathology and to have pro-inflammatory properties via the regulation of Th2 cytokines [86][87]. The phosphorylation of STAT6 was observably increased in the tissue of UC patients [88][89].
As shown above, STAT proteins could be attractive targets for the regulation of intestinal inflammation in addition to JAK for the treatment of IBD. Indeed, direct inhibitors that block STAT proteins have long been studied for treating inflammatory and autoimmune diseases, including IBD [90]. Small molecule compounds inhibiting STAT1 signaling have been shown to improve disease in experimental colitis by selective sequestering of STAT1 from the receptor [91]. STAT3 inhibitors reduce DNA binding of STAT3, thus blocking cell transformation [92]. In particular, drug discovery targeting STAT3 has been extensively undertaken in various diseases, and a large amount of evidence has supported the therapeutic potential of STAT3 inhibitors [93][94][95][96]. Nevertheless, to date, there have been no direct STAT inhibitors in clinics for the treatment of IBD. It has been reported that C188-9 has preventive effects in murine IBD models [22] (
). Thus, the identification and development of phytochemicals targeting STAT proteins could have a significant role in the development of therapeutic drugs for IBD.
Targeting the JAK–STAT pathway for IBD treatment.
| Compound | Target | Preclinical/Clinical Model | Dose/Daily | Ref. |
|---|
| JAK inhibitor | Tofacitinib | JAK1, JAK3 | Approved | 10, 20 mg | [97] | |||||||
| [ | 106 | ] | [ | 108 | ] | Filgotinib | JAK1 | PhaseII, III | 200 mg | [98] | ||
EGCG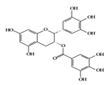 |
DSS-induced | 50, 100 | 4.0, 8.1 | STAT3 | green tea | [115] | Upadacitinib | |||||
| JAK1 | PhaseIII | Ellagic acid 24 mg |
 |
DSS-induced[99] | ||||||||
| 100 | 8.1 | STAT3 | Pomegranate ( | Punica granatum | L., | Lythraceae) | [120] | Peficitinib | JAK1, JAK2, JAK3, TYK2 | PhaseII | 25, 75, 150 mg | [100] |
Gallic acid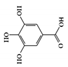 |
DSS-induced | TD-1473 | JAK1, JAK2, JAK3 | PhaseII, III | 20, 80, 270 mg | [52] | ||||||
| STAT inhibitor | C188-9 | STAT3 | DSS- or TNBS induced IBDmurine model | Not designated | [22] |
3. Phytochemicals Targeting the JAK–STAT Pathway
Recently, phytochemicals have been highlighted as alternative/potent candidates for the management of IBD. Many studies have reported that plant-derived natural compounds are considered to have protective and therapeutic effects as dietary supplements for IBD [24]. It has also been suggested that phytochemicals can improve the intestinal barrier through various action mechanisms, including cytokine regulation and reduction of oxidative stress [25][101]. So far, it has been mainly focused on the ability of phytochemicals to downregulate the production of cytokines in IBD [23]. As discussed in the previous section, inhibition of JAK–STAT pathways prevents multiple pro-inflammatory cytokine signaling pathways, which can be considered a new therapy in IBD. Herein, we discuss the present evidence that phytochemicals could induce IBD remission by affecting the JAK–STAT pathway in animal model systems of IBD (
). In addition, we analyzed human-relevant equivalent doses of each phytochemicals modulating JAK–STAT pathways, which suggests that these phytochemicals could be considered as candidates for new JAK–STAT inhibitors and have the potential for combinatorial use with current JAK inhibitors or other therapeutic drugs in IBD.
3.1. Phenolics
Phenolic compounds are secondary metabolites produced by plant cells, which possess a variety of bioactivities such as anti-inflammatory, antioxidant, antibacterial, and antiviral [102]. In particular, phenolic compounds have been well known to play anti-inflammatory roles by modulating diverse intracellular signaling pathways of inflammation and to have beneficial effects in various chronic inflammatory diseases [103].
3.1.1. Curcumin
Curcumin is the most well studied phenolic compound derived from
and is known to have anti-mutagenic and anti-tumorigenic effects [104]. Several studies have shown that curcumin has anti-inflammatory effects against IBD. Accumulating evidence has revealed that curcumin has an anti-inflammatory effect on chronic inflammatory diseases by modulating JAK–STAT pathways [105]. In particular, some studies have shown that curcumin has beneficial effects in murine models of IBD. It reduces the severity of dextran sulfate sodium (DSS)-induced colitis by blocking the DNA binding of STAT3 and suppressing STAT3 phosphorylation in the mouse colon [106]. STAT3 has been reported to be highly phosphorylated in IBD patients and to be further involved in colitis-associated cancer as well as colonic inflammation [107]. Another study reported that curcumin can ameliorate trinitrobenzene sulfonic acid (TNBS)-induced severe colitis by downregulating the phosphorylation of JAK2, STAT3, and STAT6 in colonic tissues [108]. It was also found that pretreatment with curcumin in TNBS-induced IBD reduced inflammatory tissue damage by inhibiting the phosphorylation of STAT1 [108]. This suggests that curcumin could be used as a therapeutic intervention for human intestinal inflammation. Although further clinical studies are needed, such as precise dose of administration, curcumin could reduce clinical symptoms of IBD patients and be an effective therapy in humans [109].
3.1.2. EGCG
Epigallocatechin-3-gallate (EGCG), a major bioactive polyphenol in green tea, is known to suppress inflammation and oxidative stress [110]. Many studies have shown that EGCG has anti-inflammatory effects on chronic inflammatory diseases, such as neurodegenerative diseases and cancers, in a multifactorial manner [111][112]. It improves acetic acid-induced colitis by reducing oxidative stress by decreasing nitric oxide (NO) production, increasing superoxide dismutase (SOD) expression, and inhibiting the production of TNF-α and IFN-γ in rats [113]. Treatment of DSS-induced colitis mice with EGCG ameliorated colitis by reducing malondialdehyde (MDA) caused by reactive oxygen species (ROS), which is one of the effector mechanisms of inflammation [114]. A recent study showed that EGCG downregulated cytokine IL-6 and reduced the expression of STAT3 protein in the colon tissue of colitis-induced mice. Therefore, EGCG reduced UC-like disease activity [115].
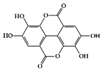
3.1.3. Ellagic Acid
Ellagic acid, found in a wide range of fruits and vegetables, including
(pomegranate), is known to have various biological activities [116]. In particular, many experimental studies have reported that ellagic acid has significant anti-inflammatory activities in the GI tract [117]. The ellagic acid and ellagic acid-rich fraction of pomegranate have antiulcerative effects in DSS-induced mice and rats [118][119]. Regarding specific anti-IBD molecular mechanisms, ellagic acid decreased inflammatory cytokines (IL-6, TNF-α, and IFN-γ) and crucial inflammatory mediators such as cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS), and blocked the expression and activation of STAT3 signaling pathways along with mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κB), resulting in a decrease in disease severity in both acute and chronic colitis [120][121].
3.1.4. Gallic Acid
Gallic acid, also known as 3,4,5-trihydroxybenzoic acid, is a naturally occurring phenolic compound found in fruits, nuts, and vegetables, and has been shown to have anti-inflammatory properties in a variety of chronic inflammatory disorders [122][123]. Gallic acid ameliorated UC-like clinical symptoms by reducing the phosphorylation of STAT3 and decreasing p65-NF-κB expression in the colon of DSS-induced mice [124]. Another study showed that gallic acid improved disease severity in DSS-induced mice by upregulating nuclear factor erythroid 2-related factor 2 (Nrf2) protein expression and downregulating the production of IL-21 and IL-23 [125].
3.1.5. Paeonol
Paeonol, 2-hydroxy-4-methoxy acetophenone, is a major phenolic compound found in
, which has been used in traditional medicine and has been reported to have various bioactivities [126]. In particular, several studies have shown that paeonol exhibits anti-inflammatory effects in many inflammation-related diseases [127]. It reduced TNBS-induced colitis and suppressed IFN-γ-induced STAT1 activation in colon cancer-derived CW-2 cells and T cell leukemia-derived Jurkat cells [128]. Oral administration of paeonol in colitis animal models (mice or rats) reduced colitis symptoms, suggesting that paeonol could be a therapeutic intervention for the treatment of IBD [129][130].
3.1.6. Piceatannol
Piceatannol is a hydroxylated derivative of resveratrol, which is a natural stilbene of phenolic compounds in grapes, berries, and passion fruits [131]. It is well known to have anti-inflammatory and suppressive effects on tumors [132][133]. Its anti-inflammatory activities target various inflammatory mediators such as iNOS, COX2, and NK-κB in vivo [134][135]. In DSS-induced colitis mice, piceatannol has been reported to ameliorate clinical signs in colonic tissue [136]. In this case, it exerted anti-inflammatory effects in the colon by decreasing the phosphorylation of STAT3, in addition to reducing the expression of iNOS, COX2, and crucial inflammatory cytokines such as TNF-α and IL-6.
3.1.7. Shikonin
Shikonin is a major component extracted from the root of
and has been studied as a potential anticancer and anti-inflammatory drug [137]. The anti-inflammatory effect of shikonin was verified in several in vivo model systems, which attenuated the pathological symptoms by reducing inflammation by inhibiting NF-κB pathways [138][139][140]. Furthermore, shikonin reduced disease symptoms by blocking the activation of STAT3 and reducing colonic inflammatory cytokines, including IL-1β, IL-6, and TNF-α in a DSS-induced UC model [141]. This suggests that shikonin can be used as a therapeutic agent for treating IBD.
3.2. Terpenoid
Terpenoids are secondary metabolites widely distributed in plants and are known to have significant therapeutic potential against inflammatory diseases [142]. Many studies have verified that terpenoids exert anti-inflammatory effects by targeting various inflammatory mediators in in vivo inflammatory disease model systems [143].
Triptolide
Triptolide is a bioactive diterpenoid extracted from
[144]. It suppresses colitis symptoms by inhibiting the IL-6/STAT3 signaling pathway in IL-10 deficient mice as well as in an in vitro culture of colonic explants of patients with CD [145]. It also inhibits the progression from colitis to colon cancer in a 1, 2-dimethylhydrazine (DMH)/DSS-induced mouse model and downregulates the JAK–STAT3 pathway by the phosphorylation of STAT3 in colorectal cancer cells [146].
3.3. Nitrogen-Containing Alkaloids and Sulfur-Containing Compounds
3.3.1. Boldine
Boldine, an aporphine alkaloid found in the boldo tree, has been used as a traditional remedy in several diseases and is well known for its anti-tumor, anti-atherogenic, and anti-diabetic effects [147][148][149]. In terms of anti-inflammatory activity, boldin has been reported to attenuate DSS-induced colon damage in mice by inhibiting inflammatory processes by increasing antioxidant enzymes SOD and catalase (CAT) and decreasing the expression and activation of STAT3 protein as well as p65 NF-κB in the colon [150].
3.3.2. Berberine
Berberine, a benzylisoquinoline alkaloid found in the
species, has been reported to have beneficial effects on DSS- or TNBS-induced colitis mouse models through various molecular mechanisms [151]. Berberine showed anti-colitic activity in TNBS-treated mice by blocking the expansion of Th1 and T helper type 17 (Th17) cells by reducing the phosphorylation of STAT1 and STAT3 in CD4+ T cells isolated from mice [152]. In addition, it ameliorated clinical severity in DSS-induced chronic relapsing mice by inhibiting the differentiation of Th17 cells by downregulating the phosphorylation of STAT3 [153]. Another study reported that berberine is involved in the protection of the intestinal barrier, not only by reducing pro-inflammatory cytokines and oxidative stress mediators such as myeloperoxidase (MPO), but also by increasing the expression of tight junction proteins such as zonula occludin-1 (ZO-1), resulting in relieving colitic symptoms in DSS-induced colitis mice [154]. In addition, a recent study demonstrated that berberine inhibited the phosphorylation of JAK1/2 and STAT1/3/4/5/6 through oncostatin M, belonging to the IL-6 cytokine family, leading to the attenuation of gut inflammation in DSS-induced mice [155].
3.3.3. Garlic Organosulfur Compounds (Allicin, Diallyl Trisulfide, and Alliin)
Garlic has long been used as food or traditional medicine for many diseases, and many studies have verified through in vitro and in vivo model systems that garlic organosulfur compounds have various biological activities, including anti-inflammatory, anti-oxidative, anti-diabetic, and anticancer properties [156]. Garlic is composed of several major organosulfur compounds, some of which have been reported to have anti-inflammatory activity in vivo [157].
Allicin is a sulfur-containing natural compound obtained from garlic,
, which has various biological activities [158]. In DSS-induced colitis mice, allicin reduced colon damage by suppressing the phosphorylation of STAT3 and inhibiting the expression of NF-κB [159].
Diallyl trisulfide (DATS) is an organic polysulfide from garlic that has been reported to ameliorate disease symptoms in DSS-induced colitis mice by blocking the DNA binding and phosphorylation of STAT3 [160].
Alliin is a well-known sulfur-containing compound in garlic. Treatment with alliin in DSS-induced colitis mice reduced colon damage, showing a decrease in pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) in colonic tissue [161]. In addition, alliin downregulated the activation of STAT1, MAPK, and NF-κB in lipopolysaccharide (LPS)-stimulated macrophages.
3.3.4. Phenethylisothiocyanate
Phenethylisothiocyanate (PEITC) is enriched in many cruciferous vegetables and is well known for its anti-cancer activity [162]. Relatively few studies have investigated the anti-inflammatory effects of PEITC. PEITC has been reported to alleviate histopathological signs in the acute and chronic inflammatory colon induced by DSS and attenuate the activation of STAT1 in LPS-activated macrophages. Therefore, blocking the activation of STAT1 by PEITC is associated with a reduction in IBD [163].
Phytochemicals targeting JAK–STAT pathways in inflammatory bowel disease models.
| Class of Phytochemicals | Phytochemical Name | Experimental System | Effective Doses (mg/kg Body Weight, Daily) |
Translated into Human-Relevant Equivalent (mg/kg) | Target of JAK–STAT Pathway | Main Source | Ref. |
|---|
| Phenolic | Curcumin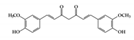 |
DSS-induced TNBS-induced |
36.8, 92 100 |
2.9, 7.4 8.1 |
JAK2, STAT1, 3, 6 | Curcuma longa Linn (turmeric) | |
| 10 | |||||||
| 0.8 | STAT3 | Green tea, strawberries, grapes, bananas, and many other fruits | [ | 124 | ] | ||
Paeonol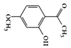 |
TNBS-induced | 0.5 mg/kg treated intrarectally | 0.04 | STAT1 | Moutan Cortex | [128] | |
Piceatannol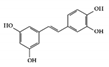 |
DSS-induced | 10 | 0.8 | STAT3 | Grapes, rheum undulatum, rhubarb, and sugar cane | [136] | |
Shikonin |
DSS-induced | 25 | 2.0 | STAT3 | Lithospermum erythrorhizon | [141] | |
| Terpenoid | Triptolide |
IL-10 deficient colitis mice | 0.07 mg/kg treated intraperitoneally | 0.005 | STAT3 | Tripterygium Wilfordii Hook. f | [146] |
| Nitrogen containing alkaloid | Boldin |
DSS-induced | 50 | 4.0 | STAT3 | Boldo tree | [150] |
Berberine |
DSS-induced | 20 50 |
1.6 4.0 |
STAT3 JAK1, 2 and STAT1, 3, 4, 5, 6 |
Berberis species | [153] [155] |
|
| Organosulfur compounds | Allicin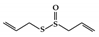 |
DSS-induced | 10 | 0.8 | STAT3 | Garlic | [159] |
Diallyl trisulfide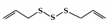 |
DSS-induced | 45, 90 | 3.6, 7.2 | STAT3 | Garlic | [160] | |
Alliin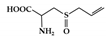 |
DSS-induced | 500 | 40.5 | STAT1 | Garlic | [161] | |
Phenethylisothiocyanate (PEITC)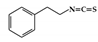 |
DSS-induced | 75 | 6.0 | STAT1 | cruciferous vegetables | [163] |
References
- Schmidt, B.M.; Ribnicky, D.M.; Lipsky, P.E.; Raskin, I. Revisiting the ancient concept of botanical therapeutics. Nat. Chem. Biol. 2007, 3, 360–366.
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201.
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—A review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58.
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of antioxidants and natural products in inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130.
- Zhu, F.; Du, B.; Xu, B. Anti-Inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1260–1270.
- Hnatyszyn, A.; Hryhorowicz, S.; Kaczmarek-Rys, M.; Lis, E.; Slomski, R.; Scott, R.J.; Plawski, A. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered. Cancer Clin. Pract. 2019, 17, 18.
- Podolsky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429.
- Kalla, R.; Ventham, N.T.; Satsangi, J.; Arnott, I.D. Crohn’s disease. BMJ 2014, 349, g6670.
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619.
- Kaplan, G.G. The global burden of ibd: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727.
- Rogler, G. Gastrointestinal and liver adverse effects of drugs used for treating ibd. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 157–165.
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342.
- Yu, H.; MacIsaac, D.; Wong, J.J.; Sellers, Z.M.; Wren, A.A.; Bensen, R.; Kin, C.; Park, K.T. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment. Pharmacol. Ther. 2018, 47, 364–370.
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68, s1–s106.
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002, 20, 495–549.
- Bevivino, G.; Monteleone, G. Advances in understanding the role of cytokines in inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 907–915.
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. Jak-stat pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337.
- Schreiner, P.; Neurath, M.F.; Ng, S.C.; El-Omar, E.M.; Sharara, A.I.; Kobayashi, T.; Hisamatsu, T.; Hibi, T.; Rogler, G. Mechanism-Based treatment strategies for ibd: Cytokines, cell adhesion molecules, jak inhibitors, gut flora, and more. Inflamm. Intest. Dis. 2019, 4, 79–96.
- Coskun, M.; Salem, M.; Pedersen, J.; Nielsen, O.H. Involvement of jak/stat signaling in the pathogenesis of inflammatory bowel disease. Pharmacol. Res. 2013, 76, 1–8.
- Soendergaard, C.; Bergenheim, F.H.; Bjerrum, J.T.; Nielsen, O.H. Targeting jak-stat signal transduction in ibd. Pharmacol. Ther. 2018, 192, 100–111.
- Flamant, M.; Rigaill, J.; Paul, S.; Roblin, X. Advances in the development of janus kinase inhibitors in inflammatory bowel disease: Future prospects. Drugs 2017, 77, 1057–1068.
- Kasembeli, M.M.; Bharadwaj, U.; Robinson, P.; Tweardy, D.J. Contribution of stat3 to inflammatory and fibrotic diseases and prospects for its targeting for treatment. Int. J. Mol. Sci. 2018, 19, 2299.
- Hur, S.J.; Kang, S.H.; Jung, H.S.; Kim, S.C.; Jeon, H.S.; Kim, I.H.; Lee, J.D. Review of natural products actions on cytokines in inflammatory bowel disease. Nutr. Res. 2012, 32, 801–816.
- Farzaei, M.H.; Bahramsoltani, R.; Abdolghaffari, A.H.; Sodagari, H.R.; Esfahani, S.A.; Rezaei, N. A mechanistic review on plant-derived natural compounds as dietary supplements for prevention of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 745–758.
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Yanping, C.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z.; et al. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1321–1345.
- Cao, S.Y.; Ye, S.J.; Wang, W.W.; Wang, B.; Zhang, T.; Pu, Y.Q. Progress in active compounds effective on ulcerative colitis from chinese medicines. Chin. J. Nat. Med. 2019, 17, 81–102.
- Favoino, E.; Prete, M.; Catacchio, G.; Ruscitti, P.; Navarini, L.; Giacomelli, R.; Perosa, F. Working and safety profiles of jak/stat signaling inhibitors. Are these small molecules also smart? Autoimmun. Rev. 2021, 20, 102750.
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. Jak-Stat signaling as a target for inflammatory and autoimmune diseases: Current and future prospects. Drugs 2017, 77, 521–546.
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288.
- Mitsuyama, K.; Suzuki, A.; Tomiyasu, N.; Takaki, K.; Toyonaga, A.; Sata, M. Transcription factor-targeted therapies in inflammatory bowel disease. Digestion 2001, 63 (Suppl. 1), 68–72.
- O’Shea, J.J.; Pesu, M.; Borie, D.C.; Changelian, P.S. A new modality for immunosuppression: Targeting the jak/stat pathway. Nat. Rev. Drug Discov. 2004, 3, 555–564.
- Menet, C.J.; Rompaey, L.V.; Geney, R. Advances in the discovery of selective jak inhibitors. Prog. Med. Chem. 2013, 52, 153–223.
- Galien, R. Janus kinases in inflammatory bowel disease: Four kinases for multiple purposes. Pharmacol. Rep. PR 2016, 68, 789–796.
- Hedl, M.; Proctor, D.D.; Abraham, C. Jak2 disease-risk variants are gain of function and jak signaling threshold determines innate receptor-induced proinflammatory cytokine secretion in macrophages. J. Immunol. 2016, 197, 3695–3704.
- Roskoski, R., Jr. Janus kinase (jak) inhibitors in the treatment of inflammatory and neoplastic diseases. Pharmacol. Res. 2016, 111, 784–803.
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the jak/stat pathway. Protein Sci. Publ. Protein Soc. 2018, 27, 1984–2009.
- Johnston, J.A.; Kawamura, M.; Kirken, R.A.; Chen, Y.Q.; Blake, T.B.; Shibuya, K.; Ortaldo, J.R.; McVicar, D.W.; O’Shea, J.J. Phosphorylation and activation of the jak-3 janus kinase in response to interleukin-2. Nature 1994, 370, 151–153.
- Rochman, Y.; Spolski, R.; Leonard, W.J. New insights into the regulation of t cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009, 9, 480–490.
- Brizzi, M.F.; Aronica, M.G.; Rosso, A.; Bagnara, G.P.; Yarden, Y.; Pegoraro, L. Granulocyte-Macrophage colony-stimulating factor stimulates jak2 signaling pathway and rapidly activates p93fes, stat1 p91, and stat3 p92 in polymorphonuclear leukocytes. J. Biol. Chem. 1996, 271, 3562–3567.
- Han, X.; Uchida, K.; Jurickova, I.; Koch, D.; Willson, T.; Samson, C.; Bonkowski, E.; Trauernicht, A.; Kim, M.O.; Tomer, G.; et al. Granulocyte-Macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn’s disease. Gastroenterology 2009, 136, 1261–1271.
- Hyams, J.S.; Fitzgerald, J.E.; Treem, W.R.; Wyzga, N.; Kreutzer, D.L. Relationship of functional and antigenic interleukin 6 to disease activity in inflammatory bowel disease. Gastroenterology 1993, 104, 1285–1292.
- Guschin, D.; Rogers, N.; Briscoe, J.; Witthuhn, B.; Watling, D.; Horn, F.; Pellegrini, S.; Yasukawa, K.; Heinrich, P.; Stark, G.R.; et al. A major role for the protein tyrosine kinase jak1 in the jak/stat signal transduction pathway in response to interleukin-6. EMBO J. 1995, 14, 1421–1429.
- Liu, X.; Jones, G.W.; Choy, E.H.; Jones, S.A. The biology behind interleukin-6 targeted interventions. Curr. Opin. Rheumatol. 2016, 28, 152–160.
- Bacon, C.M.; McVicar, D.W.; Ortaldo, J.R.; Rees, R.C.; O’Shea, J.J.; Johnston, J.A. Interleukin 12 (il-12) induces tyrosine phosphorylation of jak2 and tyk2: Differential use of janus family tyrosine kinases by il-2 and il-12. J. Exp. Med. 1995, 181, 399–404.
- Kastelein, R.A.; Hunter, C.A.; Cua, D.J. Discovery and biology of il-23 and il-27: Related but functionally distinct regulators of inflammation. Annu. Rev. Immunol. 2007, 25, 221–242.
- Schindler, C.; Plumlee, C. Inteferons pen the jak-stat pathway. Semin. Cell Dev. Biol. 2008, 19, 311–318.
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory cytokines: From discoveries to therapies in ibd. Expert Opin. Biol. Ther. 2019, 19, 1207–1217.
- Tran, V.; Shammas, R.M.; Sauk, J.S.; Padua, D. Evaluating tofacitinib citrate in the treatment of moderate-to-severe active ulcerative colitis: Design, development and positioning of therapy. Clin. Exp. Gastroenterol. 2019, 12, 179–191.
- Karaman, M.W.; Herrgard, S.; Treiber, D.K.; Gallant, P.; Atteridge, C.E.; Campbell, B.T.; Chan, K.W.; Ciceri, P.; Davis, M.I.; Edeen, P.T.; et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008, 26, 127–132.
- Bousoik, E.; Montazeri Aliabadi, H. “Do we know jack” about jak? A closer look at jak/stat signaling pathway. Front. Oncol. 2018, 8, 287.
- Ito, M.; Yamazaki, S.; Yamagami, K.; Kuno, M.; Morita, Y.; Okuma, K.; Nakamura, K.; Chida, N.; Inami, M.; Inoue, T.; et al. A novel jak inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J. Pharmacol. Sci. 2017, 133, 25–33.
- Sandborn, W.J.; Nguyen, D.D.; Beattie, D.T.; Brassil, P.; Krey, W.; Woo, J.; Situ, E.; Sana, R.; Sandvik, E.; Pulido-Rios, M.T.; et al. Development of gut-selective pan-janus kinase inhibitor td-1473 for ulcerative colitis: A translational medicine programme. J. Crohn’s Colitis 2020, 14, 1202–1213.
- Van Rompaey, L.; Galien, R.; van der Aar, E.M.; Clement-Lacroix, P.; Nelles, L.; Smets, B.; Lepescheux, L.; Christophe, T.; Conrath, K.; Vandeghinste, N.; et al. Preclinical characterization of glpg0634, a selective inhibitor of jak1, for the treatment of inflammatory diseases. J. Immunol. 2013, 191, 3568–3577.
- Parmentier, J.M.; Voss, J.; Graff, C.; Schwartz, A.; Argiriadi, M.; Friedman, M.; Camp, H.S.; Padley, R.J.; George, J.S.; Hyland, D.; et al. In vitro and in vivo characterization of the jak1 selectivity of upadacitinib (abt-494). BMC Rheumatol. 2018, 2, 23.
- Clark, J.D.; Flanagan, M.E.; Telliez, J.B. Discovery and development of janus kinase (jak) inhibitors for inflammatory diseases. J. Med. Chem. 2014, 57, 5023–5038.
- Mudter, J.; Weigmann, B.; Bartsch, B.; Kiesslich, R.; Strand, D.; Galle, P.R.; Lehr, H.A.; Schmidt, J.; Neurath, M.F. Activation pattern of signal transducers and activators of transcription (stat) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 2005, 100, 64–72.
- Miklossy, G.; Hilliard, T.S.; Turkson, J. Therapeutic modulators of stat signalling for human diseases. Nat. Rev. Drug Discov. 2013, 12, 611–629.
- Schreiber, S.; Rosenstiel, P.; Hampe, J.; Nikolaus, S.; Groessner, B.; Schottelius, A.; Kuhbacher, T.; Hamling, J.; Folsch, U.R.; Seegert, D. Activation of signal transducer and activator of transcription (stat) 1 in human chronic inflammatory bowel disease. Gut 2002, 51, 379–385.
- Azuma, Y.T.; Matsuo, Y.; Kuwamura, M.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Nakajima, H.; Karow, M.; Takeuchi, T. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm. Bowel Dis. 2010, 16, 1017–1028.
- Diegelmann, J.; Olszak, T.; Goke, B.; Blumberg, R.S.; Brand, S. A novel role for interleukin-27 (il-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (stat) protein signaling and induction of antibacterial and anti-inflammatory proteins. J. Biol. Chem. 2012, 287, 286–298.
- Meraz, M.A.; White, J.M.; Sheehan, K.C.; Bach, E.A.; Rodig, S.J.; Dighe, A.S.; Kaplan, D.H.; Riley, J.K.; Greenlund, A.C.; Campbell, D.; et al. Targeted disruption of the stat1 gene in mice reveals unexpected physiologic specificity in the jak-stat signaling pathway. Cell 1996, 84, 431–442.
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the jak/stat pathway, recent advances and future challenges. Gene 2002, 285, 1–24.
- Steen, H.C.; Gamero, A.M. Stat2 phosphorylation and signaling. Jak Stat 2013, 2, e25790.
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. Jak inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78.
- Wu, F.; Dassopoulos, T.; Cope, L.; Maitra, A.; Brant, S.R.; Harris, M.L.; Bayless, T.M.; Parmigiani, G.; Chakravarti, S. Genome-Wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: Insights into distinctive pathogenesis. Inflamm. Bowel Dis. 2007, 13, 807–821.
- Musso, A.; Dentelli, P.; Carlino, A.; Chiusa, L.; Repici, A.; Sturm, A.; Fiocchi, C.; Rizzetto, M.; Pegoraro, L.; Sategna-Guidetti, C.; et al. Signal transducers and activators of transcription 3 signaling pathway: An essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm. Bowel Dis. 2005, 11, 91–98.
- Bai, A.; Hu, P.; Chen, J.; Song, X.; Chen, W.; Peng, W.; Zeng, Z.; Gao, X. Blockade of stat3 by antisense oligonucleotide in tnbs-induced murine colitis. Int. J. Colorectal Dis. 2007, 22, 625–635.
- Han, X.; Sosnowska, D.; Bonkowski, E.L.; Denson, L.A. Growth hormone inhibits signal transducer and activator of transcription 3 activation and reduces disease activity in murine colitis. Gastroenterology 2005, 129, 185–203.
- Atreya, R.; Mudter, J.; Finotto, S.; Mullberg, J.; Jostock, T.; Wirtz, S.; Schutz, M.; Bartsch, B.; Holtmann, M.; Becker, C.; et al. Blockade of interleukin 6 trans signaling suppresses t-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in crohn disease and experimental colitis in vivo. Nat. Med. 2000, 6, 583–588.
- Brand, S.; Beigel, F.; Olszak, T.; Zitzmann, K.; Eichhorst, S.T.; Otte, J.M.; Diepolder, H.; Marquardt, A.; Jagla, W.; Popp, A.; et al. Il-22 is increased in active crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G827–G838.
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564.
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. Il-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 2008, 118, 534–544.
- Alonzi, T.; Newton, I.P.; Bryce, P.J.; Di Carlo, E.; Lattanzio, G.; Tripodi, M.; Musiani, P.; Poli, V. Induced somatic inactivation of stat3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine 2004, 26, 45–56.
- Takeda, K.; Clausen, B.E.; Kaisho, T.; Tsujimura, T.; Terada, N.; Forster, I.; Akira, S. Enhanced th1 activity and development of chronic enterocolitis in mice devoid of stat3 in macrophages and neutrophils. Immunity 1999, 10, 39–49.
- Hunter, C.A. New il-12-family members: Il-23 and il-27, cytokines with divergent functions. Nat. Rev. Immunol. 2005, 5, 521–531.
- Zundler, S.; Neurath, M.F. Immunopathogenesis of inflammatory bowel diseases: Functional role of t cells and t cell homing. Clin. Exp. Rheumatol. 2015, 33, S19–S28.
- Zundler, S.; Neurath, M.F. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015, 26, 559–568.
- Diaz-Gallo, L.M.; Palomino-Morales, R.J.; Gomez-Garcia, M.; Cardena, C.; Rodrigo, L.; Nieto, A.; Alcain, G.; Cueto, I.; Lopez-Nevot, M.A.; Martin, J. Stat4 gene influences genetic predisposition to ulcerative colitis but not crohn’s disease in the spanish population: A replication study. Hum. Immunol. 2010, 71, 515–519.
- Pang, Y.H.; Zheng, C.Q.; Yang, X.Z.; Zhang, W.J. Increased expression and activation of il-12-induced stat4 signaling in the mucosa of ulcerative colitis patients. Cell. Immunol. 2007, 248, 115–120.
- Simpson, S.J.; Shah, S.; Comiskey, M.; de Jong, Y.P.; Wang, B.; Mizoguchi, E.; Bhan, A.K.; Terhorst, C. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/signal transducer and activator of transcription (stat)-4 pathway, but is not conditional on interferon gamma expression by t cells. J. Exp. Med. 1998, 187, 1225–1234.
- Wirtz, S.; Finotto, S.; Kanzler, S.; Lohse, A.W.; Blessing, M.; Lehr, H.A.; Galle, P.R.; Neurath, M.F. Cutting edge: Chronic intestinal inflammation in stat-4 transgenic mice: Characterization of disease and adoptive transfer by tnf-plus ifn-gamma-producing cd4+ t cells that respond to bacterial antigens. J. Immunol. 1999, 162, 1884–1888.
- Arai, K.I.; Lee, F.; Miyajima, A.; Miyatake, S.; Arai, N.; Yokota, T. Cytokines: Coordinators of immune and inflammatory responses. Annu. Rev. Biochem. 1990, 59, 783–836.
- Gilbert, S.; Nivarthi, H.; Mayhew, C.N.; Lo, Y.H.; Noah, T.K.; Vallance, J.; Rulicke, T.; Muller, M.; Jegga, A.G.; Tang, W.; et al. Activated stat5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Rep. 2015, 4, 209–225.
- Burchill, M.A.; Yang, J.; Vogtenhuber, C.; Blazar, B.R.; Farrar, M.A. Il-2 receptor beta-dependent stat5 activation is required for the development of foxp3+ regulatory t cells. J. Immunol. 2007, 178, 280–290.
- Fantini, M.C.; Becker, C.; Tubbe, I.; Nikolaev, A.; Lehr, H.A.; Galle, P.; Neurath, M.F. Transforming growth factor beta induced foxp3+ regulatory t cells suppress th1 mediated experimental colitis. Gut 2006, 55, 671–680.
- Rosen, M.J.; Chaturvedi, R.; Washington, M.K.; Kuhnhein, L.A.; Moore, P.D.; Coggeshall, S.S.; McDonough, E.M.; Weitkamp, J.H.; Singh, A.B.; Coburn, L.A.; et al. Stat6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and th2-inducing cytokines. J. Immunol. 2013, 190, 1849–1858.
- Van Kampen, C.; Gauldie, J.; Collins, S.M. Proinflammatory properties of il-4 in the intestinal microenvironment. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G111–G117.
- Li, Y.; Deuring, J.; Peppelenbosch, M.P.; Kuipers, E.J.; de Haar, C.; van der Woude, C.J. Stat1, stat6 and adenosine 3′,5′-cyclic monophosphate (camp) signaling drive socs3 expression in inactive ulcerative colitis. Mol. Med. 2012, 18, 1412–1419.
- Rosen, M.J.; Frey, M.R.; Washington, M.K.; Chaturvedi, R.; Kuhnhein, L.A.; Matta, P.; Revetta, F.L.; Wilson, K.T.; Polk, D.B. Stat6 activation in ulcerative colitis: A new target for prevention of il-13-induced colon epithelial cell dysfunction. Inflamm. Bowel Dis. 2011, 17, 2224–2234.
- Lai, P.S.; Rosa, D.A.; Magdy Ali, A.; Gomez-Biagi, R.F.; Ball, D.P.; Shouksmith, A.E.; Gunning, P.T. A stat inhibitor patent review: Progress since 2011. Expert Opin. Ther. Pat. 2015, 25, 1397–1421.
- Wu, X.; Guo, W.; Wu, L.; Gu, Y.; Gu, L.; Xu, S.; Wu, X.; Shen, Y.; Ke, Y.; Tan, R.; et al. Selective sequestration of stat1 in the cytoplasm via phosphorylated shp-2 ameliorates murine experimental colitis. J. Immunol. 2012, 189, 3497–3507.
- Turkson, J.; Ryan, D.; Kim, J.S.; Zhang, Y.; Chen, Z.; Haura, E.; Laudano, A.; Sebti, S.; Hamilton, A.D.; Jove, R. Phosphotyrosyl peptides block stat3-mediated DNA binding activity, gene regulation, and cell transformation. J. Biol. Chem. 2001, 276, 45443–45455.
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Zoheir, K.M.A.; Bakheet, S.A.; Alsaad, A.M.S.; Al-Shabanah, O.A.; Attia, S.M. Sta-21, a stat-3 inhibitor, attenuates the development and progression of inflammation in collagen antibody-induced arthritis. Immunobiology 2017, 222, 206–217.
- Gavino, A.C.; Nahmod, K.; Bharadwaj, U.; Makedonas, G.; Tweardy, D.J. Stat3 inhibition prevents lung inflammation, remodeling, and accumulation of th2 and th17 cells in a murine asthma model. Allergy 2016, 71, 1684–1692.
- Odate, S.; Veschi, V.; Yan, S.; Lam, N.; Woessner, R.; Thiele, C.J. Inhibition of stat3 with the generation 2.5 antisense oligonucleotide, azd9150, decreases neuroblastoma tumorigenicity and increases chemosensitivity. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 1771–1784.
- Oh, D.Y.; Lee, S.H.; Han, S.W.; Kim, M.J.; Kim, T.M.; Kim, T.Y.; Heo, D.S.; Yuasa, M.; Yanagihara, Y.; Bang, Y.J. Phase i study of opb-31121, an oral stat3 inhibitor, in patients with advanced solid tumors. Cancer Res. Treat. 2015, 47, 607–615.
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2017, 376, 1723–1736.
- Vermeire, S.; Schreiber, S.; Petryka, R.; Kuehbacher, T.; Hebuterne, X.; Roblin, X.; Klopocka, M.; Goldis, A.; Wisniewska-Jarosinska, M.; Baranovsky, A.; et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the fitzroy study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017, 389, 266–275.
- Ma, C.; Lee, J.K.; Mitra, A.R.; Teriaky, A.; Choudhary, D.; Nguyen, T.M.; Vande Casteele, N.; Khanna, R.; Panaccione, R.; Feagan, B.G.; et al. Systematic review with meta-analysis: Efficacy and safety of oral janus kinase inhibitors for inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 50, 5–23.
- Sands, B.E.; Sandborn, W.J.; Feagan, B.G.; Lichtenstein, G.R.; Zhang, H.; Strauss, R.; Szapary, P.; Johanns, J.; Panes, J.; Vermeire, S.; et al. Peficitinib, an oral janus kinase inhibitor, in moderate-to-severe ulcerative colitis: Results from a randomised, phase 2 study. J. Crohn’s Colitis 2018, 12, 1158–1169.
- Sung, M.K.; Park, M.Y. Nutritional modulators of ulcerative colitis: Clinical efficacies and mechanistic view. World J. Gastroenterol. 2013, 19, 994–1004.
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573.
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618.
- Duvoix, A.; Blasius, R.; Delhalle, S.; Schnekenburger, M.; Morceau, F.; Henry, E.; Dicato, M.; Diederich, M. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 2005, 223, 181–190.
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Afshar, E.G.; Farkhondeh, T.; Samarghandian, S. Potential therapeutic effects of curcumin mediated by jak/stat signaling pathway: A review. Phytother. Res. PTR 2020, 34, 1745–1760.
- Yang, J.Y.; Zhong, X.; Yum, H.W.; Lee, H.J.; Kundu, J.K.; Na, H.K.; Surh, Y.J. Curcumin inhibits stat3 signaling in the colon of dextran sulfate sodium-treated mice. J. Cancer Prev. 2013, 18, 186–191.
- Bromberg, J.; Wang, T.C. Inflammation and cancer: Il-6 and stat3 complete the link. Cancer Cell 2009, 15, 79–80.
- Zhao, H.M.; Xu, R.; Huang, X.Y.; Cheng, S.M.; Huang, M.F.; Yue, H.Y.; Wang, X.; Zou, Y.; Lu, A.P.; Liu, D.Y. Curcumin suppressed activation of dendritic cells via jak/stat/socs signal in mice with experimental colitis. Front. Pharmacol. 2016, 7, 455.
- Bright, J.J. Curcumin and autoimmune disease. Adv. Exp. Med. Biol. 2007, 595, 425–451.
- Negah, S.S.; Ghazavi, H.; Vafaee, F.; Rashidi, R.; Aminian, A.R.; Forouzanfar, F. The potential role of green tea and its main constituent (epigallocatechin—3-gallate) in pain relief: A mechanistic review. Curr. Drug Discov. Technol. 2020.
- Ding, S.; Xu, S.; Fang, J.; Jiang, H. The protective effect of polyphenols for colorectal cancer. Front. Immunol. 2020, 11, 1407.
- Zhang, S.; Zhu, Q.; Chen, J.Y.; OuYang, D.; Lu, J.H. The pharmacological activity of epigallocatechin-3-gallate (egcg) on Alzheimer’s disease animal model: A systematic review. Phytomed. Int. J. Phytother. Phytopharmacol. 2020, 79, 153316.
- Ran, Z.H.; Chen, C.; Xiao, S.D. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed. Pharmacother. 2008, 62, 189–196.
- Bruckner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lugering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohn’s Colitis 2012, 6, 226–235.
- Xu, Z.; Wei, C.; Zhang, R.U.; Yao, J.; Zhang, D.; Wang, L. Epigallocatechin-3-gallate-induced inhibition of interleukin-6 release and adjustment of the regulatory t/t helper 17 cell balance in the treatment of colitis in mice. Exp. Ther. Med. 2015, 10, 2231–2238.
- Baradaran Rahimi, V.; Ghadiri, M.; Ramezani, M.; Askari, V.R. Antiinflammatory and anti-cancer activities of pomegranate and its constituent, ellagic acid: Evidence from cellular, animal, and clinical studies. Phytother. Res. PTR 2020, 34, 685–720.
- Colombo, E.; Sangiovanni, E.; Dell’agli, M. A review on the anti-inflammatory activity of pomegranate in the gastrointestinal tract. Evid. Based Complement. Altern. Med. eCAM 2013, 2013, 247145.
- Ogawa, Y.; Kanatsu, K.; Iino, T.; Kato, S.; Jeong, Y.I.; Shibata, N.; Takada, K.; Takeuchi, K. Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci. 2002, 71, 827–839.
- Singh, K.; Jaggi, A.S.; Singh, N. Exploring the ameliorative potential of punica granatum in dextran sulfate sodium induced ulcerative colitis in mice. Phytother. Res. PTR 2009, 23, 1565–1574.
- Marin, M.; Maria Giner, R.; Rios, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934.
- Rosillo, M.A.; Sanchez-Hidalgo, M.; Cardeno, A.; de la Lastra, C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745.
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of gallic acid on gut health: Focus on the gut microbiome, immune response, and mechanisms of action. Front. Immunol. 2020, 11, 580208.
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985.
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043.
- Pandurangan, A.K.; Mohebali, N.; Norhaizan, M.E.; Looi, C.Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in balb/c mice. Drug Des. Dev. Ther. 2015, 9, 3923–3934.
- Wang, J.; Wu, G.; Chu, H.; Wu, Z.; Sun, J. Paeonol derivatives and pharmacological activities: A review of recent progress. Mini Rev. Med. Chem. 2020, 20, 466–482.
- Zhang, L.; Li, D.C.; Liu, L.F. Paeonol: Pharmacological effects and mechanisms of action. Int. Immunopharmacol. 2019, 72, 413–421.
- Ishiguro, K.; Ando, T.; Maeda, O.; Hasegawa, M.; Kadomatsu, K.; Ohmiya, N.; Niwa, Y.; Xavier, R.; Goto, H. Paeonol attenuates tnbs-induced colitis by inhibiting nf-kappab and stat1 transactivation. Toxicol. Appl. Pharmacol. 2006, 217, 35–42.
- Jin, X.; Wang, J.; Xia, Z.M.; Shang, C.H.; Chao, Q.L.; Liu, Y.R.; Fan, H.Y.; Chen, D.Q.; Qiu, F.; Zhao, F. Anti-Inflammatory and anti-oxidative activities of paeonol and its metabolites through blocking mapk/erk/p38 signaling pathway. Inflammation 2016, 39, 434–446.
- Zong, S.Y.; Pu, Y.Q.; Xu, B.L.; Zhang, T.; Wang, B. Study on the physicochemical properties and anti-inflammatory effects of paeonol in rats with tnbs-induced ulcerative colitis. Int. Immunopharmacol. 2017, 42, 32–38.
- Cao, Y.; Smith, W.; Yan, L.; Kong, L. Overview of cellular mechanisms and signaling pathways of piceatannol. Curr. Stem Cell Res. Ther. 2020, 15, 4–10.
- Banik, K.; Ranaware, A.M.; Harsha, C.; Nitesh, T.; Girisa, S.; Deshpande, V.; Fan, L.; Nalawade, S.P.; Sethi, G.; Kunnumakkara, A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020, 153, 104635.
- Dvorakova, M.; Landa, P. Anti-Inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145.
- Liu, L.; Li, J.; Kundu, J.K.; Surh, Y.J. Piceatannol inhibits phorbol ester-induced expression of cox-2 and inos in hr-1 hairless mouse skin by blocking the activation of nf-kappab and ap-1. Inflamm. Res. 2014, 63, 1013–1021.
- Yum, S.; Jeong, S.; Lee, S.; Nam, J.; Kim, W.; Yoo, J.W.; Kim, M.S.; Lee, B.L.; Jung, Y. Colon-Targeted delivery of piceatannol enhances anti-colitic effects of the natural product: Potential molecular mechanisms for therapeutic enhancement. Drug Des. Dev. Ther. 2015, 9, 4247–4258.
- Kim, Y.H.; Kwon, H.S.; Kim, D.H.; Cho, H.J.; Lee, H.S.; Jun, J.G.; Park, J.H.; Kim, J.K. Piceatannol, a stilbene present in grapes, attenuates dextran sulfate sodium-induced colitis. Int. Immunopharmacol. 2008, 8, 1695–1702.
- Guo, C.; He, J.; Song, X.; Tan, L.; Wang, M.; Jiang, P.; Li, Y.; Cao, Z.; Peng, C. Pharmacological properties and derivatives of shikonin-a review in recent years. Pharmacol. Res. 2019, 149, 104463.
- Lu, L.; Qin, A.; Huang, H.; Zhou, P.; Zhang, C.; Liu, N.; Li, S.; Wen, G.; Zhang, C.; Dong, W.; et al. Shikonin extracted from medicinal chinese herbs exerts anti-inflammatory effect via proteasome inhibition. Eur. J. Pharmacol. 2011, 658, 242–247.
- Yang, J.; Wang, Z.; Chen, D.L. Shikonin ameliorates isoproterenol (iso)-induced myocardial damage through suppressing fibrosis, inflammation, apoptosis and er stress. Biomed. Pharmacother. 2017, 93, 1343–1357.
- Lin, M.X.; Yi, Y.X.; Fang, P.P.; Huang, S.S.; Pan, C.W.; Jin, L.X.; Zhang, T.; Zhou, G.Y. Shikonin protects against d-galactosamine and lipopolysaccharide-induced acute hepatic injury by inhibiting tlr4 signaling pathway. Oncotarget 2017, 8, 91542–91550.
- Andujar, I.; Rios, J.L.; Giner, R.M.; Miguel Cerda, J.; Recio Mdel, C. Beneficial effect of shikonin on experimental colitis induced by dextran sulfate sodium in balb/c mice. Evid. Based Complement. Altern. Med. eCAM 2012, 2012, 271606.
- Salminen, A.; Lehtonen, M.; Suuronen, T.; Kaarniranta, K.; Huuskonen, J. Terpenoids: Natural inhibitors of nf-kappab signaling with anti-inflammatory and anticancer potential. Cell. Mol. Life Sci. CMLS 2008, 65, 2979–2999.
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 2187.
- Tong, L.; Zhao, Q.; Datan, E.; Lin, G.Q.; Minn, I.; Pomper, M.G.; Yu, B.; Romo, D.; He, Q.L.; Liu, J.O. Triptolide: Reflections on two decades of research and prospects for the future. Nat. Prod. Rep. 2020, 843–860.
- Li, Y.; Yu, C.; Zhu, W.M.; Xie, Y.; Qi, X.; Li, N.; Li, J.S. Triptolide ameliorates il-10-deficient mice colitis by mechanisms involving suppression of il-6/stat3 signaling pathway and down-regulation of il-17. Mol. Immunol. 2010, 47, 2467–2474.
- Wang, Z.; Jin, H.; Xu, R.; Mei, Q.; Fan, D. Triptolide downregulates rac1 and the jak/stat3 pathway and inhibits colitis-related colon cancer progression. Exp. Mol. Med. 2009, 41, 717–727.
- Speisky, H.; Cassels, B.K. Boldo and boldine: An emerging case of natural drug development. Pharmacol. Res. 1994, 29, 1–12.
- Stevigny, C.; Bailly, C.; Quetin-Leclercq, J. Cytotoxic and antitumor potentialities of aporphinoid alkaloids. Curr. Med. Chem. Anticancer Agents 2005, 5, 173–182.
- O’Brien, P.; Carrasco-Pozo, C.; Speisky, H. Boldine and its antioxidant or health-promoting properties. Chem. Biol. Interact. 2006, 159, 1–17.
- Pandurangan, A.K.; Mohebali, N.; Hasanpourghadi, M.; Looi, C.Y.; Mustafa, M.R.; Mohd Esa, N. Boldine suppresses dextran sulfate sodium-induced mouse experimental colitis: Nf-kappab and il-6/stat3 as potential targets. BioFactors 2016, 42, 247–258.
- Habtemariam, S. Berberine and inflammatory bowel disease: A concise review. Pharmacol. Res. 2016, 113, 592–599.
- Li, C.; Xi, Y.; Li, S.; Zhao, Q.; Cheng, W.; Wang, Z.; Zhong, J.; Niu, X.; Chen, G. Berberine ameliorates tnbs induced colitis by inhibiting inflammatory responses and th1/th17 differentiation. Mol. Immunol. 2015, 67, 444–454.
- Li, Y.H.; Xiao, H.T.; Hu, D.D.; Fatima, S.; Lin, C.Y.; Mu, H.X.; Lee, N.P.; Bian, Z.X. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in c57bl/6 mice by suppressing th17 responses. Pharmacol. Res. 2016, 110, 227–239.
- Zhang, L.C.; Wang, Y.; Tong, L.C.; Sun, S.; Liu, W.Y.; Zhang, S.; Wang, R.M.; Wang, Z.B.; Li, L. Berberine alleviates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. Exp. Ther. Med. 2017, 13, 3374–3382.
- Li, H.; Feng, C.; Fan, C.; Yang, Y.; Yang, X.; Lu, H.; Lu, Q.; Zhu, F.; Xiang, C.; Zhang, Z.; et al. Intervention of oncostatin m-driven mucosal inflammation by berberine exerts therapeutic property in chronic ulcerative colitis. Cell Death Dis. 2020, 11, 271.
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.A.; Al-Sagan, A.A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872.
- Trio, P.Z.; You, S.; He, X.; He, J.; Sakao, K.; Hou, D.X. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833–844.
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618.
- Pandurangan, A.K.; Ismail, S.; Saadatdoust, Z.; Esa, N.M. Allicin alleviates dextran sodium sulfate- (dss-) induced ulcerative colitis in balb/c mice. Oxidative Med. Cell. Longev. 2015, 2015, 605208.
- Lee, H.J.; Lee, H.G.; Choi, K.S.; Surh, Y.J.; Na, H.K. Diallyl trisulfide suppresses dextran sodium sulfate-induced mouse colitis: Nf-kappab and stat3 as potential targets. Biochem. Biophys. Res. Commun. 2013, 437, 267–273.
- Shi, L.; Lin, Q.; Li, X.; Nie, Y.; Sun, S.; Deng, X.; Wang, L.; Lu, J.; Tang, Y.; Luo, F. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through mapk-nf-kappab/ap-1/stat-1 inactivation and ppar-gamma activation. Mol. Nutr. Food Res. 2017, 61, 1601013.
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018, 23, 2983.
- Dey, M.; Kuhn, P.; Ribnicky, D.; Premkumar, V.; Reuhl, K.; Raskin, I. Dietary phenethylisothiocyanate attenuates bowel inflammation in mice. BMC Chem. Biol. 2010, 10, 4.
