Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Zawani Mazlan.
Functionalized biomaterials which are able to be implanted regardless of the wound deformity and potentially embark the skin wound management by the ability to be bioinert, self-degradable and non invasive. These 3D network hold a unique characteristics which are able to mimic the skin extracellular matrix (ECM) and has been successfully proven to promote wound healing and skin regeneration.
- injectable hydrogels
- skin wound
- diabetic foot ulcer
- advanced dressings
1. Introduction
The human skin is the body’s first defense mechanism as it acts as a shield from external pathogens as well as initiates Vitamin D synthesis, thermal regulation and body hydration. As the largest organ in the body, the skin plays a vital role in maintaining the physiological haemostasis of the body hence, chronic skin damage may be lethal if left untreated. Wound healing can be delayed by chronic conditions such as diabetes mellitus or peripheral vascular disease [1].
Wound, defined as an injury usually occurs within the skin, connective tissues or the mucus membrane which may result in the structural and/or functional defects of the organs [2]. A wound can be categorized into two main types namely; acute wound and chronic wound. Traumatic or surgical wound is classified under acute wounds as it naturally surpass the wound healing phase and repair arrangement within the expected period [3]. The regulation of protease in this category is maintained to assist proliferation, hence regulating extracellular regeneration. In contrast with chronic wounds, this type of wound involves a much more distorted healing process that can be classified as various type of ulcers, which include pressure, venous, arterial, vascular and diabetic foot ulcers (DFU). These chronic wounds demonstrate stalled inflammatory phase resulting in the development of biofilm, bacterial clusters, and elevation of protease at the site of the wound. This could be due to the extended period of pro-inflammatory cytokines (interleukin-1; IL-1) and tumor necrosis factor (TNF) accumulation [1][4]. The domination of protease above the inhibitors leads to extracellular matrix destruction, thus accelerating the proliferation and inflammation phase. This process then triggers reactive oxygen species to rise hence resulting in premature cells and defective extracellular matrix proteins [5].
Skin ulcers are often being related to diabetes, which is defined as the loss of the epithelium lining continuity, a dry type of stratified squamous epithelium which causes the underlying tissue surface to be bare open. These ulcers breach the skin protective barrier that is keratin and its keratinocytes known as the anchor of the skin membrane [2]. In response to the injury, the body will endeavor to repair the damage locally and systemically according to the depth of the injury inflicted on the tissues. The healing process manifests an astonishing cellular mechanism distinctive in nature as every unique cell found in the tissue is capable of holding different aptitudes to replicate, divide and differentiate [2][6]. The interaction of cells, growth factors, and cytokines are involved in wound repair, which is a crucial part of the healing process.
2. Commercialized Hydrogels for Diabetic Foot Management
DFU is the primary cause of diabetes mellitus (DM) complication, often causes a number of medical complications. Therefore, advancement in hydrogel-based biomaterial has shed light to provide a better quality of life for patients with DFU. State of the art in hydrogel is the newly commercialized Apligraf® and Leucopatch® a cellular hydrogel-based biomaterials that have been approved by the FDA for the management of DFU [7][8]. Apligraf® is a bilayer skin substitute bioengineered with living cells in helping to accelerate DFU wound healing. Apligraf® is composed of the dermal and epidermal layer that is made of bovine collagen type 1 incorporated with human dermal fibroblast. These composites promote suitable hydration and protein to accelerate wound healing as well as to support cell attachment [8]. Meanwhile, Integra is a collagen-glycosaminoglycan hydrogel that has been approved in the market and has been applied in clinics for fibroblast and endothelial invasion and capillary growth. Integra can support epithelialization in the absence of vascularization [9].
As a consequence of the accessibility of skin tissue and its inert capacity to regenerate, skin wound healing has long been a subject of regenerative medicine [10]. Immediate full-thickness skin wound management is a realistic approach to improving the healing rate and minimizing the risk of complications. Hence the tissue engineering method was applied to combat these difficulties by shedding light on a novel approach of developing bioinert injectable hydrogels. The unique characteristics of the hydrogel-based skin substitutes have attracted immense attention as they are able to mimic the skin extracellular matrix and its microenvironment [11].
3. Development of Injectable Hydrogel for Skin Wound
Hydrogels are a three-dimensional (3D) network of crosslinked polymers, predominantly water based. The hydrophilicity of these hydrogels makes them ideal to mimic the ECM as it is considered non-toxic, biocompatible, and self-degradable [12][13]. Several different types of hydrogels are categorized based on the materials used; natural or synthetic hydrogels or rather based on the synthesis methods which namely; physical or chemical crosslinking methods. Hydrogels hold unique characteristics for study including a high degree of flexibility due to the versatile chemical and physical properties to develop the best hydrogel for a given application. The ability of the hydrogel to shape swollen 3D networks allows it to diffuse molecules and cells, whereas the resemblance and the ability of mimicking the ECM makes them appealing for biomedical and tissue engineering applications [14].
There are a variety of biomaterials that can be used as matrices for wound healing purposes which can be categorized as either natural or synthetic. The most utilized natural biomaterials for wound management are collagen, gelatin, hyaluronic acid, alginate, chitosan, fibrin, cellulose and silk fibroin [15][16][17][18][19][20][21][22]. They are substantially discovered in the ECM arrangements with properties of bioinert, biodegradable, and possess strength and elasticity that are applicable for the use of tissue engineering and cell culture [11][12][13][14].
4.1. Mechanism of Injectable Hydrogel
3.1. Mechanism of Injectable Hydrogel
A number of mechanisms have been utilized in the process of developing injectable hydrogels. However, the crosslinking methods are not only significant on the polymerization of this injectable biomatrix, yet also play an important role in synthesizing hydrogels. Although the crosslinking process is the same, the main difference in the preparation of injectable gels and in situ forming gels is the flexibility in the regulation of the gelling kinetics. The sol-gel transformation of an injectable hydrogel should be managed to occur within a given time interval. Injectability or mass transfer and bulk gel molding can be affected by the rate of gelation kinetics. Table 21 summarized the crosslinking methods utilize in the fabrication of injectable hydrogel [23].
Table 21. Types of crosslinking method for injectable hydrogel utilization.
| Crosslinking Method | Mechanism | External Stimuli |
|---|---|---|
| Physical | Electrostatic interactions | pH Ultrasound Electric Field Magnetic Field Temperature Light sensitivity Biomolecular species |
| Hydrophobic interactions | ||
| Host-gest interactions | ||
| Van Der Waals forces | ||
| Chemical | Diel-Alder | |
| Michael addition | ||
| Schiff base reaction | ||
| Enzyme-mediation | ||
| Photopolymerization |
Injectable hydrogels for tissue engineering applications utilize several natural polymers, synthetic polymers, peptides, and proteins. These building blocks can be crosslinked physically or chemically while being incorporated with cells, tissues or biological materials [24]. Figure 21 illustrates the schematic diagram of sol-gel transition, a mechanism of injectable hydrogel that occurs by the interaction of the basic building block with the precursor (crosslinking agents). As shown, the mixture of the polymer and the based or therapeutic agents is injected onto the wound in a solution manner and polymerize once administered via crosslinking reaction [24]. This transition in viscosity of the solution as it turns into a gel form can be calculated by rheology test. Furthermore, this polymerization method often utilized various external stimuli such as changes in temperature, pH, electromagnetic field or even enzyme and lights conditions. Chemical crosslinking can be divided into multiple reactions such as Schiff base reactions and Diels-Alder reactions, while physical crosslinking involves hydrogen bonding, van der Waals forces, π-interactions and hydrophobic interactions [25].
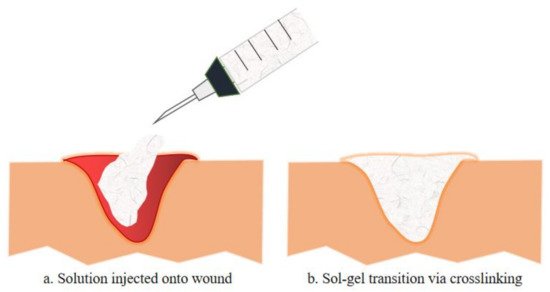

Figure 21. The exemplify of injectable hydrogel injected onto a wound is illustrated in a. whereas b. illustrates the sol-gel transition of the injectable hydrogel with the incorporation of therapeutic compositions, either by chemical or physical crosslinking. Such injectable hydrogels formed in situ have been used to deliver various therapeutic cells or biologics (e.g., growth factors, chemokines for modulating the function of endogenous cells) to promote tissue regeneration[23]
External factors such as temperature, pH, electric/magnetic fields, light, biomolecular species (such as enzymes) and others can affect and control the physical crosslinking, morphology, and properties of the injectable hydrogels. These stimuli-responsive hydrogels can be promising “smart” biomaterials for tissue engineering and disease therapy. The most widely published studies are on temperature-responsive hydrogels. A sol-gel transformation at a lower critical solution temperature is usually used to shape physical crosslinking [24].
43.1.1. Physical Crosslinking
Physical crosslinking, one of the crosslinking reaction mechanism used to make hydrogels, is caused by non-covalent interactions such as electrostatic interactions between two oppositely charged ions (such as ionic bonds, hydrogen bonds, and others), hydrophobic interactions, -interactions, and van der Waals forces such as dipole-dipole interactions and London dispersion forces. Furthermore, without the use of a crosslinker or catalyst, some injectable hydrogels can be made by physically self-crosslinking through interactions such as host-guest interactions [24][25][26]. Moreover, various external stimuli often influence crosslinking-induced gelation. The following is a study of the injectable hydrogels that have been recently produced using various physical crosslinking techniques.
43.1.2. Chemical Crosslinking
Click chemistry or chemical crosslinking is highly efficient and possesses rapid crosslinking ability. The most commonly utilized chemical is azide-alkyne catalyzed from copper, however despite its outstanding chemistry in gel formation, it may be toxic to cell hence limiting its application for cell delivery and encapsulation. There are also many other chemicals or click reactions that are being utilized for crosslinking purpose including thiol-ene, diels-alder and oxime reactions [24][25][26].
4.2. Development of Injectable Hydrogel for Diabetic Ulcer Management
3.2. Development of Injectable Hydrogel for Diabetic Ulcer Management
Several mechanisms need to be taken into consideration during the development of these injectable hydrogels in order to combat the complication of DFU. Several in vivo studies stated complex molecular mechanism that could hinder wound healing in diabetic-induce animals including sustained pro-inflammatory cytokines, impede angiogenesis reaction, vascular drawback, impaired dermal skin cells migration and proliferation [27].
Chronic wounds are often stalled in the inflammatory phase of wound healing causing severe persistent complications that could lead to amputation. In a study, Lee et al. used an electrospinning technique to create a nanofibrous collagen/poly-D-L-lactide–glycolide (PLGA) scaffold membrane. This scaffold could be applied to diabetic wounds and filled with medications to allow for long-term Glucophage release and wound healing [28]. In an in vivo study utilizing female Wistar rats demonstrated the ability of AgNP-loaded hydrogels to minimize wound size compared to injuries, encouraging histological changes in the healing tissue over the course of wound healing, as in earlier production and maturation of granulation tissue [29].
The next section discuss the future application of novel injectable hydrogel for diabetic ulcer including the development of thermosensitive injectable hydrogel, sustained-release or tissue-specific hydrogel and multifunctional injectable hydrogel. A team of researcher incorporated multi-active ingredients which are stated in Figure 32 that demonstrate antibacterial and anti-inflammation properties to diminish bacterial-induced inflammation and support as well as accelerating the wound healing process of chronic diabetic ulcer.
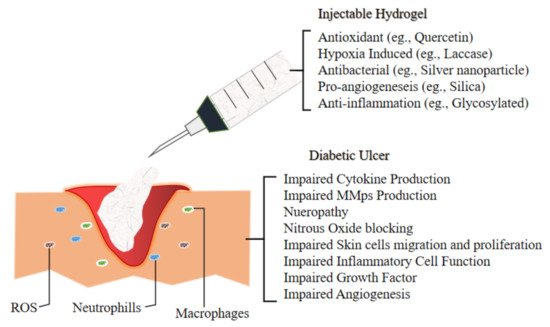

Figure 32. Illustrate the environment of Diabetic Ulcer and the impairment which lead to its slow recovery. Hence the development of novel injectable hydrogel for the management of Diabetic Foot Ulcer are incorporating various materials which exert antioxidant (to combat ROS), hypoxia induced, antibacterial (to overcome bacterial infection), pro angiogenesis (to accelerate wound healing by promoting new vessels for better blood flow) and anti-inflammation mechanism as inflammatory cells in the ulcer elevate to reactive oxygen species (ROS) level, hence ensued in extracellular matrix injuries as well as premature decrepitude of dermal cells[23].
43.2.1. Development of pH-Sensitive Injectable Hydrogel
The development of pH-sensitive injectable hydrogels has also been extensively studied. In a study by Qu et al., pH-sensitive and electric field responsive hydrogels were prepared using antibacterial and conductive chitosan-graft-polyaniline (CP) copolymers and oxidized dextrans (OD) via Schiff base reaction which are utilized as smart drug delivery systems. Amoxicillin and ibuprofen (active ingredients) were loaded into the hydrogels and the release rate of the drugs was simultaneously increased with the elevation of electric field voltage. The hydrogels showed pH-dependent degradation and morphology, outstanding cytocompatibility, and the release regulated fields were suggested as an ideal biomaterials for smart drug delivery [30].
43.2.2. Development of Thermosensitive Injectable Hydrogel
A group of researchers incorporated a combination of chitosan, collagen and β-glycerophosphate into their hydrogels. 3D mesenchymal stem cell sphere was then added to enhance wound healing activity by exerting the paracrine and neovascularize effects. These hydrogel mixtures are thermosensitive and polymerize when in contact with body temperature via physical crosslinking by filling the defect area despite the shape and depth. This biomatrix showed to exert an excellent therapeutic effect compared to other control groups. This technique was then examined by the elevation of cells attachment and proliferation for further investigation for the future use of chronic and venous wound management [31].
43.2.3. Development of Sustained Release/Tissue-Specific Injectable Hydrogel
In order to achieve the tissue-specific and sustained delivery of siMMP-9 for diabetic wounds, Lan et al. developed a thermosensitive hybrid hydrogel biomatrix with the combination of GT/siMMP-910. In this biomatrix, they utilize the combination of Pluronic F-127 (PF) and methylcellulose (MC) to create a thermosensitive hydrogel dressing. The PF and MC percentage were adjusted to match the different desired longevities of the therapeutic substance to be released. Consistent with diabetic wound dressing interval, 7 days of sustained release were achieved without reapplication. This will assist in accelerating the wound healing process, hence elevating the patient’s quality of life [32].
High blood glucose levels in chronic or diabetic wounds are known to cause microvascular endothelial injury and diastolic vascular dysfunction. Therefore, glucose-responsive injectable hydrogels are recompensed to combat this type of wound. A novel study by Zhao et al. utilizes a glucose-responsive biomatrix as an injectable hydrogel. They incorporated two main active components namely insulin and fibroblast (L929) and the hydrogels are engineered to possess a fast release at higher glucose concentration hence sanction cell proliferation in the biomatrix. An in-vivo study on diabetes-induced rats revealed that the injectable biomatrix stimulates neovascularization and collagen deposition in the wound [33].
43.2.4. Development of Multifunctional Injectable Hydrogel
Novel biomaterials are anticipated to possess a hybrid biofunction to accelerate wound healing. Hence an in vivo study by Qu et al. sheds light on the multifunctional injectable hydrogel that exhibits the properties of antibacterial, antioxidant, and electro responsive. The hydrogel encapsulates amoxicillin as the main composition, which assists the wound healing process by preventing wound infection (inhibition of ROS in the wound area). This significantly stimulates the wound healing process with elevated tissue granulation, collagen configuration, neovascularization, and angiogenesis in the full-thickness diabetic wound rats. Also, 2 mg amoxicillin/mL of hydrogel is able to cause wound contraction, which surpasses 18% of the commercialized film (p < 0.05) while the highest cumulative zone of inhibition was observed on day 3 (p < 0.05) against both negative and gram-positive bacteria [34].
43.2.5. Development of Hypoxic Injectable Hydrogel
Injectable hydrogels with the capability to cast a hypoxic microenvironment possess a great potential to develop novel therapies for tissue regeneration. However, the relative research remains at the conceptual phase. Jin et al. chose diabetic wound as a representative injury model to explore the actual therapeutic results of tissue injury by injectable hypoxia-induced hydrogels. Briefly, adipose-derived stem cells were encapsulated in the biomatrix, which later showed an acceleration of neovascularization and immunoregulation. It also stimulates the reconstruction of blood vessels, hair follicles, and dermal collagen matrix ushering to the recovery of the diabetes-induced wound and reconstruction of skin functions. The wound was assessed for 21 days and the study demonstrated an 80% ratio of wound closure in 14 days and 95% in 21 days [35].
43.2.6. Development of Self-Healing Injectable Hydrogel
Li et al. reported a bioactive self-healing antibacterial injectable dual-network silica-based nanocomposite hydrogel scaffolds that can significantly enhance diabetic wound healing/skin tissue formation through promoting early angiogenesis without adding any bioactive factors. The nanocomposite scaffold comprises the main network of polyethylene glycol diacrylate (PEGDA) forming scaffolds with an auxiliary dynamic network between bioactive glass nanoparticles containing copper (BGNC) and sodium alginate (ALG) (PABC scaffolds). PABC scaffolds exhibit biomimetic elastomeric mechanical properties, excellent injectability, self-healing behavior and robust broad-spectrum antibacterial activity. Importantly, PABC hydrogel significantly promoted the viability, proliferation and angiogenic ability of endothelial progenitor cells (EPCs) via in vitro. An in vivo study showed that PABC hydrogel could efficiently restore blood vessel networks through enhancing HIF-1α/VEGF expression, collagen matrix deposition in the full-thickness diabetic wound, and significantly accelerate wound healing and skin tissue regeneration. The prominent multifunctional properties and angiogenic capacity of PABC hydrogel scaffolds enable their promising applications in angiogenesis-related regenerative medicine [36].
Furthermore, Zhu et al. highlighted the benefit of fibroblasts on skin regeneration. They provided a very facile way to make tissue adhesive injectable hydrogel by mixing silica nanoparticles in an aqueous solution glycol chitosan. Hydrogel was formed due to the interaction between the nanoparticles and the polysaccharides, which has confined the latter’s movement. The gel’s lap-shear stretching force to adhere to the mouse skin pieces is up to 90 kPa allowing it to be pasted onto the wound of mice without the use of additional bandage. Whenever fibroblasts are loaded into the injectable hydrogel, it is discovered that the fibroblasts favoured the generation of micro vessels and hair follicles in the neo-skin tissue and inhibited the formation of scar [37]. Compared to bioinert silica, bioactive glass (BG) has the function of promoting angiogenesis. Kong et al. showed that the combination of BG with desferrioxamine (DFO) in an alginate-gluconolactone injectable hydrogel benefited the expression of vascular growth factors during the treatment of excisional wound on a diabetic rat model. This led to an improved prognosis of diabetic chronic skin defects [38].
Zhao et al. utilise Schiff’s based method by allying chitosan-g-polyaniline (QCSP) and benzaldehyde with the addition of functional poly (ethylene glycol)-co-poly (glycerol sebacate) as the antibacterial agents to combat feasible wound infection. The polyaniline exerts antioxidant properties hence act as free radical scavengers, which are an advantage in accelerating chronic skin defects in the diabetes-induced mouse. The results showed the synergistic effect on the expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1 (HIF-1α) to promote revascularisation [38][39]. Hence, Table 32 summarized the current studies of injectable hydrogel for the management of diabetic foot ulcer.
Table 32. Injectable hydrogels for future diabetic foot ulcer (DFU) management.
| Reference | Composition | Main | Aim | Study Design | Result | Conclusion |
|---|---|---|---|---|---|---|
| Qu et al. 2019 | N-carboxyethyl chitosan (CEC) Oxidized hyaluronic acid-graft-aniline tetramer (OHA-AT) |
Amoxicillin | To develop multifunctional injectable hydrogel -anti-oxidant -antibacterial -electroactive |
In vivo In vitro |
Wound: (Day 15) amoxicillin loaded hydrogel (p < 0.05) Antimicrobial: (Day 3) Highest cumulative zone of inhibition (p < 0.05) |
In vivo: accelerate wound healing rate than commercialized product In vitro: Effective antibacterial effect |
| Zhao et al. 2017 | pH and Glucose Dual-Responsive | Bovine insulin | To develop sustained and pH/glucose-triggered drug release | In vivo | Wound: 58 ± 2% of collagen deposition, 2.41-fold population of red CD31-positive cells compared to control | In vivo: Infiltration of inflammation, accelerate neovascularization, collagen disposition |
| Qian et al. 2020 | Platelet-Rich Plasma Release | Platelet-rich plasma (PRP) | To develop self-healing injectable hydrogel | In vivo In vitro |
Wound: (day 21) increased the nerve density (p > 0.05) and (day 7) higher healing rate | In vivo: Accelerate collagen deposition, wound healing, angiogenesis, neovascularization In vitro: support human dermal fibroblast (HDF), Human Umbilical Vein Endothelial cells (HUVEC), Human Umbilical Mesenchymal Stem Cells (HUMSC) proliferation. |
| Jin et al., 2020 | Hypoxia-Induced Conductive | Vanillin-grafted gelatin Laccase (Lac) |
To develop injectable hydrogel with hypoxic microenvironment ability to assist tissue regeneration. | In vivo | Wound: HIF-1α pathway activation, 95% wound closure rate (21 days) compared to control < 75% Subcutaneous study: proangiogenic factors secretion < 0.05 (day 7) |
Regulate stem cell plasticity, neovascularization, collagen deposition, hair follicle reconstruction, gene expression acceleration |
| Wang et al., 2019 | Antibacterial exosomes | Adipose mesenchymal stem cells exosomes (AMSCs-Exo) | Evaluate angiogenesis and antibacterial ability of FHE@exo hydrogel | In vitro In vivo |
HUVEC: formation of 45 vessels compared to controlled group (20 vessels), elevated alpha-smooth muscle actin (α-SMA) expression Wound: smaller wound closure, thickest granulation tissue (day 14) in the treatment group Antibacterial study: no bacterial infection during the experimental period compared to control |
In vitro: accelerate proliferation, migration, angiogenesis In vivo: less scar formation, wound healing acceleration. |
| Chen et al. 2019 | Thiolated polyethylene glycol (SH-PEG) Silver nitrate (AgNO3) |
Desferrioxamine (DFO) | Evaluate angiogenesis and antibacterial abilities of DFO on HUVEC and diabetic-induced rats. | In vitro In vivo |
HUVEC: extensive vascular tubule formations after treatment Wound: dry and 50% reduction compared to control (day 7) Antibacterial study: minimal intensity Staphylococcus aureus compared to control |
Invitro: Show antibacterial and angiogenic capability. In vivo: Proven antibacterial and enhance angiogenesis. |
| Bai et al. 2020 | Bone marrow mesenchymal stem cells (BM-MSCs) growth factors. | Hyaluronic acid (HA) Adipic acid dihydrazide (ADH) |
Evaluate inflammatory microenvironment in diabetic induce rats | In vivo | Wound: Significantly smaller (p < 0.05) wound, growth factors elevate (p < 0.01) at day 15. | In vivo: Formation of granulation tissue, collagen deposition, nucleated cell proliferation, neovascularization |
| Li et al. 2020 | Polyethylene glycol diacrylate (PEGDA) | Nanoparticles (copper + sodium alginate) |
Evaluate angiogenic properties of hydrogel on diabetic induce mice. | In vitro In vivo |
Antibacterial study: inhibit * p < 0.05 and ** p < 0.01 of Staphylococcus aureus and Escherichia coli, respectively. Wound: Peaked blood flow at day 7 with the treatment group. |
In vitro: Accelerate proliferation and angiogenesis property of endothelia cells (EPCs) In vivo: Promote neovascularization, collagen deposition, and wound healing acceleration |
| Wang et al. 2020 | Nanoezyme- Reinforced |
Insulin manganese dioxide (MnO2) nanosheet |
To develop multifunctional injectable hydrogel | In vivo | Wound: (Day 14) No scar tissue Antibacterial: (Day 14) nearly 100% reduction of bacterial colonies |
In vivo: synergistically diminished inflammatory responses, stimulated angiogenesis, accelerated cell proliferation, promoted granulation tissue formation and extracellular matrix (ECM) deposition |
References
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94.
- Zubair, M.; Ahmad, J.; Malik, A.; Talluri, M.R. Diabetic Foot Ulcer: An Update; Springer: New York, NY, USA, 2020.
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15.
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735.
- Mccarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447.
- Borena, B.M.; Martens, A.; Broeckx, S.Y.; Meyer, E.; Chiers, K.; Duchateau, L.; Spaas, J.H. Regenerative Skin Wound Healing in Mammals: State-of-the-Art on Growth Factor and Stem Cell Based Treatments. Cell. Physiol. Biochem. 2015, 36, 1–23.
- Maarof, M.; Busra, M.F.M.; Lokanathan, Y.; Idrus, R.B.H.; Rajab, N.F.; Chowdhury, S.R. Safety and efficacy of dermal fibroblast conditioned medium (DFCM) fortified collagen hydrogel as acellular 3D skin patch. Drug Deliv. Transl. Res. 2018, 9, 144–161.
- Reyes-Martínez, J.E.; Ruiz-Pacheco, J.A.; Flores-Valdéz, M.A.; ElSawy, M.A.; Vallejo-Cardona, A.A.; Castillo-Díaz, L.A. Advanced hydrogels for treatment of diabetes. J. Tissue Eng. Regen. Med. 2019, 13, 1375–1393.
- Jeschke, M.G.; Rose, C.; Angele, P.; Füchtmeier, B.; Nerlich, M.N.; Bolder, U. Development of New Reconstructive Techniques: Use of Integra in Combination with Fibrin Glue and Negative-Pressure Therapy for Reconstruction of Acute and Chronic Wounds. Plast. Reconstr. Surg. 2004, 113, 525–530.
- Gurtner, G.C.; Chapman, M.A. Regenerative Medicine: Charting a New Course in Wound Healing. Adv. Wound Care 2016, 5, 314–328.
- Tavakoli, S.; Klar, A. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169.
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018.
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv. Sci. 2019, 6, 1801664.
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 1–10.
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C 2019, 99, 1509–1522.
- Cheng, N.C.; Lin, W.J.; Ling, T.Y.; Young, T.H. Sustained release of adipose-derived stem cells by thermosensitive chitosan/gelatin hydrogel for therapeutic angiogenesis. Acta Biomater. 2017, 51, 258–267.
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressing for promoting spontaneous wound healing. Mater. Sci. Eng. C 2019, 101, 487–498.
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J. Drug Deliv. Sci. Technol. 2020, 55, 101379.
- Zhang, N.; Gao, T.; Wang, Y.; Liu, J.; Zhang, J.; Yao, R.; Wu, F. Modulating cationicity of chitosan hydrogel to prevent hypertrophic scar formation during wound healing. Int. J. Biol. Macromol. 2020, 154, 835–843.
- Banerjee, J.; Seetharaman, S.; Wrice, N.L.; Christy, R.J.; Natesan, S. Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration. PLoS ONE 2019, 14, e0217965.
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659.
- Kawabata, S.; Kanda, N.; Hirasawa, Y.; Noda, K.; Matsuura, Y.; Suzuki, S.; Kawai, K. The Utility of Silk-elastin Hydrogel as a New Material for Wound Healing. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1778.
- Zawani, M.; Fauzi, M.B. Injectable Hydrogels for Chronic Skin Wound Management: A Concise Review. Biomedicines 2021, 9, 527. https://doi.org/10.3390/biomedicines9050527
- Lee, J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018, 22, 1–14.
- Zhu, J.; Han, H.; Li, F.; Wang, X.; Yu, J.; Qin, X.; Wu, D. Peptide-Functionalized Amino Acid-Derived Pseudoprotein-Based Hydrogel with Hemorrhage Control and Antibacterial Activity for Wound Healing. Chem. Mater. 2019, 31, 4436–4450.
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702.
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615.
- Lee, C.-H.; Chang, S.-H.; Chen, W.-J.; Hung, K.-C.; Lin, Y.-H.; Liu, S.-J.; Hsieh, M.-J.; Pang, J.-H.S.; Juang, J.-H. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. J. Colloid Interface Sci. 2015, 439, 88–97.
- Diniz, F.R.; Maia, R.C.A.P.; Andrade, L.R.; Andrade, L.N.; Chaud, M.V.; Da Silva, C.F.; Corrêa, C.B.; Junior, R.L.C.D.A.; Da Costa, L.P.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390.
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69.
- Yang, M.; He, S.; Su, Z.; Yang, Z.; Liang, X.; Wu, Y. Thermosensitive Injectable Chitosan/Collagen/β-Glycerophosphate Composite Hydrogels for Enhancing Wound Healing by Encapsulating Mesenchymal Stem Cell Spheroids. ACS Omega 2020, 5, 21015–21023.
- Lan, B. Sustained Delivery of MMP-9 siRNA via Thermosensitive Hydrogel Accelerates Diabetic Wound Healing. J. Nanobiotechnol. 2021, 9, 1–21.
- Zhao, L.; Niu, L.; Liang, H.; Tan, H.; Liu, C.; Zhu, F. pH and Glucose Dual-Responsive Injectable Hydrogels with Insulin and Fibroblasts as Bioactive Dressings for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 37563–37574.
- Qu, J.; Zhao, X.; Liang, Y.; Xu, Y.; Ma, P.X.; Guo, B. Degradable conductive injectable hydrogels as novel antibacterial, anti-oxidant wound dressings for wound healing. Chem. Eng. J. 2019, 362, 548–560.
- Jin, X.; Shang, Y.; Zou, Y.; Xiao, M.; Huang, H.; Zhu, S.; Liu, N.; Li, J.; Wang, W.; Zhu, P. Injectable Hypoxia-Induced Conductive Hydrogel to Promote Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 56681–56691.
- Li, Y.; Xu, T.; Tu, Z.; Dai, W.; Xue, Y.; Tang, C.; Gao, W.; Mao, C.; Lei, B.; Lin, C. Bioactive antibacterial silica-based nanocomposites hydrogel scaffolds with high angiogenesis for promoting diabetic wound healing and skin repair. Theranostics 2020, 10, 4929–4943.
- Zhu, F.; Wang, C.; Yang, S.; Wang, Q.; Liang, F.; Liu, C.; Qiu, D.; Qu, X.; Hu, Z.; Yang, Z. Injectable tissue adhesive composite hydrogel with fibroblasts for treating skin defects. J. Mater. Chem. B 2017, 5, 2416–2424.
- Kong, L.; Wu, Z.; Zhao, H.; Cui, H.; Shen, J.; Chang, J.; Li, H.; He, Y. Bioactive Injectable Hydrogels Containing Desferrioxamine and Bioglass for Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 30103–30114.
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47.
More
