Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ravindra Prajapati and Version 2 by Vivi Li.
Plastic is referred to as a “material of every application”. From the packaging and automotive industries to the medical apparatus and computer electronics sectors, plastic materials are fulfilling demands efficiently. These plastics usually end up in landfills and incinerators, creating plastic waste pollution. According to the Environmental Protection Agency (EPA), in 2015, 9.1% of the plastic materials generated in the U.S. municipal solid waste stream was recycled, 15.5% was combusted for energy, and 75.4% was sent to landfills.
- plastic wastes
- chemicals
- chemical recycling
- carbon nanomaterials
- carbonization
- biodegradable plastics
1. Introduction
Plastic waste pollution is a major threat to ocean, wildlife, and human health. The global plastic market size was valued at USD 568.9 billion in 2019 and is expected to grow at a compounded annual growth rate (CAGR) of 3.2% from 2020 to 2027 [1]. However, the recent outbreak of Coronavirus (COVID-19) is taking the plastic waste pollution problem to a whole new level. Projections have shown that the global plastic packaging market is expected to grow from USD 909.2 billion in 2019 to USD 1012.6 billion by 2021 at a CAGR of 5.5%, mainly due to the pandemic response [2]. Most of this plastic waste ends up either in landfills or incinerators and is lost forever as a resource, despite its potential for reuse and recycling. According to the Environmental Protection Agency (EPA), in 2015, 9.1% of the plastic materials generated in the U.S. municipal solid waste stream was recycled, 15.5% was combusted for energy, and 75.4% was sent to landfills [3]. Plastic waste dumping creates serious difficulties in maintaining a clean and green environment. Yet, plastic waste reuse and recycling are projected to generate a profit-pool growth of USD 60 billion for the plastic and petrochemical sectors [4]. To generate profit, a petrochemical industry should establish a waste-collection system to adapt the plastic waste recycling strategies. In addition, plastic and petrochemical industries need to implement a different business model in which they can consider plastic waste supplies from various sources rather than obtaining raw material from one source. These industries should maintain product-portfolio priorities and implement a circular economy as much as possible [4].
Researchers worldwide have taken up plastic waste as an opportunity resource and are investigating innovative technologies that promote the recycling of plastic wastes. This includes research and development to produce new raw chemical materials and develop chemical recycling strategies to create value out of waste. The most common approach is converting plastic waste into a secondary raw material such as monomers or pyrolysis oil. The recycled material can be used in the production of new plastics, and the pyrolyzed oil can be fed into a chemical production unit (such as steam cracker); this way, these plastic waste products can replace fossil-based feedstocks.
2. Production of Chemicals from Plastic Wastes
Chemical recycling incorporates sustainability principles because it can produce either new chemical raw materials or original raw materials. In chemical recycling processes, the pyrolysis method is considered a stand-alone facility for the upgradation of plastic wastes. This process is highly useful, particularly with polyolefins (POs), which contain 2/3 of the plastic wastes to produce gaseous or liquid fuels or raw chemicals, mainly light olefins and benzene, toluene, and xylene (BTX). Polyolefin pyrolysis has gained significant interest because pyrolysis can be performed in small units near the collection sites. Therefore, one can avoid the costs related to the transportation of plastic wastes. The products obtained by thermal pyrolysis at low and high temperatures from plastics are given in Table 1. A wide range of hydrocarbons (HCs), such as paraffins, olefins, and aromatics, can be produced from the pyrolysis of plastic wastes. The yields of these HCs depend on the chemical composition, structure, and decomposition of plastics. For instance, the pyrolytic product from polystyrene (PS) waste can be refined to produce styrene, while paraffins and olefins can be obtained from polyethylene (PE) and polypropylene (PP) wastes. Further, thermal pyrolysis of polymethyl methacrylate produces a monomer, i.e., methyl methacrylate, and a 98% yield was reported at 450 °C [5][13].
Table 1. Products from different plastics by thermal pyrolysis.
| Polymer | Low-Temperature Products | High-Temperature Products |
|---|---|---|
| Polyethylene (PE) | Waxes, paraffin oil, alpha-olefins |
Gases and light oils |
| Polypropylene (PP) | Vaseline, olefins | Gases and light oils |
| Polystyrene (PS) | Styrene and its oligomers | Styrene and its oligomers |
| Polymethyl methacrylate (PMMA) | Methylmethacrylate (monomer) |
Low methyl methacrylate, more decomposition products |
| Polyethylene terephthalate (PET) | Benzoic acid (BA), Vinyl terephthalate |
|
| Polyamide 6 (PA6) | ε-caprolactum (CPL) |
Pyrolysis–catalysis has proved to be a promising technology for the plastic waste conversion into high-value products. The catalysts used for the plastic waste conversion play an important role during the processing. In general, plastics do not degrade easily, due to the presence of very strong carbon−carbon bonds. Through catalytic means, we can regain the high energy that holds these bonds in plastics; this will help to convert the plastic wastes into value-added commercial products. POs are challenging to deconstruct catalytically. The catalysts consisting of nanoparticles could help to develop more robust and effective recycling methods. The catalytic hydrogenolysis of POs has been investigated using various catalytic systems. Highly electrophilic Zr-H species prepared by surface organometallic chemistry convert the high-molecular-weight polymers (Mw = 125,000 Da), with the C20−C50 carbon chain, into fuels and smaller HCs [6][14]. Pt, Ir, Ru, and Rh nanoparticles have been studied for the catalytic hydrogenolysis of C2−C10 alkanes [7][8][9][15,16,17]. The catalytic activity depends on various factors such as operating conditions, the degree of substitution at each carbon atom of n-alkanes, and the characteristics (size and metal type) of supported metal particles. The product distribution also depends on the feedstock properties. For instance, with the Ni/H-beta catalyst, a high yield of gasoline and light diesel was obtained from PP rather than low-density polyethylene (LDPE) or high-density polyethylene (HDPE) [10][18]. However, in another report, more aromatics were produced from HDPE compared to LDPE [11][19]. In addition, carbon nanomaterials that can be recycled from plastic waste have also attracted attention in recent times. More details about carbon nanomaterials are provided in Section 3.
Another promising technology is hydrothermal liquefaction (HTL). It is highly flexible in treating both pure and mixed waste streams. The HTL technique is based on fast-heating-rate reactors with moderate residence times (15–20 min), temperatures (300–360 °C), pressures near to water saturation, and the use of catalysts (based on the feedstocks) [12][13][20,21]. Passos et al. [13][21] demonstrated a total of 12 different commercial polymers such as acrylonitrile-butadiene-styrene (ABS), HDPE, LDPE, polyamide 6 (PA6), polyamide 6/6 (PA66), polyethylene terephthalate (PET), polycarbonate (PC), PP, PS, and polyurethane foam (PUR) using the subcritical HTL process. The HTL reactions were performed in a 20 mL autoclave reactor at 350 °C for 20 min. The main findings were as follows: (i) bis-phenol-A (BPA) and its derivative compounds were identified in the oil products from epoxy and PC polymers; (ii) solid terephthalic acid (TA) as the major product was obtained in noncatalytic HTL of PET; (iii) from PA6 and PA66, AP monomers were produced, and these monomers can be repolymerized, if pure feeds are used; (iv) the oil produced from PUR polymer is a complex that contains oligomers and low-boiling-point compounds; (v) the solid residues from PVC are highly dechlorinated, and this fraction can be used as a carbon source. The results suggested that each type of synthetic polymer undergoes a different type of depolymerization based on its composition under HTL processing.
Gasification is another process that can produce syngas, which can be used as a precursor to produce acetic acid, methanol, aldehyde, carbohydrates, ammonia, etc. This process is the most advantageous because it can treat even nonsegregated wastes. However, the process produces poisonous hydrogen cyanide and nitrogen oxide as gases, and the emissions can be reduced by using effective catalysts to some extent.
2.1. Polyethylene (PE) and Polypropylene (PP)
Table 2 summarizes the few recent reported studies for chemical production from plastic wastes, which are discussed in detail. In general, a conversion process of plastic yields gas, liquid, and solid residues. From PE and PP, liquid products in the range of 83 to 96% can be obtained by pyrolysis [14][15][22,23]. As said earlier, the composition of the final products depends on the type of feedstock, conditions used, catalytic or noncatalytic, reactor system, etc. [16][24]. In PE pyrolysis, the yields of aromatics increased from 3 to 6% and the yields of naphthalenes decreased from 22 to 17% [16][24], whereas in PP pyrolysis, the paraffins yield decreased from 33 to 27% with increasing temperature from 350 to 520 °C, and that of aromatics increased from 0.8 to 11% with increasing temperature from 350 to 600 °C. A two-step process involving pyrolysis and downstream catalytic cracking was applied for the light olefin production from HDPE, and the pyrolysis was performed in a conical spouted-bed reactor (CSBR) at a reaction temperature of 500 °C. The volatile stream obtained from the HDPE pyrolysis in a CSBR mainly contained waxes (>C21), and this volatile stream was passed through a fixed-bed (downflow) catalytic reactor in the presence of HZSM-5 zeolite. It was found that 67% of the waxes were converted into light olefins. This is because of the shape selectivity, low hydrogen transfer capacity, and moderate acid strength of the HZSM-5 zeolite [17][25]. Besides the acidity of the HZSM-5 zeolite, the short residence time in the reactor was found to increase the selectivity of the light olefins and decrease the coke formation. The high-value aromatic chemical raw materials such as benzene, toluene, and other aromatic HCs can also be obtained by refining the pyrolytic product. For instance, the pyrolysis of PE and PP produces a liquid product that mainly contains BTX compounds [18][26]. The BTX yield can be increased by increasing the reaction temperature and using suitable catalysts. In general, aromatic compounds are formed due to secondary reactions and shape selectivities of the catalysts. Zhang et al. [19][27] developed a low-temperature catalytic method to convert PE directly into liquid alkylaromatics using a Pt/γ-Al2O3 catalyst. The produced alkylaromatics have applications such as lubricants, surfactants, insulating oils, and refrigeration fluids.
Table 2. Summary of reaction conditions, product yields, and key findings for chemicals produced from plastic wastes.
| S. No. | Plastics | Method | Conditions | Product Yields | Key Findings | Source |
|---|---|---|---|---|---|---|
| 1. | PE | Noncatalytic pyrolysis | T = 602 °C | Paraffins 45%; Olefins 32%; Naphthalenes 17%;Aromatics 6%; | A whole spectrum of HCs, including paraffins, olefins, naphthalenes, and aromatics. | [16][24] |
| PP | T = 602 °C | Paraffins 27%; Olefins 36%; Aromatics 11%; | ||||
| PS | T = 477 °C | Styrene 63% | ||||
| 2. | HDPE | Thermal-catalytic two-step pyrolysis | T = 500 °C | Light olefin 59% | Higher efficiency of the two-step reaction system compared to the in situ catalytic pyrolysis (single-step) for production of 10 wt.% ethylene, 32 wt.% propylene, and 17 wt.% butenes. | [17][25] |
| 3. | PP and PE | Fluidized bed reactor | T = 650–750 °C |
BTX 32–53% | Higher feed rates and gaseous fluidizing medium have a positive effect on liquid oil production. | [18][26] |
| 4. | PE | Mini-autoclave reactor (unstirred) | T = 280 °C, t = 24 h, Pt/Al2O3 |
Liquid product 80% | Tandem catalytic conversion produces a high yield of low-molecular-weight liquid/wax products. | [19][27] |
| 5. | PS | Fluidized bed reactor | T = 520 °C | Styrene 83% | Complete conversion of PS to styrene oil was reported, with only traces of aliphatic compounds | [20][28] |
| 6. | PS + organic compounds | Autoclave reactor | T = 400 °C, t = 1 h |
Liquid 91%; Residue < 4% |
Maximum styrene yield in the liquid was obtained with naphthalene as an organic compound with PS | [21][29] |
| 7. | PS | Flow reactor | T = 350 °C, t = 3 h, Fe2O3 |
Liquid 83.6%; Residue 4.8% Styrene 74.3% (in liquid oil) |
Barium oxide powder was found to be most effective catallyst for chemical recyling of PS waste | [22][30] |
| T = 350 °C, t = 3 h, BaO |
Liquid 93.4%; Residue 3.2% Styrene 76.4% (in liquid oil) |
|||||
| T = 350 °C, t = 3 h, HSM5 |
Liquid 78.2%; Residue 8.5% Styrene 64.4% (in liquid oil) |
|||||
| 8. | PS | Fixed-bed reactor | T = 510 °C thermal | Liquid 91.8%; Residue 5.7% |
Other aromatic compounds can behave like a chain transfer agent and reduce the Tg of product polymer. | [23][31] |
| T = 510 °C BaO (cat.) | Liquid 91.2%; Residue 8.1% |
|||||
| T = 510 °C FCC cat. | Liquid 90.7%; Residue 7.1% | |||||
| 9. | PS | Two-stage auger and fluidized bed reactor | T = 780 °C | BTEX 26.3% | Product yields depend on the reaction temperatures and fluidizing mediums used. | [24][32] |
| 10. | PET | Glycolysis | T = 190 °C; atm pressure | BHET 100% conversion, 84% yields |
Lewis acidic ionic liquids [Bmim]ZnCl3 catalyst was found to be effective. | [25][33] |
| Hydrolysis | T = 200–250 °C; P = 1.4–2 MPa | TPA, EG | [26][34] | |||
| Methanolysis | T = 200 °C | DMT 64%; EG 63% |
The product yields depend on the solubility of PET. | [27][35] | ||
| Aminolysis | T = 70–110 °C | Diamides of TPA 66–89% | The bifunctional 1,5,7-triazabicyclo [4.4.0]dec-5-ene activates the carbonyl group and catalyzes the reaction. | [28][36] | ||
| Pyrolysis | T = 450–600 °C ZSM-5 zeolite and NiCl2 used as catalyst |
Aromatic hydroxyl groups increased by 22% | ZSM-5 facilitated the decomposition of carboxyl, aliphatic groups, and ether bonds in the primary products produced from the PET pyrolysis. | [29][37] | ||
| Pyrolysis | T = 400–700 °C | Phenyl carboxylic acid 44–79% | Pd loaded on activated carbon used as a catalyst and produced more environmentally friendly products | [29][37] | ||
| 11. | PET | Py-GCMS, EGA-MS, and TGA | T = 600 °C | 4(vinyl oxy carbonyl) BA 27%; BA 10% |
Wide range of liquid products obtained by different pyrolysis mechanisms. | [30][38] |
| 12. | PU | Glycolysis | T = 200–210 °C; t = 2 h |
Acetone-soluble products 80.8%; Residue 19%; Amines in total acetone soluble products 58.3 mgKOH/g |
Polyol products produced from the process and used as initiators to produce oxy-alkylated polyols. | [31][39] |
| 13. | PA 6 and PA66 | Aminolysis | T = 100 °C; P = 3.5 MPa; t = 5.6 h; Raney® Co 2724 |
ACN = 2; HMD = 32%; CPL = 46.2%; Other components = 13.6% | Raney® Co provided a better catalytic activity along with long catalyst life | [32][40] |
| T = 100 °C; P = 3.5 MPa; t = 5.6 h; Raney® Ni 2400 |
ACN = 19.6; HMD = 15%; CPL = 46.5%; Other components = 14.7% | |||||
| 14. | PA66 | Microwave irradiation | T = 200 °C; t = 0.16 h; HCl:PA66 = 1:0.25 |
AA 90%; HMDA 86%; with 100% purity | The rate of PA hydrolysis depended on the PA type and HCl/amide molar ratio. With microwave treatment, high-purity and high-quality products were formed. | [33][41] |
T = temperature; P = pressure; PE = polyethylene; HDPE = high-density polyethylene; PP = polypropylene; PS = polystyrene; PET = polyethylene terephthalate; PU = polyurethane; PA66 = polyamide 66; HCs = hydrocarbons; BTX = benzene, toluene, and xylene; BaO = barium oxide; FCC = fluid catalytic cracking; BTEX = benzene, toluene, ethylene, and xylene; BHET = bis(hydroxyethyl)-terephthalate; TPA = terephthalic acid; EG = ethylene glycol; DMT = dimethyl terephthalate; BA = benzoic acid; ACN = 6-aminocapronitrile; HMD = hexamethylene diamine; CPL = caprolactam; AA = adipic acid; HMDA = hexamethylene diamine; HCl = hydrochloric acid; py-GCMS = pyrolysis-gas chromatography mass spectroscopy; EGA-MS = evolved gas analyzer-mass chromatography; TGA = thermogravimetric analysis.
2.2. Polystyrene (PS)
PS, a thermoplastic, is used mainly in electrical appliances, medicines and packaging materials, thermal insulation, and in the automotive industry. In 2012, the U.S. generated ~2 million tons of PS waste, of which only 0.9% was recovered [34][42]. PS chemical recycling is mainly performed by the pyrolysis process [20][21][22][23][24][34][35][36][37][28,29,30,31,32,42,43,44,45]. A styrene monomer with high selectivity can be obtained via PS pyrolysis via thermal and catalytic routes. A 63% yield of styrene at 477 °C was observed [16][24]. This is because of the polycyclic nature of PS and the thermodynamic limitations in converting cyclic structures to aliphatic compounds. The product oil containing 83% (w/w) styrene was generated by PS pyrolysis at 520 °C using a fluidized-bed reactor [20][28]. With added organic additives such as naphthalene in the PS pyrolysis, the styrene yield can be enhanced [21][29]. Catalytic pyrolysis using ZSM-5 zeolite produces oil, which mainly contains single-ring aromatics such as ethylbenzene and toluene [35][43]. Zhang et al. [22][30] reported that the various basic catalysts helped to increase the monomer yield compared to thermal and/or acid-catalyzed pyrolysis. In addition to aromatic chemicals, the direct re-polymerization of the PS pyrolysis product to synthesize a polymer comparable to the original PS was also reported [23][31]. Productions of monomers such as benzene, toluene, ethylene, and xylenes (BTEX) were reported by using the two-stage pyrolysis process, which includes an auger and a fluidized-bed reactor [24][32]. A high value, i.e., 26%, of BTEX was obtained [24][32]. The microwave-assisted pyrolysis of PS with coal was investigated, and aromatic liquid products in the narrow range with acetylene and hydrogen sulfide were produced [36][44].
2.3. Polyethylene Terephthalate (PET)
Polyethylene terephthalate (PET) is one of the top four thermoplastics used throughout the world primarily in food packaging, the textile industry, and the production of bottles [37][45]. The production of PET worldwide in 2014 was approximately 41.6 million metric tons (MMT) and is forecasted to be approximately 73.4 MMT by 2020 [38][46]. In the U.S., the recycling rate for PET packaging was 31.2% in 2013, according to the Association of Postconsumer Plastic Recyclers (APR) and the National Association for PET Container Resources (NAPCOR). The recycling rate of PET waste is very low; therefore, there is a need to develop economical and low-carbon-footprint depolymerization processes to utilize this plastic waste in value-added applications. Chemical recycling methods for the PET wastes consist of glycolysis [25][33], hydrolysis [26][34], methanolysis [27][35], and aminolysis [28][36]. The most commonly used method is glycolysis because it is a simple, flexible, and low-cost process. In the glycolysis process, PET is treated by a glycol such as ethylene glycol (EG), propylene glycol, diethylene glycol, and triethylene glycol (TEA), with transesterification catalysts to yield bis(hydroxyethyl)-terephthalate (BHET) (shown in Figure 1) [25][33]. The produced BHET can be used with virgin PET or can be utilized for different PET production processes. The BHET yield and glycolysis reaction rates depend on the different reaction parameters such as temperature, type and amount of catalysts, and the PET/glycol ratio. On the contrary, via the hydrolysis method, terephthalic acid (TPA) and EG are mainly produced at high pressure (1.4–2 MPa), high temperature (200–250 °C), and longer reaction times [23][31]. Hydrolysis can be acidic (sulfuric or nitric acid), alkaline (sodium hydroxide), or neutral (metal catalysts). The cost associated with the process is high, and therefore, this process is not commercially used. The next method is methanolysis (treatment with alcohol), in which dimethyl terephthalate (DMT) and EG are mainly produced [27][35]. Finally, the aminolysis method involves the reaction of PET with amines such as allylamine, morphine, hydrazine, and polyamines to produce diamides of terephthalic acid (TPA) [28][36]. In recent times, the treatment of PET wastes with different amino alcohols, ethanolamine, has been of significant interest. This process leads to the production of phthalimide diols (low-cost polyols). The solid powder polyols (terephthalate diol) produced after the aminolysis step can be used as a building block to produce different kinds of polyurethane (PU) products with a higher economic value. PUs are the most important elastomers with extensive industrial applications. The global polyurethane market is forecasted to increase to USD 74.24 billion in 2021 from USD 58.28 billion in 2018 [39][47].
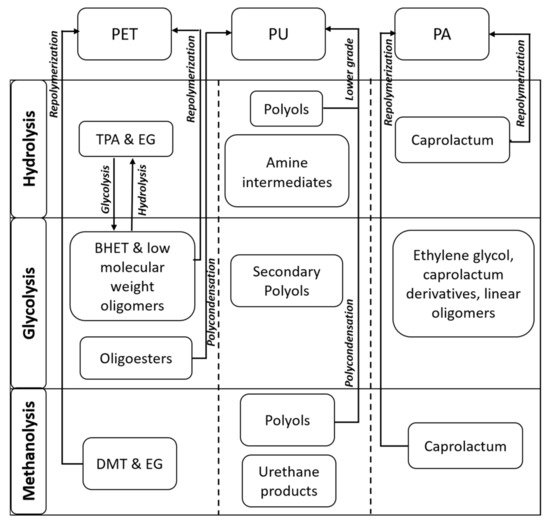
Figure 1. Products obtained through different pathways of polyethylene terephthalate (PET), polyurethane (PU), and polyamide (PA) (TPA = terephthalic acid; EG = ethylene glycol; BHET = bis(hydroxyethyl)-terephthalate; DMT = dimethyl terephthalate).
PET pyrolysis products consist of various aromatic and oxygenated compounds such as vinyl benzoate, benzoic acid (BA), and acetaldehyde. PET pyrolysis using ZSM-5 zeolite and NiCl2 catalysts was found to be effective for producing more liquid products [29][37]. The pyrolysis of waste PET takes place by cleavage of the ester linkage, leading to the formation of vinyl ester and carboxyl compounds, mainly BA. The produced vinyl ester can be decomposed into compounds such as acetophenone, acetaldehyde, and lighter HCs (C1−C3) [40][48]. BA, which is a high-value chemical around USD 4000/ton [41][49], is mainly used in the food and beverage industries. BA is also used as a feedstock for manufacturing phenols, benzoates, and other antifungal preservatives. Besides, BA is used as a feedstock for fungal ointments (medical use), plasticizers, and as a calibrating material for bomb calorimeters [42][50]. Thus, the recovery and production of BA from waste PET can produce a potential chemical. Dimitrov et al. [30][38] demonstrated that in the presence of a different medium or contaminants, different pyrolyzed products can be obtained. For instance, when the pyrolysis of PET is performed with acidic contaminants, CO2/acetaldehyde, BA, and vinyloxycarbonyl benzoic acids are formed. While in the presence of a base, tetramethylammonium hydroxide (TMAH), dimethyl terephthalate, short-chain alcohol, and trimethylamine (TMA) can be produced. TMA and short-chain alcohol are formed from the dissociation of TMAH. In another study, TPA was produced from the pyrolysis of PET, which can later be converted to benzene in the presence of CaO under controlled conditions.
The use of mechanically recycled PET as an additive in asphaltic mixtures has been explored. Modified asphalt prepared using PET wastes has shown advantages with respect to rutting and fatigue parameters and creep deformation as well [43][44][51,52]. Merkel et al. reported the use of chemically deconstructed mixed PET waste as an additive for asphalt [45][53]. The proposed approach utilizes the aminolysis process in which PET waste was treated with various amine nucleophiles to generate terephthalic amides with distinct structures such as polar, nonpolar, and lipophilic. For the activity demonstration, the generated terephthalic amides were added to the road-grade asphalt binder at 5 wt.% and the performance was investigated. Parameters such as rutting, fatigue characteristics, and thermochemical and creep performances were evaluated. The results revealed that the addition of these additives increases the performance by as much as 18%. Asphalt, mainly used in road construction and roofing, is the most expensive part of the road-paving material, although asphalt makes up only 5 wt.% of the pavement mixture. However, the cost of asphalt was approximately USD 610 per ton in 2012 [46][54]. Thus, recycling PET waste can produce high-performance asphalt paving mixtures.
2.4. Polyurethane (PU)
Polyurethane (PU) represents 8% of the total plastics, mainly used in coatings, adhesives, sealants, elastomers, mattresses, and automotive seats. Chemical compounds such as polyols and amine intermediates can be produced from PU (flexible foams) hydrolysis. The hydrolysis process is difficult to use at a larger scale because of the use of high temperature and high pressure. This process is also uneconomical because the time taken for hydrolysis reaction is quite long, conversion is relatively low, and product purification is challenging. The reaction of PU foams in the presence of water, glycols, and basic catalysts is widely used [31][47][48][49][39,55,56,57]. Multifunctional alcohols and amines can also be obtained by processing PU with diamines or amino alcohols. For this process, PU is dissolved in suitable solvents such as cyclic ether, a chlorinated HC solvent, or N-methyl pyrrolidone. The reaction temperature for this reaction ranges from 200 to 210 °C with catalysts [31][39]. Phosphorous containing oligourethanes can also be produced by treating PU with esters of phosphoric and phosphonic acids [50][58]. This technology has been less explored. These oligourethanes can be used to make new PUs with enhanced flame retardant, UV resistance, and adhesive properties.
2.5. Polyamides (PA)
Polyamides (PA) are utilized for various applications such as fibers in carpets and textiles, electrical and electronic industries, engineering plastic in the automotive and construction industries, and the coating and packaging sectors. Cyclic ε-caprolactam (CPL) as a monomer can be recovered from PA depolymerization (Figure 1). The depolymerization of PAs is mainly carried out by alcohols/glycols, ammonia, water, and in the presence of catalytic agents [51][52][59,60]. The major challenge associated with PA depolymerization is the harsh reaction conditions, which lead to the formation of undesired side products that create problems in purification. For instance, a 78% CPL yield was obtained from PA6 by hydrolysis in the presence of phosphotungstic acid at a reaction temperature of 300 °C and reaction time of 85 min. Products such as 6-amino-caproic acid and water-soluble oligomers were produced as side products [52][60]. PA glycolysis using EG with a diammonium hydrogen phosphate catalyst at 190 °C for 1.5 h led to incomplete degradation. A blend of glycosylates obtained was used as a replacement for industrial polyols in PU production [53][61]. The combination of diols and diesters was also produced from PA-based wastes in supercritical methanol at 330 °C [54][55][62,63]. Aminolysis can convert PA 66 and PA 6 plastics to hexamethylenediamine (HMDA). This occurs via the conversion of carboxylic groups through the amides to nitrile, and then these can be hydrogenated to provide a final amine group [32][40]. Cesarek et al. [33][41] demonstrated the use of microwave irradiation for the efficient depolymerization of PAs into a monomer without any side-product formation. The complete hydrolysis of PAs was demonstrated at a temperature of 200 °C in a relatively short time, and the high-quality monomers were recovered.
The production of waxes from plastic wastes has also been reported. These waxes have some special characteristics compared to waxes obtained directly from petroleum. The unique characteristics are excellent distribution, smooth flow behavior, high softening point, chemical- and water-resistant properties, and better chemical stability. The waxes produced have a large market and are used for applications such as an antioxidant additive for rubber, candles, shine products for wood floors and cars, paint cans, lubrication, and as an additive in the fabricating and processing of POs. The waxes can also be used for asphalt roads [56][57][64,65] and roofs and as additives for plastics, coatings, and adhesives. Wax as high as 90 wt.% can be produced from PE under suitable pyrolysis conditions [58][66]. HDPE pyrolysis produced waxes (HCs > C21) selectively using a CSBR reactor at a 500 °C reaction temperature [18][26]. The production of high-value chemicals such as different grades of microcrystalline wax, paraffin wax, and lube and grease base stocks were reported by the conversion process, including low polymer wax or polymer mud [59][67]. These low polymer waxes were obtained as a by-product during HDPE production. The conversion involves thermal treatment in the presence of organic peroxides, such as butyl peroxide and benzoyl peroxide, and metal oxides such as magnesium oxide and calcium oxide. The product composition was found to be dependent on the process parameters used, such as the type of peroxides, metal oxides, reaction temperature, and reaction time. Low polymer waxes can also be converted to gasoline, diesel, and aromatics along with liquified petroleum gas (LPG) via a conversion process that includes pyrolysis followed by a vapor-phase catalytic conversion in the presence of zeolites. The products obtained from the reaction contain HCs in the range of C5−C16. Celik et al. [60][68] developed a stable nanoparticle-support catalyst for the upcycling of single-use polyethylene into high-quality liquid products. The developed catalyst consisted of strontium titanate (SrTiO3), an archetypical cubic perovskite, as a support for the deposition of PtNPs to form a Pt/SrTiO3 catalyst. The used SrTiO3 was single crystal nanocuboids having a sub-100 nm average size, with {100} facets and rounded stepped edges. The hydrogenolysis was performed at 300 °C and 170 psi of H2 under solvent-free conditions. The results suggest that PE adsorption is more favorable on Pt sites compared to the SrTiO3 support. Pt edge sites were found to be highly reactive for PE hydrogenolysis compared to Pt facets.
The production of high-quality lubricating oils from plastic waste has also been investigated by a few researchers [19][61][27,69]. Lubricating oil without added additives is called base oil and has a viscosity index (VI) in the range of 95–105; these are called conventional base oils. Base oils with VI values > 115 are known as unconventional base oils (UCBO). Miller [61][69] developed a new process for the conversion of plastic waste and Fischer–Tropsch (FT) wax to lube range molecules, and these can be hydroisomerized to low-pour-point base oils with UCBO quality. Different types of feedstocks were used such as PE, 96% PE + 4% PET, FT wax, and a 50/50 mixture of PE and FT wax. In this work, the pyrolysis process converts high-molecular-weight compounds into lower-molecular-weight compounds in the lube oil range HCs. After pyrolysis, the hydroisomerization process was used to produce low-pour-point oils of UCBO quality. The authors reported that hydrotreatment of feed prior to the hydroisomerization step did not significantly affect the lubricating oil yield and quality.
Overall, the recovery of chemicals from plastic wastes is challenging because of the difficulty in separating catalysts (mainly homogeneous catalysts) from the products and purification from other products. Another difficulty is the slow reaction rates with low selectivity that generate significant challenges in scaling up to a commercially applicable process.
