Selenium (Se) is an essential micronutrient for mammals, and its deficiency seriously threatens human health. A series of biofortification strategies have been developed to produce Se-enriched foods for combating Se deficiency. Although there have been some inconsistent results, extensive evidence has suggested that Se supplementation is beneficial for preventing and treating several chronic diseases. Understanding the association between Se and chronic diseases is essential for guiding clinical practice, developing effective public health policies, and ultimately counteracting health issues associated with Se deficiency. The current review will discuss the food sources of Se, biofortification strategies, metabolism and biological activities, clinical disorders and dietary reference intakes, as well as the relationship between Se and health outcomes, especially cardiovascular disease, diabetes, chronic inflammation, cancer, and fertility.
- selenium biofortification
- chronic diseases
- baseline selenium status
- methylated selenium compounds
1. Introduction
2. Food Sources of Se
2.1. The Overview of Se Contents and Forms in Different Foods
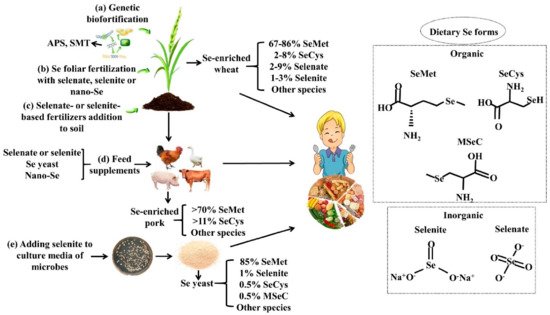
2.2. Se Biofortification
2.2.1. Agronomic Biofortification
2.2.2. Genetic Biofortification
2.2.3. Se-Biofortified Agricultural Products
2.3. Se Nutritional Fortifiers and Se Fortified Foods
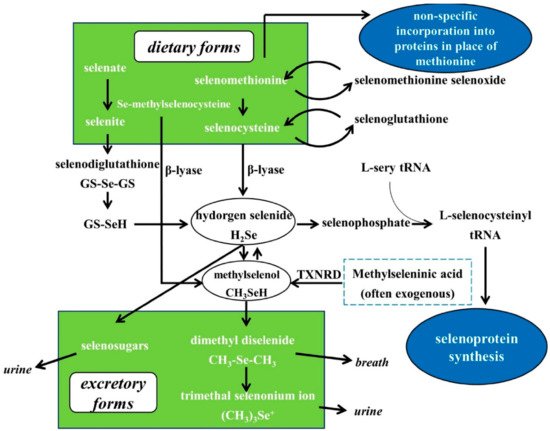
3. Se Nutritional Status Assessment, Metabolism, Bioavailability and Biological Functions
| Selenoprotein (Abbreviation) | Tissue Distribution a | Localization | Functions |
|---|---|---|---|
| Glutathione peroxidase 1 (GPX1) | Blood, kidney, liver, placenta | Cytosol | Reduces cellular H2O2 and lipid peroxides |
| Glutathione peroxidase 2 (GPX2) | Gastrointestinal tract, liver, mammary |
Cytosol | Reduces peroxide in the gut |
| Glutathione peroxidase 3 (GPX3) | Epididymis, kidney, plasma | Plasma | Reduces peroxide in blood |
| Glutathione peroxidase 4 (GPX4) | Liver, testis | Cytosol; mitochondria; nucleus (testis-specific) |
Reduces phospholipid peroxide |
| Glutathione peroxidase 6 (GPX6) | Embryos, olfactory epithelium | Cytosol | Reduces cellular H2O2 in the olfactory epithelium |
| Thioredoxin reductase 1 (TXNRD1) | Heart, kidney, liver | Cytosol | Regenerates reduced thioredoxin |
| Thioredoxin reductase 2 (TXNRD2) | Adrenal gland, heart, kidney, liver | Cytosol | Catalyzes a variety of reactions, specific for thioredoxin and glutaredoxin systems |
| Thioredoxin reductase 3 (TXNRD3) | Testis, heart, kidney, liver | Mitochondria | Reduces the oxidized form of thioredoxin and glutaredoxin 2 |
| Iodothyronine deiodinase 1 (DIO1) | Kidney, liver, thyroid | Plasma membrane | Important for systemic active thyroid hormone levels |
| Iodothyronine deiodinase 2 (DIO2) | Brain, brown adipose tissue, pituitary |
Endothelial reticulum | Important for local active thyroid hormone levels |
| Iodothyronine deiodinase 3 (DIO3) | Brain, placenta, skin | Plasma membrane | Inactivates thyroid hormone |
| Methionine sulfoxide reductase B1 (MSRB1) | Liver, kidney | Cytosol | Reduces methionine- R-sulfoxide residues in proteins to methionine |
| Selenophosphate synthetase 2 (SEPHS2) | Kidney, liver, testis | Cytosol | Synthesis of selenophosphate |
| Selenoprotein F (SELENOF) | Liver, prostate | Endoplasmic reticulum (ER) | Involved in protein folding |
| Selenoprotein H (SELENOH) | Unknown b | Nucleus | Involved in redox sensing and transcription |
| Selenoprotein I (SELENOI) |
Unknown b | Membrane | Involved in phospholipid biosynthesis |
| Selenoprotein K (SELENOK) | Unknown b | ER membrane | Modulates Ca2+ influx that affects immune cell function; component of ER-associated degradation |
| Selenoprotein M (SELENOM) | Brain | ER | Protein folding in ER |
| Selenoprotein N (SELENON) | Brain, heart, liver, muscle | ER membrane | Proper muscle development |
| Selenoprotein O (SELENOO) | Unknown b | Mitochondria | Unknown c |
| Selenoprotein P (SELENOP) | Liver, plasma | Plasma | Se transport and antioxidant function |
| Selenoprotein S (SELENOS) |
Unknown b | ER membrane | Involved in ER-associated degradation |
| Selenoprotein T (SELENOT) |
Unknown b | ER and Golgi | Involved in redox regulation and cell anchorage |
| Selenoprotein V (SELENOV) | Testes | Cytosol | Unknown c |
| Selenoprotein W (SELENOW) | Brain, muscle, testes | Cytosol | Necessary for muscle function |
4. Chronic Diseases
4.1. Cardiovascular Disease
4.2. Metabolic Diseases
4.2.1. Diabetes Mellitus
4.2.2. Thyroid Diseases
4.3. Chronic/Acute Inflammations
4.4. Cancer
4.4.1. Human Studies on Se and Cancer
-
Epidemiological studies on Se exposure and cancer risk
- Human intervention studies with Se
4.4.2. Preclinical Studies on the Anticarcinogenic Effects of Different Forms of Se
4.4.3. Possible Mechanisms for Anticarcinogenic Actions of Se
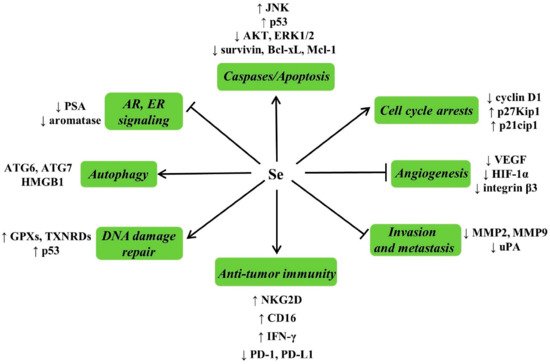
4.4.4. Se and Cancer Adjuvant Therapy
- Enhancing antitumor efficacy
- Reduction in toxicity
4.5. Fertility
References
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309.
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake and Food Sources of Zinc, Selenium, and Vitamins A, E and C in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 697.
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383.
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and De-termi-nation of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55.
- Roberge, M.T.; Borgerding, A.J.; Finley, J.W. Speciation of selenium compounds from high selenium broccoli is affected by the ex-tracting solution. J. Agric. Food Chem. 2003, 51, 4191–4197.
- Whanger, P.D. Selenocompounds in Plants and Animals and their Biological Significance. J. Am. Coll. Nutr. 2002, 21, 223–232.
- Wolf, W.R.; Goldschmidt, R.J. Updated estimates of the selenomethionine content of NIST wheat reference materials by GC–IDMS. Anal. Bioanal. Chem. 2006, 387, 2449–2452.
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Banuelos, G.S.; Liang, D. Understanding boosting selenium accumulation in Wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. Pollut. Res. Int. 2020, 27, 717–728.
- Zhang, K.; Guo, X.; Zhao, Q.; Han, Y.; Zhan, T.; Li, Y.; Tang, C.; Zhang, J. Development and application of a HPLC-ICP-MS method to determine selenium speciation in muscle of pigs treated with different selenium supplements. Food Chem. 2020, 302, 125371.
- Ip, C.; Birringer, M.; Block, E.; Kotrebai, M.; Tyson, J.F.; Uden, P.C.; Lisk, D.J. Chemical speciation influences comparative activity of selenium-enriched garlic and yeast in mammary cancer prevention. J. Agric. Food Chem. 2000, 48, 2062–2070.
- Ewu, Z.; Bañuelos, G.S.; Lin, Z.-Q.; Eliu, Y.; Eyuan, L.; Eyin, X.; Eli, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136.
- Broadley, M.R.; Alcock, J.; Alford, J.; Cartwright, P.; Foot, I.; Fairweather-Tait, S.J.; Hart, D.J.; Hurst, R.; Knott, P.; McGrath, S.P.; et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 2010, 332, 5–18.
- Lyons, G. Biofortification of Cereals with Foliar Selenium and Iodine Could Reduce Hypothyroidism. Front. Plant Sci. 2018, 9, 730.
- Lavu, R.V.S.; Du Laing, G.; Van De Wiele, T.; Pratti, V.L.; Willekens, K.; Vandecasteele, B.; Tack, F. Fertilizing Soil with Selenium Fertilizers: Impact on Concentration, Speciation, and Bioaccessibility of Selenium in Leek (Allium ampeloprasum). J. Agric. Food Chem. 2012, 60, 10930–10935.
- Ávila, F.W.; Faquin, V.; Yang, Y.; Ramos, S.J.; Guilherme, L.R.G.; Thannhauser, T.W.; Li, L. Assessment of the anticancer compounds Se-methylselenocysteine and glucosinolates in Se-biofortified broccoli (Brassica oleracea L. var. italica) sprouts and florets. J. Agric. Food Chem. 2013, 61, 6216–6223.
- Ros, G.H.; Van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112.
- Shalaby, T.; Bayoumi, Y.; Alshaal, T.; Elhawat, N.; Sztrik, A.; El-Ramady, H. Selenium fortification induces growth, antioxidant activity, yield and nutritional quality of lettuce in salt-affected soil using foliar and soil applications. Plant Soil 2017, 421, 245–258.
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84.
- Kumar, J.; Gupta, D.S.; Kumar, S.; Gupta, S.; Singh, N.P. Current Knowledge on Genetic Biofortification in Lentil. J. Agric. Food Chem. 2016, 64, 6383–6396.
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium Biofortification and Phytoremediation Phytotechnologies: A Review. J. Environ. Qual. 2017, 46, 10–19.
- Malagoli, M.; Schiavon, M.; Dall’Acqua, S.; Pilon-Smits, E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015, 6, 280.
- Van Huysen, T.; Terry, N.; Pilon-Smits, E.A. Exploring the selenium phytoremediation potential of transgenic Indian mustard overexpressing ATP sulfurylase or cystathionine-gamma-synthase. Int. J. Phytoremediat. 2004, 6, 111–118.
- Chen, M.; Zeng, L.; Luo, X.; Mehboob, M.Z.; Ao, T.; Lang, M. Identification and functional characterization of a novel selenocysteine methyltransferase from Brassica juncea L. J. Exp. Bot. 2019, 70, 6401–6416.
- Amato, R.D.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Bosco, A.D.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097.
- Djujić, I.S.; Jozanov-Stankov, O.N.; Milovac, M.; Janković, V.; Djermanović, V. Bioavailability and possible benefits of wheat intake naturally enriched with selenium and its products. Biol. Trace Elem. Res. 2000, 77, 273–285.
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 74, 449–460.
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801.
- Schiavon, M.; Dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium Fertilization Alters the Chemical Composition and Antioxidant Constituents of Tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554.
- Bachiega, P.; Salgado, J.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Schwarz, K.; Tezotto, T.; Morzelle, M.C. Antioxidant and antiproliferative activities in different maturation stages of broccoli (Brassica oleracea Italica) biofortified with selenium. Food Chem. 2016, 190, 771–776.
- Babalar, M.; Mohebbi, S.; Zamani, Z.; Askari, A.M. Effect of foliar application with sodium selenate on selenium biofortification and fruit quality maintenance of ‘Starking Delicious’ apple during storage. J. Sci. Food Agric. 2019, 99, 5149–5156.
- Zahedi, S.M.; Hosseini, M.S.; Meybodi, N.D.H.; da Silva, J.A.T. Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. S. Afr. J. Bot. 2019, 124, 350–358.
- Amato, R.D.; De Feudis, M.; Hasuoka, P.E.; Regni, L.; Pacheco, P.H.; Onofri, A.; Businelli, D.; Proietti, P. The Selenium Supplementation Influences Olive Tree Production and Oil Sta-bil-ity Against Oxidation and Can Alleviate the Water Deficiency Effects. Front. Plant Sci. 2018, 9.
- Marković, R.; Ćirić, J.; Starčević, M.; Šefer, D.; Baltić, M.Ž. Effects of selenium source and level in diet on glutathione peroxidase activity, tissue selenium distribution, and growth performance in poultry. Anim. Health Res. Rev. 2018, 19, 166–176.
- Joksimovic-Todorovic, M.; Davidovic, V.; Sretenovic, L. The effect of diet selenium supplement on meat quality. Biotechnol. Anim. Husb. 2012, 28, 553–561.
- Netto, A.S.; Zanetti, M.A.; Claro, G.R.; de Melo, M.P.; Vilela, F.G.; Correa, L.B. Effects of copper and selenium supplementation on per-formance and lipid metabolism in confined brangus bulls. Asian-Australas J. Anim. Sci. 2014, 27, 488–494.
- Lönnerdal, B.; Vargas-Fernández, E.; Whitacre, M. Selenium fortification of infant formulas: Does selenium form matter? Food Funct. 2017, 8, 3856–3868.
- Morris, J.S.; Stampfer, M.J.; Willett, W. Dietary selenium in humans toenails as an indicator. Biol. Trace Element Res. 1983, 5, 529–537.
- Gutiérrez-González, E.; García-Esquinas, E.; de Larrea-Baz, N.F.; Salcedo-Bellido, I.; Navas-Acien, A.; Lope, V.; Gómez-Ariza, J.L.; Pastor, R.; Pollán, M.; Pérez-Gómez, B. Toenails as biomarker of exposure to essential trace metals: A review. Environ. Res. 2019, 179, 108787.
- Do Nascimento Da Silva, E.; Aureli, F.; Amato, M.D.; Raggi, A.; Cadore, S.; Cubadda, F. Selenium Bioaccessibility and Speciation in Selenium-Enriched Lettuce: Investigation of the Selenocompounds Liberated after in Vitro Simulated Human Digestion Using Two-Dimensional HPLC-ICP-MS. J. Agric. Food Chem. 2017, 65, 3031–3038.
- Hu, T.; Hui, G.; Li, H.; Guo, Y. Selenium biofortification in Hericium erinaceus (Lion’s Mane mushroom) and its in vitro bio-acces-sibility. Food Chem. 2020, 331, 127287.
- Pyrzynska, K.; Sentkowska, A. Selenium in plant foods: Speciation analysis, bioavailability, and factors affecting composition. Crit. Rev. Food Sci. Nutr. 2021, 61, 1340–1352.
- Nicastro, H.L.; Dunn, B.K. Selenium and Prostate Cancer Prevention: Insights from the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Nutrients 2013, 5, 1122–1148.
- Lü, J.; Zhang, J.; Jiang, C.; Deng, Y.; Özten, N.; Bosland, M.C. Cancer chemoprevention research with selenium in the post-SELECT era: Promises and challenges. Nutr. Cancer 2016, 68, 1–17.
- Labunskyy, V.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777.
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and Cancer Prevention. Annu. Rev. Nutr. 2012, 32, 73–95.
- Avery, J.; Hoffmann, P. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203.
- Gladyshev, V.N.; Arnér, E.; Berry, M.J.; Brigelius-Flohé, R.; Bruford, E.A.; Burk, R.F.; Carlson, B.A.; Castellano, S.; Chavatte, L.; Conrad, M.; et al. Selenoprotein Gene Nomenclature. J. Biol. Chem. 2016, 291, 24036–24040.
- Liu, H.; Xu, H.; Huang, K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 2017, 9, 21–37.
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and Its Supplementation in Cardiovascular Disease—What do We Know? Nutrients 2015, 7, 3094–3118.
- Zhang, X.; Liu, C.; Guo, J.; Song, Y. Selenium status and cardiovascular diseases: Meta-analysis of prospective observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2015, 70, 162–169.
- Jenkins, D.J.; Spence, J.D.; Giovannucci, E.L.; Kim, Y.-I.; Josse, R.; Vieth, R.; Mejia, S.B.; Viguiliouk, E.; Nishi, S.; Sahye-Pudaruth, S.; et al. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J. Am. Coll. Cardiol. 2018, 71, 2570–2584.
- Rayman, M.P.; Bath, S.C.; Westaway, J.; Williams, P.; Mao, J.; Vanderlelie, J.J.; Perkins, A.V.; Redman, C.W.G. Selenium status in UK pregnant women and its relationship with hypertensive con-ditions of pregnancy. Br. J. Nutr. 2015, 113, 249–258.
- Alehagen, U.; Johansson, P.; Björnstedt, M.; Rosén, A.; Dahlström, U. Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: A 5-year prospective randomized double-blind place-bo-controlled trial among elderly Swedish citizens. Int. J. Cardiol. 2013, 167, 1860–1866.
- Alehagen, U.; Alexander, J.; Aaseth, J. Supplementation with Selenium and Coenzyme Q10 Reduces Cardiovascular Mortality in Elderly with Low Selenium Status. A Secondary Analysis of a Randomised Clinical Trial. PLoS ONE 2016, 11, e0157541.
- Alehagen, U.; Aaseth, J.; Johansson, P. Less increase of copeptin and MR-proADM due to intervention with selenium and co-en-zyme Q10 combined: Results from a 4-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Biofactors 2015, 41, 443–452.
- Alehagen, U.; Aaseth, J.; Alexander, J.; Johansson, P.; Larsson, A. Supplemental selenium and coenzyme Q10 reduce glycation along with cardiovascular mortality in an elderly population with low selenium status—A four-year, prospective, ran-dom-ised, double-blind placebo-controlled trial. J. Trace Elem. Med. Bio. 2020, 61, 126541.
- Balakumar, P.; Maung-U, K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113, 600–609.
- Steinbrenner, H.; Speckmann, B.; Pinto, A.; Sies, H. High selenium intake and increased diabetes risk: Experimental evidence for interplay between selenium and carbohydrate metabolism. J. Clin. Biochem. Nutr. 2010, 48, 40–45.
- Wang, X.-L.; Yang, T.-B.; Wei, J.; Lei, G.-H.; Zeng, C. Association between serum selenium level and type 2 diabetes mellitus: A non-linear dose–response meta-analysis of observational studies. Nutr. J. 2015, 15, 1–9.
- Stranges, S.; Marshall, J.R.; Natarajan, R.; Donahue, R.P.; Trevisan, M.; Combs, G.F.; Cappuccio, F.P.; Ceriello, A.; Reid, M.E. Effects of long-term selenium supplementation on the incidence of type 2 diabe-tes: A randomized trial. Ann. Intern. Med. 2007, 147, 217–223.
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51.
- Rayman, M.P.; Stranges, S. Epidemiology of selenium and type 2 diabetes: Can we make sense of it? Free Radic. Biol. Med. 2013, 65, 1557–1564.
- Wu, Q.; Rayman, M.P.; Lv, H.; Cui, B.; Gao, C.; Chen, P.; Zhuang, G.; Zhang, Z.; Peng, X.; Li, H.; et al. Low Population Selenium Status Is Associated with Increased Prevalence of Thyroid Disease. J. Clin. Endocrinol. Metab. 2015, 100, 4037–4047.
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692.
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44.
- Negro, R.; Greco, G.; Mangieri, T.; Pezzarossa, A.; Dazzi, D.; Hassan, H. The Influence of Selenium Supplementation on Postpartum Thyroid Status in Pregnant Women with Thyroid Peroxidase Autoantibodies. J. Clin. Endocrinol. Metab. 2007, 92, 1263–1268.
- Mantovani, G.; Isidori, A.M.; Moretti, C.; Dato, C.D.; Greco, E.; Ciolli, P.; Bonomi, M.; Petrone, L.; Fumarola, A.; Campagna, G.; et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine 2019, 66, 542–550.
- Marcocci, C.; Kahaly, G.J.; Krassas, G.E.; Bartalena, L.; Prummel, M.; Stahl, M.; Altea, M.A.; Nardi, M.; Pitz, S.; Boboridis, K.; et al. Selenium and the Course of Mild Graves’ Orbitopathy. N. Engl. J. Med. 2011, 364, 1920–1931.
- Winther, K.H.; Rayman, M.P.; Bonnema, S.J.; Hegedüs, L. Selenium in thyroid disorders—essential knowledge for clinicians. Nat. Rev. Endocrinol. 2020, 16, 165–176.
- Prabhu, K.S.; Lei, X.G. Selenium. Adv. Nutr. 2016, 7, 415–417.
- Kudva, A.K.; Shay, A.E.; Prabhu, K.S. Selenium and inflammatory bowel disease. Am. J. Physiol. Liver Physiol. 2015, 309, G71–G77.
- Kaushal, N.; Kudva, A.K.; Patterson, A.D.; Chiaro, C.; Kennett, M.J.; Desai, D.; Amin, S.; Carlson, B.A.; Cantorna, M.T.; Prabhu, K.S. Crucial Role of Macrophage Selenoproteins in Experimental Colitis. J. Immunol. 2014, 193, 3683–3692.
- Saxena, A.; Fayad, R.; Kaur, K.; Truman, S.; Greer, J.; Carson, J.A.; Chanda, A. Dietary selenium protects adiponectin knockout mice against chronic inflammation in-duced colon cancer. Cancer Biol. Ther. 2017, 18, 257–267.
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The Role of Selenium in Inflammation and Immunity: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants Redox Signal. 2012, 16, 705–743.
- Norton, R.L.; Hoffmann, P.R. Selenium and asthma. Mol. Asp. Med. 2012, 33, 98–106.
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101.
- Harthill, M. Review: Micronutrient Selenium Deficiency Influences Evolution of Some Viral Infectious Diseases. Biol. Trace Element Res. 2011, 143, 1325–1336.
- Schiavon, M.; Nardi, S.; Vecchia, F.D.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270.
- Bae, M.; Kim, H. Mini-Review on the Roles of Vitamin C, Vitamin D, and Selenium in the Immune System against COVID-19. Molecules 2020, 25, 5346.
- Moghaddam, A.; Heller, R.A.; Sun, Q.; Seelig, J.; Cherkezov, A.; Seibert, L.; Hackler, J.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Selenium Deficiency Is Associated with Mortality Risk from COVID-19. Nutrients 2020, 12, 2098.
- Zhang, J.; Taylor, E.W.; Bennett, K.; Saad, R.; Rayman, M.P. Association between regional selenium status and reported outcome of COVID-19 cases in China. Am. J. Clin. Nutr. 2020, 111, 1297–1299.
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738.
- Heller, R.A.; Sun, Q.; Hackler, J.; Seelig, J.; Seibert, L.; Cherkezov, A.; Minich, W.B.; Seemann, P.; Diegmann, J.; Pilz, M.; et al. Prediction of survival odds in COVID-19 by zinc, age and selenoprotein P as composite biomarker. Redox Biol. 2021, 38, 101764.
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early Nutritional Interventions with Zinc, Selenium and Vitamin D for Raising Anti-Viral Resistance Against Progressive COVID-19. Nutrients 2020, 12, 2358.
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of M(pro) from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293.
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213.
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2018, 1, CD005195.
- Matthews, N.H.; Fitch, K.; Li, W.Q.; Morris, J.S.; Christiani, D.C.; Qureshi, A.A.; Cho, E. Exposure to Trace Elements and Risk of Skin Cancer: A Systematic Review of Epi-demi-ologic Studies. Cancer Epidemiol. Biomark. Prev. 2019, 28, 3–21.
- Vinceti, M.; Filippini, T.; Del Giovane, C.; Dennert, G.; Zwahlen, M.; Brinkman, M.; Zeegers, M.P.A.; Horneber, M.; D’Amico, R.; Crespi, C.M. Selenium for preventing cancer. Cochrane Database Syst. Rev. 2014, 2014, CD005195.
- Gül-Klein, S.; Haxhiraj, D.; Seelig, J.; Kästner, A.; Hackler, J.; Sun, Q.; Heller, R.A.; Lachmann, N.; Pratschke, J.; Schmelzle, M.; et al. Serum Selenium Status as a Diagnostic Marker for the Prognosis of Liver Transplan-ta-tion. Nutrients 2021, 13, 619.
- Blot, W.J.; Li, J.-Y.; Taylor, P.R.; Guo, W.; Dawsey, S.; Wang, G.-Q.; Yang, C.S.; Zheng, S.-F.; Gail, M.; Li, G.-Y.; et al. Nutrition Intervention Trials in Linxian, China: Supplementation with Specific Vitamin/Mineral Combinations, Cancer Incidence, and Disease-Specific Mortality in the General Population. J. Natl. Cancer Inst. 1993, 85, 1483–1491.
- Clark, L.C.; Combs, G.J.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with car-ci-noma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963.
- Clark, L.C.; Dalkin, B.; Krongrad, A.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Witherington, R.; Herlong, J.H.; Janosko, E.; Carpenter, D.; et al. Decreased incidence of prostate cancer with selenium supplementation: Results of a double-blind cancer prevention trial. BJU Int. 1998, 81, 730–734.
- Marshall, J.R.; Tangen, C.M.; Sakr, W.A.; Wood, D.P., Jr.; Berry, D.L.; Klein, E.A.; Lippman, S.M.; Parnes, H.L.; Alberts, D.S.; Jarrard, D.F.; et al. Phase III trial of selenium to prevent prostate cancer in men with high-grade pros-tatic intraepithelial neoplasia: SWOG S9917. Cancer Prev. Res. 2011, 4, 1761–1769.
- Algotar, A.M.; Stratton, M.S.; Ahmann, F.R.; Ranger-Moore, J.; Nagle, R.B.; Thompson, P.A.; Slate, E.; Hsu, C.H.; Dalkin, B.L.; Sindhwani, P.; et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate 2013, 73, 328–335.
- Karp, D.D.; Lee, S.J.; Keller, S.M.; Wright, G.S.; Aisner, S.; Belinsky, S.A.; Johnson, D.H.; Johnston, M.R.; Goodman, G.; Clamon, G.; et al. Randomized, Double-Blind, Placebo-Controlled, Phase III Chemoprevention Trial of Selenium Supplementation in Patients with Resected Stage I Non–Small-Cell Lung Cancer: ECOG 5597. J. Clin. Oncol. 2013, 31, 4179–4187.
- Kristal, A.R.; Darke, A.; Morris, J.S.; Tangen, C.M.; Goodman, P.J.; Thompson, I.M.; Meyskens, F.L.; Goodman, G.E.; Minasian, L.M.; Parnes, H.L.; et al. Baseline Selenium Status and Effects of Selenium and Vitamin E Supplementation on Prostate Cancer Risk. J. Natl. Cancer Inst. 2014, 106, djt456.
- Thompson, P.A.; Ashbeck, E.L.; Roe, D.J.; Fales, L.; Buckmeier, J.; Wang, F.; Bhattacharyya, A.; Hsu, C.-H.; Chow, H.H.S.; Ahnen, D.J.; et al. Selenium Supplementation for Prevention of Colorectal Adenomas and Risk of Associated Type 2 Diabetes. J. Natl. Cancer Inst. 2016, 108, djw152.
- Menter, D.G.; Sabichi, A.L.; Lippman, S.M. Selenium effects on prostate cell growth. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1171–1182.
- Li, G.-X.; Lee, H.-J.; Wang, Z.; Hu, H.; Liao, J.D.; Watts, J.C.; Combs, J.G.F.; Lü, J. Superior in vivo inhibitory efficacy of methylseleninic acid against human prostate cancer over selenomethionine or selenite. Carcinogenesis 2008, 29, 1005–1012.
- Ip, C.; Thompson, H.J.; Zhu, Z.; Ganther, H.E. In Vitro and in vivo studies of methylseleninic acid: Evidence that a monome-thyl-ated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000, 60, 2882–2886.
- Abdulah, R.; Miyazaki, K.; Nakazawa, M.; Koyama, H. Chemical forms of selenium for cancer prevention. J. Trace Elements Med. Biol. 2005, 19, 141–150.
- Yan, L.; DeMars, L.C. Dietary supplementation with methylseleninic acid, but not selenomethionine, reduces spontaneous me-tastasis of Lewis lung carcinoma in mice. Int. J. Cancer 2012, 131, 1260–1266.
- Ip, C.; Zhu, Z.; Thompson, H.J.; Lisk, D.; E Ganther, H. Chemoprevention of mammary cancer with Se-allylselenocysteine and other selenoamino acids in the rat. Anticancer Res. 2000, 19, 2875–2880.
- Pi, J.; Jiang, J.; Cai, H.; Yang, F.; Jin, H.; Yang, P.; Cai, J.; Chen, Z.W. GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways. Drug Deliv. 2017, 24, 1549–1564.
- Chen, S.; Xing, C.; Huang, D.; Zhou, C.; Ding, B.; Guo, Z.; Peng, Z.; Wang, D.; Zhu, X.; Liu, S.; et al. Eradication of tumor growth by delivering novel photothermal selenium-coated tellurium nanoheterojunctions. Sci. Adv. 2020, 6, eaay6825.
- Hu, Y.; McIntosh, G.H.; Young, G.P. Selenium-rich foods: A promising approach to colorectal cancer prevention. Curr. Pharm. Biotechnol. 2012, 13, 165–172.
- Liu, J.-G.; Zhao, H.-J.; Liu, Y.-J.; Liu, Y.-W.; Wang, X.-L. Effect of two selenium sources on hepatocarcinogenesis and several angiogenic cytokines in diethylnitrosamine-induced hepatocarcinoma rats. J. Trace Elements Med. Biol. 2012, 26, 255–261.
- Li, G.X.; Hu, H.; Jiang, C.; Schuster, T.; Lu, J. Differential involvement of reactive oxygen species in apoptosis induced by two clas-ses of selenium compounds in human prostate cancer cells. Int. J. Cancer 2007, 120, 2034–2043.
- Jiang, C.; Wang, Z.; Ganther, H.; Lü, J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol. Cancer Ther. 2002, 1, 1059–1066.
- Jiang, C.; Hu, H.; Malewicz, B.; Wang, Z.; Lü, J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apopto-sis in LNCaP human prostate cancer cells. Mol. Cancer Ther. 2004, 3, 877–884.
- Hu, H.; Li, G.-X.; Wang, L.; Watts, J.; Combs, G.F.; Lü, J. Methylseleninic Acid Enhances Taxane Drug Efficacy against Human Prostate Cancer and Down-Regulates Antiapoptotic Proteins Bcl-XL and Survivin. Clin. Cancer Res. 2008, 14, 1150–1158.
- Yin, S.; Dong, Y.; Li, J.; Fan, L.; Wang, L.; Lu, J.; Vang, O.; Hu, H. Methylseleninic acid potentiates multiple types of cancer cells to ABT-737-induced apoptosis by tar-geting Mcl-1 and Bad. Apoptosis 2012, 17, 388–399.
- Guo, X.; Yin, S.; Dong, Y.; Fan, L.; Ye, M.; Lu, J.; Hu, H. Enhanced apoptotic effects by the combination of curcumin and methylseleninic acid: Potential role of mcl-1 and fak. Mol. Carcinog. 2013, 52, 879–889.
- Jiang, W.; Jiang, C.; Pei, H.; Wang, L.; Zhang, J.; Hu, H.; Lü, J. In vivo molecular mediators of cancer growth suppression and apoptosis by selenium in mammary and prostate models: Lack of involvement of gadd genes. Mol. Cancer Ther. 2009, 8, 682–691.
- Wu, X.; Zhang, Y.; Pei, Z.; Chen, S.; Yang, X.; Chen, Y.; Lin, D.; Ma, R.Z. Methylseleninic acid restricts tumor growth in nude mice model of metastatic breast cancer probably via inhibiting angiopoietin-2. BMC Cancer 2012, 12, 192.
- Sinha, I.; Null, K.; Wolter, W.; Suckow, M.A.; King, T.; Pinto, J.T.; Sinha, R. Methylseleninic acid downregulates hypoxia-inducible factor-1α in invasive prostate cancer. Int. J. Cancer 2011, 130, 1430–1439.
- Cai, Z.; Dong, L.; Song, C.; Zhang, Y.; Zhu, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Huang, Z.; et al. Methylseleninic Acid Provided at Nutritional Selenium Levels Inhibits Angiogenesis by Down-regulating Integrin β3 Signaling. Sci. Rep. 2017, 7, 9445.
- Roomi, M.W.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Modulation of uPA, MMPs and their inhibitors by a novel nutrient mix-ture in human glioblastoma cell lines. Int. J. Oncol. 2014, 45, 887–894.
- Sundaram, S.; Yan, L. Dietary Supplementation with Methylseleninic Acid Inhibits Mammary Tumorigenesis and Metastasis in Male MMTV-PyMT Mice. Biol. Trace Elem. Res. 2018, 184, 186–195.
- Yoon, S.-O.; Kim, M.-M.; Chung, A.-S. Inhibitory Effect of Selenite on Invasion of HT1080 Tumor Cells. J. Biol. Chem. 2001, 276, 20085–20092.
- Hu, Y.; Liu, T.; Li, J.; Mai, F.; Li, J.; Chen, Y.; Jing, Y.; Dong, X.; Lin, L.; He, J.; et al. Selenium nanoparticles as new strategy to potentiate γδ T cell anti-tumor cytotoxicity through up-reg-ulation of tubulin-α acetylation. Biomaterials 2019, 222, 119397.
- Nair, D.; Rådestad, E.; Khalkar, P.; Diaz-Argelich, N.; Schröder, A.; Klynning, C.; Ungerstedt, J.; Uhlin, M.; Fernandes, A.P. Methylseleninic Acid Sensitizes Ovarian Cancer Cells to T-Cell Mediated Killing by De-creasing PDL1 and VEGF Levels. Front. Oncol. 2018, 8, 407.
- Bera, S.; De Rosa, V.; Rachidi, W.; Diamond, A.M. Does a role for selenium in DNA damage repair explain apparent controversies in its use in chemoprevention? Mutagenesis 2013, 28, 127–134.
- Gudkov, A.V. Converting p53 from a killer into a healer. Nat. Med. 2002, 8, 1196–1198.
- Seo, Y.R.; Kelley, M.R.; Smith, M.L. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc. Natl. Acad. Sci. USA 2002, 99, 14548–14553.
- Dong, Y.; Lee, S.O.; Zhang, H.; Marshall, J.; Gao, A.C.; Ip, C. Prostate Specific Antigen Expression Is Down-Regulated by Selenium through Disruption of Androgen Receptor Signaling. Cancer Res. 2004, 64, 19–22.
- Lee, S.O.; Nadiminty, N.; Wu, X.X.; Lou, W.; Dong, Y.; Ip, C.; Onate, S.A.; Gao, A.C. Selenium Disrupts Estrogen Signaling by Altering Estrogen Receptor Expression and Ligand Binding in Human Breast Cancer Cells. Cancer Res. 2005, 65, 3487–3492.
- Gao, R.; Zhao, L.; Liu, X.; Rowan, B.G.; Wabitsch, M.; Edwards, D.P.; Nishi, Y.; Yanase, T.; Yu, Q.; Dong, Y. Methylseleninic acid is a novel suppressor of aromatase expression. J. Endocrinol. 2011, 212, 199–205.
- Kim, E.H.; Sohn, S.; Kwon, H.J.; Kim, S.U.; Kim, M.-J.; Lee, S.-J.; Choi, K.S. Sodium selenite induces superoxide-mediated mitochondrial damage and subsequent au-tophagic cell death in malignant glioma cells. Cancer Res. 2007, 67, 6314–6324.
- Pan, X.; Song, X.; Wang, C.; Cheng, T.; Luan, D.; Xu, K.; Tang, B. H2Se Induces Reductive Stress in HepG2 Cells and Activates Cell Autophagy by Regulating the Redox of HMGB1 Protein under Hypoxia. Theranostics 2019, 9, 1794–1808.
- Zhang, Y.; Zheng, S.; Zheng, J.-S.; Wong, K.-H.; Huang, Z.; Ngai, S.-M.; Zheng, W.; Wong, Y.-S.; Chen, T. Synergistic Induction of Apoptosis by Methylseleninic Acid and Cisplatin, The Role of ROS-ERK/AKT-p53 Pathway. Mol. Pharm. 2014, 11, 1282–1293.
- Tarrado-Castellarnau, M.; Cortés, R.; Zanuy, M.; Tarragó-Celada, J.; Polat, I.H.; Hill, R.; Fan, T.W.; Link, W.; Cascante, M. Methylseleninic acid promotes antitumour effects via nuclear FOXO3a translocation through Akt inhibition. Pharmacol. Res. 2015, 102, 218–234.
- Cao, S.; Durrani, F.A.; Rustum, Y.M. Selective Modulation of the Therapeutic Efficacy of Anticancer Drugs by Selenium Containing Compounds against Human Tumor Xenografts. Clin. Cancer Res. 2004, 10, 2561–2569.
- Cao, S.; A Durrani, F.; Tóth, K.; Rustum, Y.M. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br. J. Cancer 2014, 110, 1733–1743.
- Hu, H.; Jiang, C.; Ip, C.; Rustum, Y.M.; Lü, J. Methylseleninic Acid Potentiates Apoptosis Induced by Chemotherapeutic Drugs in Androgen-Independent Prostate Cancer Cells. Clin. Cancer Res. 2005, 11, 2379–2388.
- Li, S.; Zhou, Y.; Wang, R.; Zhang, H.; Dong, Y.; Ip, C. Selenium sensitizes MCF-7 breast cancer cells to doxorubicin-induced apopto-sis through modulation of phospho-Akt and its downstream substrates. Mol. Cancer Ther. 2007, 6, 1031–1038.
- Yamaguchi, K.; Uzzo, R.G.; Pimkina, J.; Makhov, P.; Golovine, K.; Crispen, P.; Kolenko, V.M. Methylseleninic acid sensitizes prostate cancer cells to TRAIL-mediated apoptosis. Oncogene 2005, 24, 5868–5877.
- Hu, H.; Jiang, C.; Schuster, T.; Li, G.-X.; Daniel, P.T.; Lü, J. Inorganic selenium sensitizes prostate cancer cells to TRAIL-induced apoptosis through superoxide/p53/Bax-mediated activation of mitochondrial pathway. Mol. Cancer Ther. 2006, 5, 1873–1882.
- Shin, S.H.; Yoon, M.J.; Kim, M.; Kim, J.-I.; Lee, S.-J.; Lee, Y.-S.; Bae, S. Enhanced lung cancer cell killing by the combination of selenium and ionizing radiation. Oncol. Rep. 2007, 17, 209–216.
- Lafin, J.T.; Sarsour, E.H.; Kalen, A.L.; Wagner, B.A.; Buettner, G.R.; Goswami, P.C. Methylseleninic Acid Induces Lipid Peroxidation and Radiation Sensitivity in Head and Neck Cancer Cells. Int. J. Mol. Sci. 2019, 20, 225.
- Asfour, I.A.; Fayek, M.; Raouf, S.; Soliman, M.; Hegab, H.M.; El-Desoky, H.; Saleh, R.; Moussa, M.A.R. The Impact of High-dose Sodium Selenite Therapy on Bcl-2 Expression in Adult Non-Hodgkin’s Lymphoma Patients: Correlation with Response and Survival. Biol. Trace Element Res. 2007, 120, 1–10.
- Muecke, R.; Schomburg, L.; Glatzel, M.; Berndt-Skorka, R.; Baaske, D.; Reichl, B.; Buentzel, J.; Kundt, G.; Prott, F.J.; Devries, A.; et al. Multicenter, Phase 3 Trial Comparing Selenium Supplementation with Observation in Gynecologic Radiation Oncology. Int. J. Radiat. Oncol. 2010, 78, 828–835.
- Muecke, R.; Micke, O.; Schomburg, L.; Glatzel, M.; Reichl, B.; Kisters, K.; Schaefer, U.; Huebner, J.; Eich, H.T.; Fakhrian, K.; et al. Multicenter, phase III trial comparing selenium supplementation with observation in gynecologic radiation oncology: Follow-up analysis of the survival data 6 years after cessation of randomization. Integr. Cancer Ther. 2014, 13, 463–467.
- Lobb, R.J.; Jacobson, G.M.; Cursons, R.T.; Jameson, M.B. The Interaction of Selenium with Chemotherapy and Radiation on Normal and Malignant Human Mononuclear Blood Cells. Int. J. Mol. Sci. 2018, 19, 3167.
- Muecke, R.; Micke, O.; Schomburg, L.; Buentzel, J.; Kisters, K.; Adamietz, I.A. Selenium in Radiation Oncology-15 Years of Expe-ri-ences in Germany. Nutrients 2018, 10, 483.
- Vieira, M.L.D.S.; Fonseca, F.L.A.; Costa, L.G.; Beltrame, R.L.; Chaves, C.M.D.S.; Cartum, J.; Alves, S.I.P.M.d.N.; Azzalis, L.A.; Junqueira, V.B.C.; Pereria, E.C.; et al. Supplementation with Selenium Can Influence Nausea, Fatigue, Physical, Renal, and Liver Function of Children and Adolescents with Cancer. J. Med. Food 2015, 18, 109–117.
- Fakih, M.G.; Pendyala, L.; Brady, W.; Smith, P.F.; Ross, M.E.; Creaven, P.J.; Badmaev, V.; Prey, J.D.; Rustum, Y.M. A Phase I and pharmacokinetic study of selenomethionine in combination with a fixed dose of irinotecan in solid tumors. Cancer Chemother. Pharmacol. 2007, 62, 499–508.
- Qazi, I.H.; Angel, C.; Yang, H.; Zoidis, E.; Pan, B.; Wu, Z.; Ming, Z.; Zeng, C.-J.; Meng, Q.; Han, H.; et al. Role of Selenium and Selenoproteins in Male Reproductive Function: A Review of Past and Present Evidences. Antioxidants 2019, 8, 268.
- Taghizadeh, L.; Eidi, A.; Mortazavi, P.; Rohani, A.H. Effect of selenium on testicular damage induced by varicocele in adult male Wistar rats. J. Trace Elements Med. Biol. 2017, 44, 177–185.
- Qazi, I.H.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.-J.; Han, H.; Zhou, G.-B. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053.
- Grieger, J.A.; Grzeskowiak, L.E.; Wilson, R.L.; Bianco-Miotto, T.; Leemaqz, S.Y.; Jankovic-Karasoulos, T.; Perkins, A.V.; Norman, R.J.; Dekker, G.A.; Roberts, C.T. Maternal Selenium, Copper and Zinc Concentrations in Early Pregnancy, and the Association with Fertility. Nutrients 2019, 11, 1609.
