绝大多数胰腺导管腺癌患者的肿瘤中含有 KRAS 突变。在功能上,突变的KRAS不仅致力于肿瘤细胞的增殖,存活和侵袭性,而且还导致该癌症的免疫抑制。 The vast majority of patients with pancreatic ductal adenocarcinomas harbor KRAS mutations in their tumors. Functionally, mutated KRAS is not only dedicated to tumor cell proliferation, survival and invasiveness, but also causing the immunosuppression in this cancer.
- KRAS gene
- pancreatic ductal adenocarcinoma
- cancer immunity
- immune checkpoint blockade
一、简介
1. Introduction
In humans, patients with pancreatic ductal adenocarcinomas (PDACs) commonly have a poor prognosis. As reported in 2018, the five-year survival rate of PDAC patients is only 9% [1]. The biology of PDAC is aggressive, and a certain portion of patients will die from disease-related complications rather than this disease itself [2]. Traditional approaches for managing this cancer include surgery, radiotherapy and chemotherapy. To exploit the genomic characteristics of PDAC, some molecular targeted approaches have been developed. These approaches have exhibited therapeutic effects in a small portion of metastatic cases carrying specific driver alterations, such as the treatment of cases with a germline BRCA1 or BRCA2 mutation using olaparib, a PARP inhibitor, or the treatment of cases with NTRK gene fusions using larotrectinib or entrectinib [3]. Recently, immune checkpoint blockade (ICB) therapy has opened a new era in the comprehensive treatment of cancers. In metastatic PDAC, only patients with the high microsatellite instability (MSI-H) or deficient mismatch repair (dMMR) phenotype in their tumors are reported to benefit from the ICB therapy with pembrolizumab, an anti-PD-1 drug [4]. However, the MSI-H/dMMR phenotype is rarely detected in PDAC. For those patients without the MSI-H/dMMR phenotype, available data indicate that their responses to monotherapy by using ICB drugs are extremely poor [5].
The existing immune environment in tumors will impact the effectiveness of ICB therapy [6]. In PDAC, the tumor milieu is generally immunosuppressive [7]. Recently, driver oncogenes have been recognized to play a convincing role in the cancer immune status [8]. In PDAC, the Kirsten rat sarcoma virus oncogene homolog (KRAS) gene is broadly mutated [9]. KRAS mutations in PDAC include those induced by a missense mutation in codon 12 or codon 13, leading to a replacement of the original glycine (G) by other amino acids, thus causing persistent activation of the KRAS protein in this setting [9]. The KRAS mutation acts as a driver to cause PDAC occurrence and progression together with the concomitant inactivation of other genes, such as TP53, CDKN2A and SMAD4 [10, 11] (Figure 1). In this process, the KRAS mutation will also lead to activation of downstream pathways that can improve cancer cell survival, proliferation, immune evasion and drug resistance [7, 9]. Concerning immunosuppression in PDAC, the KRAS mutation utilizes several routes to achieve this goal, such as activating the yes-associated protein (YAP)-TAZ pathway and its downstream JAK-STAT3 signaling [12], inducing cell autophagy-associated MHC-I degradation by reprogramming glucose metabolism [13, 14], and synergizing with other genetic alterations (e.g., TP53 inactivation) [15] (Figure 1). Consequently, PDAC tumors can be infiltrated by myeloid cells with pro-cancer functions, such as neutrophils, myeloid-derived suppressive cells (MDSCs) and M2-like macrophages [7].
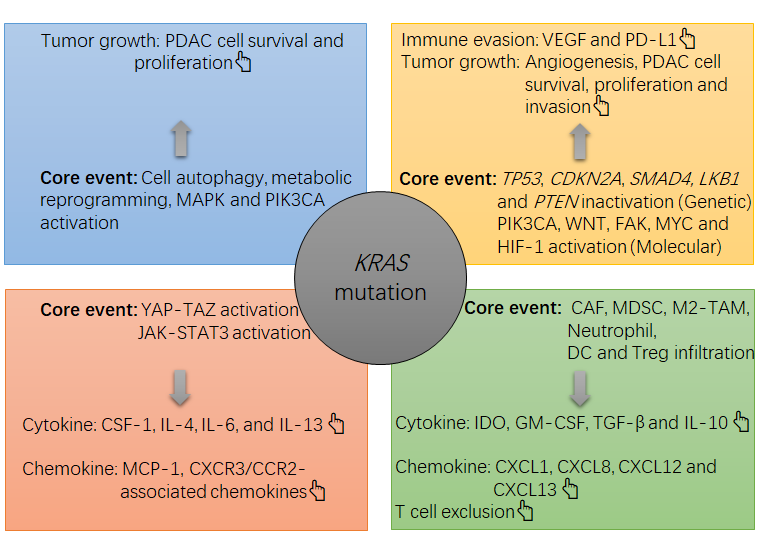
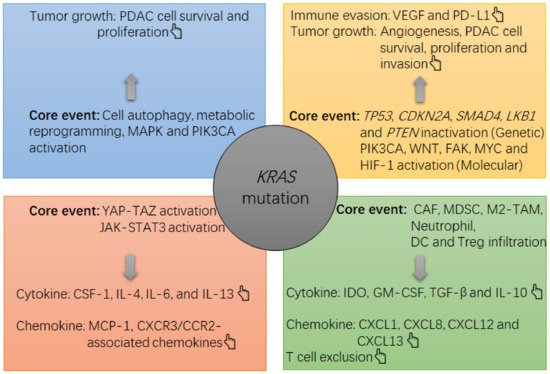
Figure 1. The note chart of KRAS mutation-induced growth and immunosuppression in PDAC tumors. The KRAS mutation causes a suppressive milieu in PDAC tumors mainly via the following routes, such as activation of MAPK and PI3K-Akt, activation of YAP-TAZ and JAK-STAT3, and induction of cell autophagy and metabolic reprogramming in PDAC cells. In this context, the survival and proliferation of PDAC cells will be accelerated, and an overgrowth of tumor cells can cause a hypoxia within the tumor, which then activates HIF-1α to upregulate the expression of gene encoding VEGF by PDAC cells. VEGF is a potent cytokine that induces angiogenesis and immune evasion (e.g. PD-L1 upregulation and tumoricidal T cell exhaustion). Meanwhile, PDAC cells can increase their production of suppressive cytokines and chemokines, such as IL-4, IL-6, IL-13, CSF-1 and MCP-1, which then recruit and increase the survival and suppressive function of immune infiltrates including CAFs, MDSCs, M2-like TAMs, IDO-producing DCs and Treg cells. In this context, an overload of suppressive cells will increase the local levels of suppressive cytokines and chemokines, such as TGF-β, IDO, IL-10, GM-CSF, CXCL1, CXCL8, CXCL12 and CXCL13, thus strengthening the immunosuppression in the tumor (e.g. tumoricidal T cell exclusion). In concert with the KRAS mutation, other alterations at genetic and molecular levels, such as LKB1 inactivation, TP53 inactivation, PTEN inactivation, FAK activation, PIK3CA activation or WNT activation, also contribute to the tumor growth (e.g. PDAC cell survival, proliferation and invasion) and immune evasion (PD-L1 upregulation). .
In addition to PDAC, other cancers in humans, such as colorectal adenocarcinomas (CRACs) and lung adenocarcinomas (LUACs), also harbor a high prevalence of KRAS mutations [9]. Although KRAS mutation has been revealed to correlate with immune evasion in PDAC [13, 14], the situation in LUAC appears to be different because LUAC tumors with KRAS mutation plus TP53 inactivation commonly have massive infiltration of tumoricidal T cells and PD-L1 upregulation [16]. Moreover, clinical data support that LUAC patients with this pattern of tumor immunity can largely benefit from anti-PD-1 monotherapy [17]. Similarly, KRAS mutation is able to cause immunosuppression in CRAC tumors as well. However, unlike in PDAC, the published data suggest that CRAC patients with this phenotype can benefit from a combinational strategy featuring conventional therapy plus an ICB drug [18]. Importantly, despite having KRAS mutation, PDACs, CRACs and LUACs differ in their tumor immune status (Table 1).
在人类中,胰腺导管腺癌(PDAC)患者通常预后较差。肿瘤中现有的免疫环境会影响ICB治疗的有效性[ 6 ]。在 PDAC 中,肿瘤环境通常具有免疫抑制作用 [ 7 ]。最近,人们已经认识到驱动癌基因在癌症免疫状态中发挥着令人信服的作用 [ 8 ]。在 PDAC 中,Kirsten 大鼠肉瘤病毒癌基因同源物 ( KRAS ) 基因发生广泛突变 [ 9 ]。PDAC 中的KRAS突变包括由密码子 12 或密码子 13 中的错义突变引起的突变,导致原始甘氨酸 (G) 被其他氨基酸取代,从而导致持续激活在这种情况下的KRAS蛋白 [ 9 ]。的KRAS突变作为驾驶员原因PDAC发生,发展与其它基因,如肿瘤蛋白P53基因(的伴随灭活TP53),细胞周期蛋白依赖性激酶抑制剂2A基因(CDKN2A)和SMAD家族成员4基因(SMAD4) [ 10 , 11 ](图 1)。在这个过程中,KRAS突变也会导致下游通路的激活,这些通路可以提高癌细胞的存活、增殖、免疫逃避和耐药性[ 7 , 9 ]。关于 PDAC 中的免疫抑制,KRAS突变利用多种途径来实现这一目标,例如激活 yes 相关蛋白 (YAP)-tafazzin (TAZ) 通路及其下游 Janus 激酶信号转导和转录激活因子 3 (JAK-STAT3) 信号传导 [ 12 ],诱导细胞通过重新编程的葡萄糖代谢[自噬相关的主要组织相容性复合体I(MHC-I)降解13,14 ],并与其他的遗传改变协同(例如,TP53失活)[ 15 ](图1)。因此,PDAC 肿瘤可以被具有促癌功能的骨髓细胞浸润,例如中性粒细胞、骨髓源性抑制细胞 (MDSCs) 和 M2 样巨噬细胞 [ 7]]。Table 1. The comparison of immune-related characteristics among KRAS-mutant adenocarcinomas
|
Cancer Characters [Ref.] |
PDAC |
CRAC |
LUAC |
|
Prevalence of KRAS mutation |
97.7% [9] |
44.7% [9] |
30.9% [9] |
|
Hottest missense mutation in KRAS |
G12D [9] |
G12D [9] |
G12C [9] |
|
Sensitive to glucose restriction vs. KRASwt |
Yes [24] |
Yes [93] |
No [94] |
|
Common alteration with KRAS |
TP53 inactivation [10] |
TP53 and APC inactivation [77] |
TP53 or LKB1 inactivation [95] |
|
General milieu of KRAS-mutant tumors |
Immune-cold [7] |
Immune-cold [86] |
KRAS-only: Immune-cold or hot [95] TP53 inactivation: Immune-hot [95] LKB1 inactivation: Immune-cold [95] |
|
Number/function of tumoricidal T cells in KRAS-mutant tumors |
Decrease/Decrease [7] |
Decrease/Decrease [86] |
KRAS-only: Slight increase/Decrease [95] TP53 inactivation: Significant increase/Decrease [95] LKB1 inactivation: Significant decrease/Decrease [95] |
|
Major type of immune infiltrates in KRAS-mutant tumors |
Myeloid suppressive cell [7] |
Myeloid suppressive cell [86] |
KRAS-only: T cell, Macrophage, Neutrophil [95] TP53 inactivation: CD8+ T cell, CD45RO+ T cell [95] LKB1 inactivation: Myeloid suppressive cell [95] |
|
Common presentation of the ICB therapy biomarker if KRAS mutation |
pMMR/MSS [59] |
pMMR/MSS [81] |
KRAS-only: PD-L1 expression↑ [95] TP53 inactivation: PD-L1 expression ↑↑ [95] LKB1 inactivation: PD-L1 expression ↓↓ [95] |
|
Biomarker associated with the effectiveness of ICB therapy |
dMMR/MSI-H [4] |
dMMR/MSI-H [84] |
PD-L1 [95] |
|
Prevalence of dMMR/MSI-H in all cases |
1% ~ 2% [59] |
14% [81] |
NM |
|
Prevalence of positive expression of PD-L1 by tumor cells |
NM |
NM |
Among KRAS-only tumors: 37.5 % [95] Among TP53 inactivation tumors: 68.8 % [95] Among LKB1 inactivation tumors: 10 % [95] |
|
General response to monotherapy using ICB drugs |
Poor [5] |
Poor [85] |
KRAS-only tumor: Fair [95] TP53 inactivation tumor: Excellent [95] LKB1 inactivation tumor: Poor [95] |
|
Core molecular events associated with KRAS mutation-induced immunosuppression |
1. YAP-TAZ activation[12]; 2. JAK-STAT3 activation [12]; 3. Metabolic reprogramming of glucose and cell autophagy [13, 14]; 4. In concert with other events, TP53 inactivation [15], LKB1 mutation [33, 34], PTEN loss [33, 34], WNT/β-catenin activation [33, 34], FAK activation [33, 34], PIK3CA activation [33, 34] and MYC activation [33, 34]; |
1. In concert with APC and TP53 inactivation: TGF-β1 upregulation and EMT [79]; 2. TGF-β-induced immune suppression [78]; 3. IRF2 inactivation [86, 87]; 4. Metabolic dysregulation in glucose, glutamine, fatty acid and lipid [81, 92]; 5. MAPK and HIF-1-related cascade activation [92]; |
1. ERK activation-induced PD-L1 upregulation [95] 2. Metabolic reprogramming of glucose [94] 3. In concert with LKB1 inactivation: strengthening metabolic reprogramming of glucose and JAK-STAT3 activation [95] |
图 1. PDAC 肿瘤中KRAS突变诱导生长和免疫抑制的注释图。该KRAS突变主要通过以下途径在 PDAC 肿瘤中引起抑制环境,例如激活丝裂原活化蛋白激酶 (MAPK) 和磷脂酰肌醇 3-激酶 (PI3K)-Akt,激活 YAP-TAZ 和 JAK-STAT3,以及诱导PDAC细胞中的细胞自噬和代谢重编程。在这种情况下,PDAC细胞的存活和增殖会加速,肿瘤细胞的过度生长会导致肿瘤内缺氧,进而激活缺氧诱导因子1(HIF-1)α,上调编码基因的表达。血管内皮生长因子 (VEGF) 通过 PDAC 细胞。VEGF 是一种有效的细胞因子,可诱导血管生成和免疫逃避(例如,程序性死亡配体 1 (PD-L1) 上调和杀伤肿瘤性 T 细胞耗竭)。同时,PDAC细胞可以增加抑制性细胞因子和趋化因子的产生,例如白介素4(IL-4),IL-6,IL-13,巨噬细胞集落刺激因子1(CSF-1)和单核细胞趋化蛋白1(MCP-1) ),然后招募并增加免疫浸润物的存活和抑制功能,包括癌症相关成纤维细胞 (CAF)、MDSC、M2 样肿瘤相关巨噬细胞 (TAM)、产生吲哚胺 2, 3-双加氧酶 (IDO) 的树突细胞(DC) 和调节性 T 细胞 (Treg 细胞)。在这种情况下,抑制性细胞的过载将增加抑制性细胞因子和趋化因子的局部水平,例如转化生长因子-β (TGF-β)、IDO、IL-10、粒细胞-巨噬细胞集落刺激因子 (GM-CSF) , 趋化因子 CXC 基序配体 1 (CXCL1)、CXCL8、CXCL12 和 CXCL13,从而加强肿瘤中的免疫抑制(例如,杀瘤性 T 细胞排除)。与KRAS突变,基因和分子水平的其他改变,例如肝激酶 B1 基因 ( LKB1 ) 失活、TP53失活、磷酸酶和张力蛋白同源基因 ( PTEN ) 失活、粘着斑激酶 (FAK) 激活、磷脂酰肌醇 4, 5-二磷酸3-激酶催化亚基 α (PIK3CA) 激活或 Wingless/整合 (WNT) 激活也有助于肿瘤生长(例如 PDAC 细胞存活、增殖和侵袭)和免疫逃避(PD-L1 上调)。PDAC: pancreatic ductal adenocarcinoma; CRAC: colorectal adenocarcinoma; EMT: epithelial-mesenchymal transition; LUAC: lung adenocarcinoma; pMMR: proficient mismatch repair; MAPK: mitogen-activated protein kinase; MSS: microsatellite stability; dMMR: deficient mismatch repair; MSI-H: high microsatellite instability; ICB: immune checkpoint blockade; NM: no mention; PD-L1: programmed death-ligand 1; TP53: tumor protein P53 gene; LKB1: liver kinase B1 gene.
2. KRAS突变在 PDAC 中的致癌作用
Given the above information, this review will focus on the role of KRAS mutation in dictating pancreatic carcinogenesis and the cancer immune status in PDAC, aiming to illustrate the response of PDAC to ICB therapy in published data and to provide new insights into the use of ICB therapy in PDAC treatment. In addition, we will consider other KRAS-mutant cancers, such as CRAC and LUAC, and compare them with PDAC, aiming to uncover the mechanism by which KRAS mutation dictates the cancer immune status across these adenocarcinomas.
人PDAC只具有KRAS突变而不是神经母细胞瘤RAS病毒癌基因同系物基因(NRAS)或哈维大鼠肉瘤病毒原癌基因同源基因(HRAS)突变[ 9 ]。总体而言,97.7% 的 PDAC 病例被检测到具有KRAS突变 [1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95]。G12D、G12V和G12R是PDAC中三种最常见的KRAS突变错义形式,其中以G12D错义突变最为常见[ 9 ](表1)。生理上,正常的KRAS蛋白具有 GTPase 活性,但这些错义变体会产生与 GTP 稳定结合的 KRAS 蛋白,从而组成性激活 MAPK 和 PI3K-Akt 通路,这两条经典通路负责维持细胞存活和增殖 [ 35 ](图 1)。
2. The carcinogenic role of KRAS mutation in PDAC
Human PDAC exclusively has KRAS mutation rather than NRAS or HRAS mutation [9]. Overall, 97.7% of PDAC cases are detected to have the KRAS mutation [9]. G12D, G12V and G12R are the three most common missense forms of KRAS mutation in PDAC, while the G12D missense mutation is the most frequent among them [9] (Table 1). Physiologically, the normal KRAS protein has GTPase activity, but these missense variants generate a KRAS protein that stably binds with GTP, thus constitutively activating MAPK and PI3K-Akt pathways, two classical pathways responsible for maintaining cell survival and proliferation [19] (Figure 1). For example, in mice bearing PDAC, the KRASG12D mutation was revealed to activate the MAPK and PI3K-Akt pathways to increase the cellular content of Krüppel-like factor 5 (KLF5), which was required for PDAC cell proliferation [20]. Consistently, in human cell lines, MAPK activation upon KRAS mutation was revealed to induce posttranscriptional modification of YAP, and KRAS mutation was able to augment the transcriptional activity of YAP on its target genes [21]. Functionally, YAP-TAZ activation was demonstrated to be required for pancreatic carcinogenesis in mice carrying the KRASG12D mutation: YAP and TAZ protein levels were upregulated in each stage of PDAC pathogenesis, including pancreatitis, acinar-to-ductal metaplasia (ADM) and pancreatic intraepithelial neoplasia (PanIN), and double knockout of Yap and Taz genes significantly mitigated KRASG12D mutation-induced ADM and PanIN lesions [12]. In fact, YAP is essential for maintaining glucose metabolism in normal pancreatic epithelial cells [21]. This means that the KRAS mutation potentially induces a metabolic dysregulation of glucose. In a previous study, the KRASG12D mutation was revealed to induce an upregulation of the gene encoding NIX, a critical protein for inducing mitophagy, thus restricting glucose flux into mitochondria (Figure 1). Via this mechanism, glucose metabolism in PDAC cells could be switched to favor glycolysis, and the antioxidant program could be activated, thus facilitating cell proliferation [13]. To understand the relationship among KRAS mutation, the antioxidant program and cell proliferation in PDAC, another study conducted by the same team reported that the KRASG12D mutation could activate the Nrf2-related antioxidant program in pancreatic epithelial cells of mice; in addition, PanIN cells from Nrf2-deficient mice were less proliferative than those without Nrf2 deficiency [22]. Consistent with this finding, inhibiting glutathione synthesis in PanIN cells without Nrf2 deficiency decreased their proliferation [22]. Collectively, these results show that KRAS mutation impacts the proliferation of PDAC cells in a metabolic manner (Figure 1).
| 癌症 | 数模转换器 | CRAC | LUAC | |
|---|---|---|---|---|
| 字符 [参考] | ||||
| KRAS突变的流行率 | 97.7% [ 9 ] | 44.7% [ 9 ] | 30.9% [ 9 ] | |
| KRAS 中最热门的错义突变 | G12D [ 9 ] | G12D [ 9 ] | G12C [ 9 ] | |
| Sensitive to glucose restriction vs. KRASwt | Yes [19] | Yes [20] | No [21] | |
| Common alteration with KRAS | TP53 inactivation [10] | TP53 and APC inactivation [22] | TP53 or LKB1 inactivation [23] | |
| General milieu of KRAS-mutant tumors | Immune-cold [7] | Immune-cold [24] | KRAS-only: immune-cold or hot [23] TP53 inactivation: immune-hot [23] LKB1 inactivation: immune-cold [23] |
|
| Number/function of tumoricidal T cells in KRAS-mutant tumors | Decrease/Decrease [7] | Decrease/Decrease [24] | KRAS-only: slight increase/decrease [23] TP53 inactivation: significant increase/decrease [23] LKB1 inactivation: significant decrease/decrease [23] |
|
| Major type of immune infiltrates in KRAS-mutant tumors | Myeloid suppressive cell [7] | Myeloid suppressive cell [24] | KRAS-only: T cell, macrophage, neutrophil [23] TP53 inactivation: CD8+ T cell, CD45RO+ T cell [23] LKB1 inactivation: myeloid suppressive cell [23] |
|
| Common presentation of the ICB therapy biomarker if KRAS mutation | pMMR/MSS [25] | pMMR/MSS [26] | KRAS-only: PD-L1 expression ↑ [23] TP53 inactivation: PD-L1 expression ↑↑ [23] LKB1 inactivation: PD-L1 expression ↓↓ [23] |
|
| Biomarker associated with the effectiveness of ICB therapy | dMMR/MSI-H [4] | dMMR/MSI-H [27] | PD-L1 [23] | |
| Prevalence of dMMR/MSI-H in all cases | 1~2% [25] | 14% [26] | NM | |
| Prevalence of positive expression of PD-L1 by tumor cells | NM | NM | Among KRAS-only tumors: 37.5% [23] Among TP53 inactivation tumors: 68.8% [23] Among LKB1 inactivation tumors: 10% [23] |
|
| General response to monotherapy using ICB drugs | Poor [5] | Poor [28] | KRAS-only tumor: Fair [23] TP53 inactivation tumor: Excellent [23] LKB1 inactivation tumor: Poor [23] |
|
| Core molecular events associated with KRAS mutation-induced immunosuppression | 1. YAP-TAZ activation [12]; 2. JAK-STAT3 activation [12]; 3. Metabolic reprogramming of glucose and cell autophagy [13,14]; 4. In concert with other events, TP53 inactivation [15], LKB1 mutation [29,30], PTEN loss [29,30], WNT/β-catenin activation [29,30], FAK activation [29,30], PIK3CA activation [29,30] and MYC activation [29,30]; |
1. In concert with APC and TP53 inactivation: TGF-β1 upregulation and EMT [31]; 2. TGF-β-induced immune suppression [32]; 3. IRF2 inactivation [24,33]; 4. Metabolic dysregulation in glucose, glutamine, fatty acid and lipid [26,34]; 5. MAPK and HIF-1-related cascade activation [34]; |
1. ERK activation-induced PD-L1 upregulation [23] 2. Metabolic reprogramming of glucose [21] 3. In concert with LKB1 inactivation: strengthening metabolic reprogramming of glucose and JAK-STAT3 activation [23] |
|
3. The KRAS mutation and immune environment in PDAC
3. The KRAS Mutation and Immune Environment in PDAC
In addition to impacting cell survival, proliferation and nutrient metabolism during pancreatic carcinogenesis, KRAS mutations also function in controlling the cancer immune environment. As documented, competition for glucose between cancer cells and stromal immune cells serves as a route for immune evasion of tumors [23]. As evidenced in mice, pancreatic epithelial cells carrying the KRASG12D mutation and Lkb1 inactivation were revealed to enhance their proliferation by overly consuming glucose [24]. In addition, in mice bearing pancreatitis-induced ADM, KLF5 deficiency was revealed to suppress STAT3 activation [3620]. Generally, STAT3 activation correlates with immune suppression in cancers [25]. In the presence of KRASG12D mutation, Stat3 was revealed to be required for the development of ADM and PanIN during pancreatic carcinogenesis in mice [26]. In this model, IL-6 family cytokines were found to serve as inflammatory stimuli for STAT3 activation [26]. In another mechanism, KRASG12D mutation-induced upregulation of YAP and TAZ was revealed to potently activate the downstream JAK-STAT3 pathway during pancreatic carcinogenesis in mice [12] (Figure 1). In fact, mutant KRAS KRAS can cooperate with extracellular stimuli, such as inflammation, the gut microbiota and gastrointestinal peptides, to persistently activate downstream YAP-TAZ signaling, which undermines immune surveillance against PDAC cells in addition to improving their proliferation, invasion, survival and metabolism [4227]. In PDAC, a high expression of YAP was revealed to correlate with a poor histological grade of tumor cells [4328], a high risk of metastasis and a poor prognosis of patients [4429].
Mechanistically, KRAS mutation-induced activation of YAP enables PDAC cells to release IL-4, IL-6, IL-13, MCP-1 and CSF-1, which promote the recruitment of tumor-associated macrophages (TAMs) into tumors and induce them to proliferate and polarize into an M2-like phenotype [4530] (Figure 1). In addition, the prevalence of TP53 inactivation is only second to the prevalence of KRAS mutation in PDAC [10], meaning that a large portion of patients concomitantly harbor KRASKRAS mutation and TP53 inactivation [10]. To evaluate the function of this genetic alteration pattern in pancreatic carcinogenesis, concomitantly transgenic mutations of KRASG12D and Tp53R172H were introduced into the pancreas of mice, resulting in the PDAC formation and metastasis [31]. In this research, the Tp53R172H mutation was found to accelerate chromosomal instability in the presence of the KRASG12D mutation compared with wild-type Tp53 [31]. In addition, the Tp53 inactivation cooperated with the KRAS mutation to induce PDAC cells to secrete CXCR3/CCR2-associated chemokines and CSF-1, thus recruiting myeloid-derived suppressive cells (MDSCs) into PDAC tumors and promoting the expansion of MDSCs [15]. In addition, PDAC tumors with KRAS mutation plus Tp53 inactivation had increased numbers of Treg cells compared with PDAC tumors with only KRAS mutation [15]. In tumors anwith both alterations, the Treg cells presented TP53upregulation of CD25, GITR and KLRG1, indicating increased suppressive ability [15]. In addition, Th1 and CD8+ T cell-mediated anticancer responses were attenuated [15]. Conversely, pancreas-specific knockout of Yap in mice carrying KRASG12D/Tp53R172H comutation restored the expression of cytotoxicity-associated genes by CD8+ T cells in addition to preventing MDSC accumulation [1032].
This result suggests that Yap is required for KRAS mutation-induced immunosuppression in PDAC tumors.
In concert with the KRAS mutation, alterations in environmental, genetic and molecular levels, such as hypoxia, LKB1 mutation, PTEN loss, PIK3CA activation, WNT/β-catenin activation, focal FAKadhesion kinase (FAK) activation and MYC MYCactivation also contribute to immune suprotopression in PDAC tumors [33, 34] (Figure 1). For example, hypoxia can activate HIF-1α, and moncogene (reover, HIF-1α activation is potent in inducing tumoral angiogenesis by increasing the expression of VEGF [35]. This event also occurs in PDAC [26]. As documented, VEGF is a potent cytokine that undermines anticancer immunity by dictating the expansion, phenotypic conversion and suppressive function of tumor-infiltrating immune cells, such as MDSCs, TAMs, dendritic cells (DCs) and Treg cells [35] (Figure 1). In response to hypoxia, some infiltrating immune cells, such as DCs and TAMs, and the endothelium can induce self-expression of PD-L1 molecule, thus impairing the infiltration, survival and effector function of tumoricidal T cells [35]. In addition to immune cells, tumor cells are critical sources of PD-L1. For example, the transcriptional activation of MYC) activation also co enables PDAC cells to upregulate PD-L1 expression [37]. In addition, mixed lineage leukemia protein-1 (MLL1) can upregulate PD-L1 expression: as a histone methyltransferase, MLL1 can accelerate H3K4 trimethylation in the promoter of the gene encoding PD-L1 [38]. Via these actions, immune evasion in PDAC tumors can be facilitated. Thus, as documented, features of the immune milieu in PDAC tumors include infiltration of cancer-supportive cells [e.g., cancer-associatried fibroblasts (CAFs), Treg cells, suppressive neutrophils, indoleamine 2,3-dioxygenase (IDO)-producing DCs, M2-like TAMs and MDSCs], upregulation of suppressive cytokines (e.g., nitric oxide, hyaluronic acid, IL-6, IL-10, VEGF, TGF-β, CSF-1, GM-CSF, CXCL1, CXCL8, CXCL12 and CXCL13), angiogenesis and ‘T cell exclusion’ [7, 39, 40] (Figure 1). In fact, both in hute to immunmans and mice, although PDAC tumors were found to harbor tumoricidal T cell infiltrates, few of them were found in the vicinity of PDAC cells, a phenomenon known as ‘T cell exclusion’ [40, 41] (Figure 1). This exclusion suppression in PDAC tumoris a critical mechanism by which intratumoral cells, such as CAFs, M2-like TAMs and MDSCs, encourage PDAC cells to escape T cell attack [39]. In support of this mechanism, CAF-derived CXCL12 was demonstrated to show a high affinity to PDAC cells, whereas inhibition of CXCR4 by using AMD3100 could significantly limit the tumor growth of mice bearing PDAC in a T cell-dependent manner [41]. Moreover, upon CXCR4 inhibition, PDAC cells could be besieged by massive numbers of T cells [41]. In addition, myeloid-derived Ly6GLow+/F4/80+ macrophages served as extratumoral cells that caused T cell exclusion from the PDAC tumors of mice [2942]. In summary, due to the lack of tumoricidal T cells and the enrichment of immunosuppressive cells and cytokines,30] the immune milieu of PDAC tumors is generally cancer-supportive (Figure 1).
4. Current Status of Immune Checkpoint Blockade Therapy for PDAC
4. Current status of immune checkpoint blockade therapy for PDAC
Since the tumoral milieu of PDAC is immunosuppressive, ICB therapy is anticipated to have low effectiveness in this cancer. In fact, several lines of clinical data have confirmed this speculation, and the effectiveness of monotherapy by using ICB drugs in patients with metastatic PDAC remains disappointing [55]. For example, a phase II study reported that as a second- or later-line therapy for metastatic PDAC, durvalumab (an anti-PD-L1 drug) alone and durvalumab plus tremelimumab (an anti-CTLA-4 drug) had ORR values of 0% and 3.1%, respectively [43] (Table 2). Prior to this study, in order to improve the effectiveness of ICB therapy, a phase I study employed stereotactic body radiation therapy (SBRT) in combination with ICB therapy (in this case pembrolizumab) to upregulate the expression of the genes encoding PD-L1 and MHC-I in tumor cells and improve the production of tumor-associated antigens (TAAs) by recruiting tumoricidal T cells and by improving the production of IFN-γ by CD8+ T cells [18] as a strategy against metastatic cancers, and this combination achieved an ORR of 13.2% among enrolled patients [44]. However, this study only included three patients with metastatic PDAC, and their ORR to this strategy was not reported. Recently, a single-center phase I study tested SBRT plus durvalumab with or without tremelimumab as a second- or later-line therapy for metastatic PDAC patients [45]. Unexpectedly, the ORR for this strategy was only 5.1% [44]. Like radiotherapy, chemotherapy agents exert cytotoxicity to induce immunogenic cell death (ICD) as well [46]. To evaluate the synergistic effect of chemotherapy plus ICB therapy, a phase I study was carried out, and 2 of 11 patients with metastatic PDAC achieved a partial response after receiving gemcitabine-based chemotherapy plus pembrolizumab [47]. Yet, these two patients were chemotherapy-naïve. In contrast, the remaining patients had received at least one line of chemotherapy before receiving this therapy combination, which had produced stable disease in most of them [47]. Consistent with this finding, another phase I study concluded that an anti-CTLA-4 drug (ipilimumab) plus gemcitabine exhibited no advantages over gemcitabine alone in increasing the ORR of patients with metastatic PDAC [48]. Notably, most patients had received at least one line therapy prior to being enrolled in the study. Hence, the above data suggest that ICB drugs are not effective in significantly shrinking the size of PDAC tumors when used as a second- or later-line therapy regardless of whether they are used alone or in combination with radiotherapy or chemotherapy (Table 2).
| Author [Ref.] |
|---|
| Year | Phase | Patient No. | ICB Drug | Other Treatment | ORR | |
|---|---|---|---|---|---|---|
| • First-line therapy | ||||||
| Aglietta M, et al. [62] | 2014 | I | 34 | Tremelimumab | Gemcitabine | 10.5% |
| Wainberg ZA, et al. [63] | 2019 | I | 50 | Nivolumab | Gemcitabine + Nab- paclitaxel | 18% |
| Wainberg ZA, et al. [64] | 2017 | I | 17 | Nivolumab | Gemcitabine + Nab- paclitaxel | 50% |
| Renouf, et al. [65] | 2018 | II | 11 | Durvalumab + Tremelimumab | Gemcitabine + Nab-paclitaxel | 73% |
| Borazanci, et al. [66] | 2018 | II | 11 | Nivolumab | Gemcitabine + Nab-paclitaxel + Cisplatin + Paricalcitol | 80% |
| • Second- or later-line therapy | ||||||
| Luke JJ, et al. [57] | 2018 | I | 3 | Pembrolizumab | SBRT: 30–50 Gy for 2–4 metastatic lesions | NR |
| O’Reilly EM, et al. [56] | 2019 | II | Arm A: 32 Arm B: 32 |
Durvalumab Durvalumab + Tremelimumab |
No | 0% 3.1% |
| Xie C, et al. [58] | 2020 | I | Arm A1: 14 Arm A2: 10 Arm B1: 19 Arm B2: 16 |
Durvalumab Durvalumab Durvalumab + Tremelimumab Durvalumab + Tremelimumab |
SBRT: 8 Gy/1 fraction SBRT: 25 Gy/5 fractions SBRT: 8 Gy/1 fraction SBRT: 25 Gy/5 fractions |
5.1% A |
| Weiss GJ, et al. [60] | 2017 | I | 11 | Pembrolizumab | Gemcitabine (Gem)-based chemotherapy | 18.2% |
| Kamath SD, et al. [61] | 2020 | I | 21 B | Arm A: Ipilimumab 3 mg/kg Arm B: Ipilimumab 3 mg/kg Arm C: Ipilimumab 6 mg/kg |
Gem 750 mg/m2 Gem 1g/m2 Gem 1g/m2 |
14% C |
The effectiveness of ICB therapy on PDAC
|
Author[Ref.] |
Year |
Phase |
Patient No. |
ICB drug |
Other treatment |
ORR |
|
||||||
|
Aglietta M, et al.[54] |
2014 |
I |
34 |
Tremelimumab |
Gemcitabine |
10.5 % |
|
Wainberg ZA, et al.[55] |
2019 |
I |
50 |
Nivolumab |
Gemcitabine + Nab- paclitaxel |
18 % |
|
Wainberg ZA, et al.[56] |
2017 |
I |
17 |
Nivolumab |
Gemcitabine + Nab- paclitaxel |
50 % |
|
Renouf, et al.[57] |
2018 |
II |
11 |
Durvalumab + Tremelimumab |
Gemcitabine + Nab-paclitaxel |
73 % |
|
Borazanci, et al.[58] |
2018 |
II |
11 |
Nivolumab |
Gemcitabine + Nab-paclitaxel + Cisplatin + Paricalcitol |
80 % |
|
||||||
|
Luke JJ, et al.[44] |
2018 |
I |
3 |
Pembrolizumab |
SBRT: 30-50 Gy for 2 - 4 metastatic lesions |
NR |
|
O‘Reilly EM, et al.[43] |
2019 |
II |
Arm A: 32 Arm B: 32 |
Durvalumab Durvalumab + Tremelimumab |
No |
0% 3.1 % |
|
Xie C, et al.[45] |
2020 |
I |
Arm A1: 14 Arm A2: 10 Arm B1: 19 Arm B2: 16 |
Durvalumab Durvalumab Durvalumab + Tremelimumab Durvalumab + Tremelimumab |
SBRT: 8 Gy/1 fraction SBRT: 25 Gy/5 fractions SBRT: 8 Gy/1 fraction SBRT: 25 Gy/5 fractions |
5.1 %A |
|
Weiss GJ, et al.[47] |
2017 |
I |
11 |
Pembrolizumab |
Gemcitabine (Gem)-based chemotherapy |
18.2 % |
|
Kamath SD, et al.[48] |
2020 |
I |
21B |
Arm A: Ipilimumab 3 mg/kg Arm B: Ipilimumab 3 mg/kg Arm C: Ipilimumab 6 mg/kg |
Gem 750 mg/㎡ Gem 1g/㎡ Gem 1g/㎡ |
14 %C |
Abbreviation: PDAC: pancreatic ductal adenocarcinoma; ORR: objective response rate; SBRT:
: The total ORR of four arms;
: 67% of them received at least one line of chemotherapy; C: The total ORR of three arms.
In fact, metastatic cancers commonly show clonal evolution of tumor cells as the therapies are engaged [49], and this scenario is suitable for ICB therapy [50]. As reported, first-line chemotherapy using [FOLFIRINOX] (oxaliplatin plus irinotecan plus 5-fluorouracil plus calcium folinate) [51] or [GA] (gemcitabine plus nab-paclitaxel) [52] regimens significantly prolonged the overall survival of patients with metastatic PDAC compared with gemcitabine monotherapy, implying that these combination regimens are more effective in killing tumor cells. In this regard, adding ICB drugs to intensive chemotherapy is speculated to further improve the prognosis of patients, mainly because the increased burden of neoantigens derived from lysed cancer cells can potentially improve anticancer immunity when these antigens are successfully presented by DCs to peripheral T cells [53]. When such T cells migrate into the tumor, they can recognize the cancer clones sharing the neoantigens and then kill these cancer cells [53]. Supporting this theory, recent data from several phase I and II trials indeed revealed that as a first-line therapy, chemotherapy plus ICB therapy had improved effectiveness compared with as a second- or later-line therapy in metastatic PDAC (Table 2). For example, gemcitabine plus tremelimumab achieved an ORR of 10.5% [54]. The [GA] regimen plus nivolumab (an anti-PD-1 drug) or plus pembrolizumab achieved ORRs ranging from 18% to 50% [55, 56]. More strikingly, when [GA] regimen was combined with durvalumab plus tremelimumab, the ORR was 73% [57]. In addition, an ORR of 80% was achieved when nivolumab was added to the regimen containing nab-paclitaxel, cisplatin, gemcitabine and paricalcitol [58]. These combinational strategies were tolerated by most enrolled patients. Therefore, although these trials had low patient numbers, their data at least provide new insights into the future management of metastatic PDAC by using chemotherapy plus ICB therapy in the first-line setting. Nevertheless, the prognostic value of this strategy in metastatic PDAC remains to be elucidated via randomized phase III trials.
Overall, the currently published data indicate an extremely low effectiveness of monotherapy by using ICI drugs or their combination with other conventional approaches, such as radiotherapy or chemotherapy, as second- or later-line therapies for metastatic PDAC (Table 2). In PDAC, only the MSI-H/dMMR phenotype is indicative of response to pembrolizumab. However, the prevalence of the MSI-H/dMMR phenotype in PDAC has been reported to be only 1% ~ 2% [59], but intriguingly, the MSI-H/dMMR phenotype was found to be strongly correlated with a high tumor mutational burden (TMB) and a wild-type KRAS and p53 molecular background [58]. Consequently, to achieve a breakthrough in the management of PDAC with KRAS mutation, a focus should be placed on eliminating the tumor cell- or stromal cell-induced barriers that counteract anticancer immunity. Recent studies in this field have revealed several strategies, such as adding an antiangiogenic drug [60], an anti-IL-6 antibody [61], an ATM inhibitor [62], a CD40 agonist [42], a CSF1R inhibitor [63], a YAP inhibitor plus a pan-RAF inhibitor [28], a CXCR4 inhibitor [41], a PARP inhibitor [64], a Listeria vaccine plus an anti-CD25 antibody [65], a FAK inhibitor [66], a CCR2 inhibitor [67], an IDO inhibitor plus the GM-CSF-conjugated whole-cell PDAC vaccine (GVAX) [68], or the combination of anti-PD-1 and anti-PD-L1 monoclonal antibodies [69], that have been confirmed to improve the immune milieu and the effectiveness of ICB therapy in preclinical models of PDAC. On these bases, some strategies using ICB therapy plus GVAX or other means, such as CXCR4 inhibition, CSF1R inhibition and CD40 blockade, have been designed to treat PDAC patients in clinical trials [39]. A few strategies have exhibited their effectiveness, such as the success of GVAX plus ipilimumab in prolonging the survival of PDAC patients [70].
Besides, a few small molecular compounds, such as AMG510, MRTX849, ARS-3248/JNJ-74699157 or LY3499446, have been designed to antagonize cancer cells carrying the KRASG12C mutation [71]. Among them, the data of AMG510 and MRTX849 are encouraging. For example, both in KRASG12C-mutated LUAC and CRAC models, basic experiments revealed the tumoricidal activity of AMG510 or MRTX849 both in vitro and in vivo [72, 73]; Likewise, administration of AMG510 or MRTX849 was confirmed to cause a significant shrinkage of tumors among patients with the KRASG12C-mutated LUAC, CRAC or PDAC [72-74]. Moreover, the tumoral immune milieu can be improved by using such KRASG12C inhibitors. In the model of mice bearing KRASG12C-muated CT-26 cell line-derived tumors, following AMG510 administration, T cells were found to significantly infiltrate into tumors [72]. Particularly, most of them were positive for CD8, and they presented a proliferating status upon AMG510 administration [72]. Mechanically, AMG510 administration could induce the upregulation of CXCL10 and CXCL11 by tumor cells, two crucial chemoattractant of T cells, thus causing an increasement of T cells in xenografted tumors [72]. Meanwhile, DCs including CD103+ cross-presenting pool and macrophages were found to increase their infiltration in xenografted tumors as well [72]. Functionally, CD103+ DCs are crucial for T cell priming and activation, while activated CD8+ T cells can produce IFN-γ, which enables tumor cells to increase their expression of MHC-I [72]. Thus, following AMG510 administration, the tumoral immune milieu was characterized by increased interferon signaling, antigen processing, chemokine production, cytotoxic activity and innate immune system stimulation [72]. Similar to AMG510, in the model of mice bearing KRASG12C-muated CT-26 cell line-derived tumors, MRTX849 administration was revealed to induce the polarization of TAMs from M2 to M1, the infiltration of DCs, B cells and tumoricidal T cells in tumors, as well as the reduction of MDSCs in tumors [75]. Therefore, either AMG510 or MRTX849 plus an anti-PD-1 antibody were demonstrated to cause a durable shrinkage of xenografted tumors with KRASG12C mutation [72, 75]. In fact, data associated with the potential of AMG510 or MRTX849 in shifting tumoral immune milieu from a suppressive to a tumoricidal state are mainly collected from the model of CRAC, rather than PDAC [72, 75]. In this regard, more efforts should be paid in the future to reveal whether KRASG12C inhibition can improve the tumoral immune milieu of PDAC, thus enabling the combination of KRASG12C inhibition and anti-PD-1 therapy to overcome the immunosuppression in PDAC. However, the frequency of KRASG12C mutation only accounts for less than 3% among all PDAC cases, whereas approximately 50% of PDAC cases have the missense form of G12D [9]. In fact, adaptive transfer of CD8+ T cells that react with KRASG12D-mutated tumor cells were demonstrated to be an effective approach in treating CRAC [76]. In order to benefit the majority of PDAC patients, drugs or new treatment strategies that target G12D missense mutation should deserve attention; In this scenario, the tumoricidal activity of newly developed approaches along with their potentials in improving tumoral immune milieu should be explored in the future.
5. Value of KRAS mutation for predicting cancer immune status in other adenocarcinomas
As mentioned above, PDAC, CRAC and LUAC are the top three cancers harboring a high prevalence of KRAS mutations [9] (Table 1). In CRAC, the prevalence of KRAS mutation is 44.7% [9]. As in PDAC, G12D is the most frequent missense mutation that causes consecutive activation of KRAS protein in CRAC [9] (Table 1). Among KRAS-mutant CRAC cases, 35% to 50% of them are reported to have concomitant inactivation in APC and p53 [77]. In mice bearing CRAC, the KRASG12D mutation was revealed to significantly increase the invasion and metastasis of cancer cells because conditional codeletion of Apc and Tp53 concomitant with KRASG12D mutation enabled primary and metastatic tumors to significantly upregulate the expression of the gene encoding TGF-β1, both a critical immunosuppressive cytokine [78] and a critical ligand of TGF-β/SMAD signaling that can dictate epithelial-mesenchymal transition (EMT) in CRAC cells [79]. Moreover, compared with patients with the wild-type RAS, CRAC patients harboring KRAS mutation generally have a poor prognosis [80].
However, the prevalence of the MSI-H/dMMR phenotype in CRAC is higher than that in PDAC. According to published data, the incidence of the MSI-H/dMMR phenotype in CRAC is 14% [81]. Currently, ICB therapy with pembrolizumab is recommended as the first-line therapy for metastatic CRAC with the MSI-H/dMMR phenotype, which has been confirmed as a reliable biomarker for predicting the outcome of ICB therapy by several lines of trial data [4, 82, 83]. Regardless, not all patients with this phenotype benefit from the ICB therapy [82, 83]. In the KEYNOTE-177 study, chemotherapy plus bevacizumab was still more effective than pembrolizumab monotherapy in prolonging the progression-free survival of patients with metastatic disease, the MSI-H/dMMR phenotype and KRAS mutation [84]. Conversely, those patients without KRAS mutation did benefit more from pembrolizumab than chemotherapy plus bevacizumab [84]. Hence, these results suggest that KRAS mutation can undermine the effectiveness of pembrolizumab even in the presence of the MSI-H/dMMR phenotype. Critically, KRAS mutation was revealed to be enriched in CRAC with the microsatellite stability (MSS) or proficient mismatch repair (pMMR) phenotype [81]. However, published data reveal that patients with CRAC with the MSS/pMMR phenotype respond poorly to ICB therapy alone [85].
Similar to its role in PDAC (Table 1), KRAS mutation in CRAC with the MSS/pMMR phenotype generally correlates with immune suppression in the tumor. To address this issue, a study evaluated the role of KRAS mutation in dictating the cancer immune status of CRAC tumors [86], which were mainly classified into four subgroups, namely, CMS1 (immune type), CMS2 (classical type), CMS3 (metabolic type) and CMS4 (mesenchymal type), according to which molecular pathways were enriched [81]. The results indicated that CMS2 or CMS3 tumors with KRAS mutation had a significantly reduced number of tumoricidal T cells compared with those without wild-type KRAS [86]. To explore the mechanism, experiments were performed in mice bearing CRAC with the KRASG12D mutation plus conditional depletion of Apc and Tp53, and this genetic alteration pattern was found to enable the tumors to have increased numbers of MDSCs but decreased numbers of CD4+ or CD8+ T cells compared with the pattern of conditional codeletion of Apc and Tp53 [87]. In detail, the KRASG12D mutation was able to activate ERK, which showed a negative relationship with the expression of gene encoding interferon-related factor 2 (IRF2) by tumor cells [86]. In return, IRF2 inactivation upregulated the expression of the gene encoding CXCL3, a chemokine that attracts MDSCs into tumors, thus impairing the expansion and IFN-γ-producing function of tumoricidal T cells [87]. Notably, KRAS mutation-related IRF2 inactivation was revealed to correlate with a poor response of CRAC patients to ICB therapy [87]. Conversely, in mice bearing CRAC with the KRASG12D mutation plus codeletion of Apc and Tp53, blocking CXCR2 on MDSCs improved the efficacy of anti-PD-1 therapy by increasing the number of CD8+ T cells but decreasing the number of Treg cells in tumors [87]. In fact, the CRAC tumors in these mice were revealed to resemble the CMS4 tumors in terms of some molecular signatures, such as the TGF-β/EMT signature [79]. As reported, the patients in the CMS4 subgroup commonly presented with rapid disease progression along with a poorer prognosis than the patients in other subgroups [81]. As such, KRAS mutation-induced immunosuppression potentially contributes to this process. In addition, CD8+ T cells that recognize the cancer cell clones carrying the KRASG12D mutation have been shown to exist in human CRAC tumors [88]. To our knowledge, the recognition of tumor antigens by tumoricidal T cells is as critical as having these cells infiltrate into tumors. Therefore, ICB therapy is speculated to improve the anticancer effect of T cells on KRAS-mutant CRAC.
In fact, KRAS-mutant CRAC still has several differences from PDAC in tumor biology (Table 1). As mentioned above, CRAC patients with the MSS/pMMR phenotype appear to be inherently refractory to ICB therapy [85]. Unlike in PDAC, the data from clinical trials, such as VOLTAGE (chemoradiation followed by 5 doses of nivolumab before radical surgery as a neoadjuvant therapy for locally advanced rectal cancer) [89], MEDETREME (FOLFOX regimen plus durvalumab and tremelimumab as a first-line therapy for metastatic CRAC) [90] and REGONIVO (regorafenib plus nivolumab as a third-line therapy for refractory CRAC) [91], have confirmed that patients with MSS/pMMR tumors could benefit from ICB therapy-based combinational strategies. Certainly, a portion of patients harboring KRAS mutations in their tumors are included in these studies, thus helping to elucidate the role of chemoradiation, duplet chemotherapy or molecule-targeted therapy in boosting the tumoricidal milieu. In addition to using conventional means, several new means have been developed. As documented, KRAS mutation-driven molecular alterations cause CMS3 tumor cells to have dysregulated glucose, glutamine, fatty acid and lipid metabolism [81, 92]. Targeting the metabolic abnormalities or blocking the downstream pathways affected by KRAS mutation, such as the MAPK and HIF-1-related pathways, has been shown to induce cancer cell death, potentially increasing the release of tumor antigens [92]. However, intriguingly, although KRAS-mutant CRAC cells have been revealed to consume glucose for their expansion, they are more resistant to glucose restriction than cells with wild-type KRAS [93]. This is another difference from PDAC cells, and murine pancreatic epithelial cells with the KRASG12D mutation with Lkb1 inactivation have been found to be sensitive to acute glucose restriction or glycolysis inhibition [24]. Consistent with this finding, LUAC cells in mice with homozygous KRASG12D/G12D mutation were more sensitive to glucose restriction than those with heterogeneous KRASG12D/wt mutation or KRASwt/wt, and a higher consumption of glucose occurred in LUAC cells with the KRASG12D/G12D mutation than in LUAC cells with other versions of KRAS [94]. Notably, G12C is the most common missense causing KRAS mutation in LUAC, with a prevalence of 30.9% in Western patients [9]. Nevertheless, KRAS mutation should not be regarded as a marker indicating immunosuppression in LUAC tumors because the immune milieu in KRAS-mutant LUAC tumors is heterogeneous. For example, the KRAS/LKB1 and KRAS/TP53 comutations enable LUAC patients to have dramatically different responses to ICB therapy because these two mutational patterns generally create a unique immune milieu in tumors (see details in Ref. [95]). Collectively, KRAS mutation can affect the cancer immune state in PDAC, CRAC and LUAC in different ways and contextures (Table 1).
6. Conclusion
The tumor milieu in PDAC is profoundly immunosuppressive, which renders monotherapy by using ICB drugs almost completely ineffective. Regarding the development of immunosuppression in PDAC, multiple factors are involved. Herein, KRAS mutation has been shown to be central in this process, because KRAS mutation can activate YAP-TAZ and JAK-STAT3 to elicit an immunosuppressive response, and this initial signaling can then be strengthened by coordination with TP53 inactivation and other genetic or molecular alterations. Overall, KRAS mutation generally correlates with tumor immunosuppression in PDAC. Nevertheless, in CRAC and LUAC, KRAS mutation can dictate the cancer immune environment in different ways. In these cancers, the immune milieu varies despite the commonality of KRAS mutation. This notion can be exemplified by KRAS-mutant LUAC, which exhibits a varied response to ICB therapy depending on the types of genetic alterations that cooccur with the KRAS mutation.
Abbreviation list
ADM:acinar-to-ductal metaplasia; APC: adenomatous polyposis coli protein; ATM: ataxia telaniectasia-mutated gene-coded protein; BRAC: breast cancer susceptibility gene; CAF: cancer-associated fibroblast; CCR: chemokine C-C motif receptor; CDKN2A: cyclin dependent kinase inhibitor 2A gene; CMS: consensus molecular subtype; CRAC: colorectal adenocarcinoma; CSF-1: macrophage-colony stimulating factor; CTLA-4: cytotoxic T lymphocyte-associated antigen-4; CXCL: chemokine C-X-C motif ligand; CXCR: chemokine C-X-C motif receptor; DC: dendritic cell; dMMR: deficient mismatch repair; EMT: epithelial-mesenchymal transition; FAK: focal adhesion kinase; GITR: glucocorticoid-induced tumor necrosis factor receptor; GM-CSF: granulocyte-macrophage colony stimulating factor; GVAX: GM-CSF-conjugated whole-cell PDAC vaccine; HIF-1: hypoxia-induced factor 1; HRAS: Harvey rat sarcoma viral oncogene homolog gene; H3K4: histone 3 lysine 4; ICB: immune checkpoint blockade; ICD: immunogenic cell death; IDO: indoleamine 2, 3-dioxygenase; IRF2: interferon-related factor 2; JAK: Janus kinase; KLF5: Krüppel-like factor 5; KLRG1: killer cell lectin like receptor G1; KRAS: Kristen rat sarcoma virus oncogene homolog gene; LKB1: liver kinase B1 gene; LUAC: lung adenocarcinoma; MAPK: mitogen-activated protein kinase; MCP-1: monocyte chemotactic protein 1; MDSC: myeloid-derived suppressive cell; MHC-I: major histocompatibility complex-I; MLL1: mixed lineage leukemia protein 1; MSI: microsatellite instability; MSS: microsatellite stability; MYC: MYC proto-oncogene; NRAS: neuroblastoma RAS viral oncogene homolog gene; NIX: encoded by BNIP3L, Bcl2/adenovirus E1B 19 KDa protein-interacting protein 3-like gene; Nrf2: nuclear-related factor 2; NTRK: neuro trophin receptor kinase gene; ORR: objective response rate; PARP: poly ADP-ribose polymerase; PanIN: pancreatic intraepithelial neoplasia; PDAC: pancreatic ductal adenocarcinoma; PD-1: programmed death-1; PD-L1: programmed death ligand 1; PIK3CA: phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha; PI3K: phosphatidylinositol 3-kinase; pMMR: proficient mismatch repair; PTEN: phosphatase and tensin homolog gene; RAF: RAF proto-oncogene; SBRT: stereotactic body radiation therapy; SMAD4: SMAD family member 4 gene; STAT3: signal transducers and activators of transcription 3; TAA: tumor-associated antigen; TAM: tumor-associated macrophage; TAZ: tafazzin; TGF-β: transforming growth factor-beta; Th: T helper cell; TP53: tumor protein P53 gene; Treg: regulatory T cell; TMB: tumor mutational burden; VEGF: vascular endothelial growth factor; WNT: “Wingless/Integrated”; YAP: yes-associated protein.
Conflicts of Interest: The author declared no conflict of interest.
Author Contributions: CP wrote this paper, GM and GY prepared the figure and tables.
Funding: This work was supported by National Natural Science Foundation of China [Grant No. 81874254], by Scientific and Technological Developing Scheme Foundation of Jilin Province [Grant No. 20200201400JC], and by Foundation of Scientific Research Planning Project of the 13th Five-year Plan of Jilin Provincial Department of Education [Grant No. JJKH20201043KJ].
References
[1] Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018, 68:394-424.
[2] Vincent A., Herman J., Schulik R., Hruban R. H., Goggins M. Pancreatic cancer. Lancet. 2011, 378: 607-20.
[3] Davendra P.S. Sohal, Erin B. Kennedy, Pelin Cinar, Thierry Conroy, Mehmet S. Copur, Christopher H. Crane, et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020, Epub ahead of print.
[4] Marabelle A., Le D. T., Ascierto P. A. Di Giacomo A. M., De Jesus-Acosta A. Delord Jean-Pierre, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020, 38:1-10.
[5] Henriksen A., Dyhl-Polk A., Chen I., Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treatment Rev. 2019, 78: 17-30.
[6] Ribas A., Wolchok J. D. Cancer immunotherapy using checkpoint blockade. Science. 2018, 359: 1350-1355.
[7] Liu X., Xu J., Zhang B., Liu J., Liang C., Meng Q., et al. The reciprocal regulation between host tissue and immune cells in pancreatic ductal adenocarcinoma: new insights and therapeutic implications. Mol Cancer. 2019, 18:184.
[8] Wellenstein M. D., E de Visser D. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity. 2018, 48:399-416.
[9] Cox A. D., Fesik S. W., Kimmelman A. C., Luo J., Der C. J. Drugging the undruggable RAS: Mission possible? Nat Rev Drug Discov. 2014, 13: 828-51.
[10] Bailey P., Chang D. K. Nones K., Johns A. L., Patch A. M. Gingras M. C., et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016, 531: 47-52.
[11] Kamisawa T., Wood L. D. Itoi T., Takaori K. Pancreatic cancer. Lancet. 2016, 388:73-85.
[12] Gruber R., Panayiotou R., Nye E., Spencer-Dene B., Stamp G., Behrens A. YAP1 and TAZ Control Pancreatic Cancer Initiation in Mice by Direct Up-regulation of JAK-STAT3 Signaling. Gastroenterology. 2016, 151:526-39.
[13] Humpton T. J., Alagesan B., DeNicola G. M., Lu D., Yordanov G. N. Leonhardt C. S., et al. Oncogenic KRAS Induces NIX-Mediated Mitophagy to Promote Pancreatic Cancer. Cancer Discov. 2019, 9:1268-1287.
[14] Yamamoto K., Venida A., Yano J., Biancur D. E., Kakiuchi M., Gupta S., et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020, 581:100-105.
[15] Blagih J., Zani F., Chakravarty P., Hennequart M., Pilley S., Hobor S., et al. Cancer-Specific Loss of p53 Leads to a Modulation of Myeloid and T Cell Responses. Cell Rep. 2020, 30:481-496.
[16] Dong Z.Y., Zhong W. Z., Zhang X. C., Su J., Xie Z., Liu S. Y., et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res. 2017, 23:3021-3024.
[17] Skoulidis F., Goldberg M. E., Greenawalt D. M., Hellman M. D. Awad M. M. Gainor J. F. et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8:822-835.
[18] Liang T., Tong W., Ma S., Chang P. Standard therapies: solutions for improving therapeutic effects of immune checkpoint inhibitors on colorectal cancer. OncoImmunology. 2020, 9:1773205.
[19] Waters A. M., Der C. J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med. 2018, 8: a0311435.
[20] He P., Yang J. W., Yang V. W., Bialkowska. A. B. Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Promotes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice. Gastroenterology. 2018, 154: 1494-1508.
[21] Zhang W., Nandakumar N., Shi Y., Manzano M., Smith A., Graham G., et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014, 7:ra42.
[22] DeNicola G. M., Karreth F. A., Humpton T. J., Gopinathan A., Wei C., Frese K., Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011, 475:106-9.
[23] Chang C., Qiu J., O’Sullivan D., Buck M. D. Noguchi T., Curtis J. D., et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015, 162:1229-41.
[24] Kottakis F., Nicolay B. N., Roumane A., Karnik R., Gu H., Nagle J. M., et al. LKB loss links serine metabolism to DNA methylation and tumorigenesis. Nature. 2016, 539: 390-395.
[25] Jones S. A., Jenkins B. J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018, 18:773-789.
[26] Corcoran R. B., Contino G., Deshpande V., Tzatsos A., Conrad C., Benes C. H. et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011, 71:5020-9.
[27] Eibl G., Rozengurt E. KRAS, YAP, and obesity in pancreatic cancer: A signaling network with multiple loops. Semin Cancer Biol. 2019, 54: 50-62.
[28] Zhao X., Wang X., Fang L., Lan C., Zheng X., Wang Y., et al. A combinatorial strategy using YAP and pan-RAF inhibitors for treating KRAS-mutant pancreatic cancer. Cancer Lett. 2017, 402: 61-70.
[29] Allende M., Zeron-Medina J., Hernandez J., Macarulla T., Balsells J., Merino X., et al. Overexpression of Yes Associated Protein 1, an Independent Prognostic Marker in Patients With Pancreatic Ductal Adenocarcinoma, Correlated With Liver Metastasis and Poor Prognosis. Pancreas. 2017, 46:913-920.
[30] Yang W., Yang S., Zhang F., Cheng F., Wang X., Rao J. Influence of the Hippo-YAP signaling pathway on tumor associated macrophages (TAMs) and its implications on cancer immunosuppressive microenvironment. Ann Transl Med. 2020, 8:399.
[31] Hingorani S. R., Wang L., Multani A. S., Combs C., Deramaudt T. B. Hruban R. H., et al. Trp53R172H and KRASG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005, 7:469-83.
[32] Murakami S., Shabhazian D., Surana R., Zhang W., Chen H., Graham G. T., Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene. 2017, 36:1232-1244.
[33] Gan L. L., Hii L.W., Wong S. F., Leong C. O., Mai C. W. Molecular Mechanisms and Potential Therapeutic Reversal of Pancreatic Cancer-Induced Immune Evasion. Cancers (Basel). 2020, 12:1872.
[34] Sivaram N., McLaughlin P. A., Han H. V., Petrenko O., Jiang Y. P., Ballou L. M., et al. Tumor-intrinsic PIK3CA represses tumor immunogenecity in a model of pancreatic cancer. J Clin Invest. 2019, 129:3264-3276.
[35] Fukumura D., Kloepper J., Amoozgar Z., Duda D. G., Jain R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018, 15:325-340.
[36] Li S., Xu H. X., Wu C. T., Wang W. Q., Jin W., Gao H. L., et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019, 22:15-36.
[37] Sodir N.M., Kortlever R. M., Barthet V. J. A., Campos T., Pellegrinet L., Kupczak S., et al. MYC Instructs and Maintains Pancreatic Adenocarcinoma Phenotype. Cancer Discov. 2020, 10:588-607.
[38] Lu C., Paschall A. V., Shi H., Savage N., Waller J.L., Sabbatini M. E., et al. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expression and Pancreatic Cancer Immune Evasion. J. Natl. Cancer Inst. 2017, 109: djw283.
[39] Johnson B. A. 3rd., Yarchoan M., Lee V., Laheru D. A., Jaffee E. M. Strategies for Increasing Pancreatic Tumor Immunogenicity. Clin Cancer Res. 2017, 23:1656-1669.
[40] Joyce J. A., Fearon D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015, 348: 74-80.
[41] Feig C., Jones J. O., Kraman M., Wells R. J. B., Deonarine A., Chan D. S., Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergized with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013, 110: 20212-7.
[42] Beatty G. L., Winograd R., Evans R. A., Long K. B., Luque S. L., Lee J. W., et al. Exclusion of T cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extracuumoral Macrophages. Gastroenterology. 2015, 149:201-10.
[43] O’Reilly E.M., Oh D.Y., Dhani N., Renouf D.J. Lee M. A., Sun W., et al. Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5: 1431-8.
[44] Luke J.J., Lemons J.M., Karrison T. G., Pitroda S.P., Melotek J. M., Zha Y. Y., et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol. 2018, 36:1611-1618.
[45] Xie C., Duffy A. G., Brar G., Fioravanti S., Mabry-Hrones D., Walker M., et al. Immune Checkpoint Blockade in Combination with Stereotactic Body Radiotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2020, 26:2318-2326.
[46] Wang Q., Ju X., Wang J., Fan Y., Ren M., Zhang H. Immunogenic cell death in anticancer chemotherapy and its impact on clinical studies. Cancer Lett. 2018, 438: 17-23.
[47] Weiss G. J., Waypa J., Blaydorn L., Coats J., McGahey K., Sangal A., et al. A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PmebroPlus). Br J Cancer. 2017, 117:33-40.
[48] Kamath S. D., Kalyan A., Kircher S., Nimeiri H., Fought A.J., Benson 3rd Al., et al. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A phase Ib Study. Oncologist. 2020, 25: e808-e815.
[49] Dawood S., Austin L., Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park). 2014, 28:1101-7.
[50] Riaz N., Havel J. J., Makarov V., Desrichard A., Urba W. J., Sims J. S., et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017, 171: 934-949.
[51] Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011, 364:1817-25.
[52] Von Hoff D. D., Ervin T., Arena F. P., Chiorean E. G., Infante J., Moore M., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013, 369:1691-703.
[53] Schumacher T.N., Scheper W., Kvistborg P. Cancer Neoantigens. Annu Rev Immunol. 2019, 37:173-200.
[54] Aglietta M., Barone C., Sawyer M. B., Moore M. J., Miller Jr W. H., Bagalà C., et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014, 25:1750-5.
[55] Wainberg Z. A., Hochster H.S., Kim E., George B., Kalyan A., Chiorean E.G., et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) + gemcitabine (Gem) in advanced pancreatic cancer (APC). J Clin Oncol. 2019, 37:298.
[56] Wainberg Z. A., Hochster H. S., George B., Gutierrez M., Johns M. E., Chiorean E. G., et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) ± gemcitabine (Gem) in solid tumors: interim results from the pancreatic cancer (PC) cohorts [abstracts]. J Clin Oncol. 2017, 35: 412.
[57] Renouf D.J., Dhani N.C., Kavan P., Jonker D.J., Wei AC-C., Hsu T., et al. The Canadian Cancer Trials Group PA.7 trial: results from the safety run in of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) versus GEM, nab-P, durvalumab (D), and tremelimumab (T) as first-line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). J Clin Oncol. 2018, 36: 349.
[58] Borazanci E.H., Jameson G.S., Borad M.J., Ramanathan R.K., Korn R.L., Caldwell L., et al. A phase II pilot trial of nivolumab (N) + albumin bound paclitaxel (AP) + paricalcitol (P) + cisplatin (C) + gemcitabine (G) (NAPPCG) in patients with previously untreated metastatic pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol. 2018, 36:358.
[59] Luchini C., Brosens L. A. A., Wood L. D. Chatterjee D., Shin J. II., Sciammarella C., et al. Comprehensive characterization of pancreatic ductal adenocarcinoma with microsatellite instability: histology, molecular pathology and clinical implications. Gut. 2020. Online ahead of print.
[60] Allen E., Jabouille A., Rivera Lee B., Lodewijckx I., Missiaen R., Steri V., et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates immunity through HEV formation. Sci Transl Med. 2017, 9: eaak9679.
[61] Mace T. A., Shakya R., Pitaarresi J. R., Swanson B., McQuinn C. W., Loftus S., et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018, 67:320-332.
[62] Zhang Q., Green M. D., Lang X., Lazarus J., Parsels J. D., Wei S., Inhibition of ATM Increases Interferon Signaling and Sensitizes Pancreatic Cancer to Immune Checkpoint Blockade Therapy. Cancer Res. 2019, 79:3940-3951.
[63] Zhu Y., Knolhoff B.L., Meyer M.A., Nywening T.M., West B.L., Luo J., et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014, 74:5057-69.
[64] Stewart R. A., Pilié P.G., Yap T. A. Development of PARP and Immune-Checkpoint Inhibitor Combinations. Cancer Res. 2018, 78: 6717-6725.
[65] Keenan B. P., Saenger Y., Kafrouni M. I., Leubner A., Lauer P., Maitra A., et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology. 2014, 146: 1784-94.
[66] Jiang H., Hegde S., Knolhoff B. L., Zhu Y., Herndon J. M., Meyer M. A., et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016, 22:851-60.
[67] Sanford D. E., Belt B. A., Panni R. Z., Mayer A., Deshpande A. D., Carpenter D., et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013, 19:3404-15.
[68] Blair A.B., Kleponis J., Thomas D.L. 2nd., Muth S. T., Murphy A. G., Kim V., et al. IDO1 inhibition potentiates vaccine-induced immunity against pancreatic adenocarcinoma. J Clin Invest. 2019, 129:1742-1755.
[69] Burrack A. L., Spartz E. J., Raynor J. F., Wang I., Olson M., Stromnes I.M. Combination PD-1 and PD-L1 Blockade Promotes Durable Neoantigen-Specific T Cell-Mediated Immunity in Pancreatic Ductal Adenocarcinoma. Cell Rep. 2019, 28:2140-2155.
[70] Christenson E. S., Jaffee E., Azad N. S. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol. 2020, 21: e135-e145.
[71] Nollmann FI., Ruess DA. Targeting Mutant KRAS in Pancreatic Cancer: Futile or Promising? Biomedicines. 2020,8(8):281.
[72] Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., et al. The clinical KRAS(G12C) inhibitor AMG510 drives anti-tumour immunity. Nature. 2019, 575(7781):217-223.
[73] Hallin J., Engstrom L.D., Hargis L., Calinisan A., Aranda R., Briere D. M., et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10(1): 54-71.
[74] Hong D. S., Fakih M. G., Strickler J. H., Desai J., Durm G. A., Shapiro G.I., et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020, 383(13):1207-1217.
[75] Briere D., Calinisan A., Aranda R., Sudhakar N., Hargis L., Gatto S., The KRASG12C
5. Conclusions
PDAC 中的肿瘤环境具有很强的免疫抑制作用,这使得使用 ICB 药物的单一疗法几乎完全无效。关于 PDAC 免疫抑制的发展,涉及多种因素。在本文中,KRAS突变已被证明是该过程的核心,因为KRAS突变可以激活 YAP-TAZ 和 JAK-STAT3 以引发免疫抑制反应,然后可以通过与TP53失活和其他遗传或分子的协调来加强这种初始信号。改动。总体而言,KRAS突变通常与 PDAC 中的肿瘤免疫抑制相关。尽管如此,在 CRAC 和 LUAC 中,KRAS突变可以以不同的方式决定癌症免疫环境。在这些癌症中,尽管KRAS突变具有共性,但免疫环境各不相同。这个概念可以用KRAS 突变体 LUAC来举例说明,它对 ICB 治疗表现出不同的反应,这取决于与KRAS突变同时发生的基因改变的类型。
Inhibitor MRTX849 Reconditions the Tumor Immune Microenvironment and Leads to Durable Complete Responses in Combination with Anti-PD-1 Therapy in a Syngeneic Mouse Model. AACR 2019. [Abstract]
[76] Tran E., Robbins P.F., Lu Y.C., Prickett T.D., Gartner J.J., Jia L., et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016, 375(23):2255-2262.
[77] Vakiani E., Janakiraman M., Shen R., Sinha R., Zeng Z., Shia J., et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol. 2012, 30: 2956-2962.
[78] Yoshimura A., Muto G. TGF-β function in immune suppression. Curr Top Microbiol Immunol. 2011, 350:127-47.
[79] Boutin A. T., Liao W. T., Wang M., Hwang S. S., Karpinets T. V., Cheung H., et al. Oncogenic KRAS drives invasion and maintains metastases in colorectal cancer. Genes Dev. 2017, 31:370-382.
[80] Smeby J., Sveen A., Merok M. A., Danielssen S. A., Eilertsen I. A., Guren M. G., et al. CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer. Ann Oncol. 2018, 29:1227-1234.
[81] Guinney J., Dienstmannn R., Wang X., deReyniès A., Schlicker A., Soneson C., et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015, 21:1350-6.
[82] Le D. T., Kim T. W., Van Cutsem E., Geva R., Jäger D., Hara H., et al. Phase II Open-Label Study of Pembrolizumab in Treatment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020, 38:11-19.
[83] Overman M. J., Lonardi S., Wong K. Y. M., Lenz H. J., Gelsomino F., Aglietta M., et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018, 36:773-779.
[84] Andre T., Shiu K. K., Kim T. W., Jensen B. V., Jensen L. H., Punt C., et al. KEYNOTE-177: Phase 3, Open-label, randomized study of first-line pembrolizumab (Pembro) versus investigator-choice chemotherapy for mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) metastatic colorectal carcinoma (mCRC)[Abstract]. J Clin Oncol. 2018, 4-suppl (February 26th).
[85] Ganesh K., Stadler Z. K., Cercek A., Mendelsohn R. B., Shia J., Segal N. H., et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019, 16:361-375.
[86] Lal N., White B. S., Goussous G., Pickles O., Mason M. J., Beggs A. D., et al. KRAS Mutation and Consensus Molecular Subtypes 2 and 3 Are Independently Associated with Reduced Immune Infiltration and Reactivity in Colorectal Cancer. Clin Cancer Res. 2018, 24:224-233.
[87] Liao W., Overman M. J., Boutin A. T., Shang X., Zhao D., Dey P., et al. KRAS-IRF2 Axis Drives Immune Suppression and Immune Therapy Resistance in Colorectal Cancer. Cancer Cell. 2019, 35:559-572.
[88] Tran E., Ahmadzadeh M., Lu Y. C., Gros A., Turcotte S., Robbins P. F., et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015, 350:1387-90.
[89] Yuki S., et al. Short-term results of VOLTAGE-A: Nivolumab monotherapy and subsequent radical surgery following preoperative chemoradiotherapy in patients with microsatellite stable and microsatellite instability-high locally advanced rectal cancer. 2020 ASCO annual meeting. [Abstract 4100]
[90] Ghiringhelli F., et al. Durvalumab and tremelimumab in combination with FOLFOX in patients with RAS-mutated, microsatellite-stable, previously untreated metastatic colorectal cancer (mCRC): Results of the first intermediate analysis of the phase Ib/II MEDETREME trial. J Clin Oncol. 2020 38: 15-suppl, 3006.
[91] Shota F., et al. Regorafenib plus nivolumab in patients with advanced gastric (GC) or colorectal cancer (CRC): An open-label, dose-finding, and dose-expansion phase 1b trial (REGONIVO, EPOC1603). 2019 ASCO annual meeting. [Abstract]
[92] Wang G., Wang J. J., Yin P. H., Xu K., Wang Y. Z., Shi F., et al. Strategies to target energy metabolism in consensus molecular subtype 3 along with Kirsten rat sarcoma viral oncogene homolog mutations for colorectal cancer therapy. J Cell Physiol. 2019, 234:5601-5612.
[93] Yun J., Rago C., Cheong I., Pagliarini R., Angenendt P., Rajagopalan H., et al. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009, 325: 1555-9.
[94] Kerr E. M., Gaude E., Turrell F. K., Frezza C., Martins C. P. Mutant KRAS copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature. 2016, 531: 110-3.
[95] Gu M., Xu T., Chang P. KRAS/LKB1 and KRAS/TP53 co-mutations create divergent immune signatures in lung adenocarcinomas. Ther Adv Med Oncol. 2020, ahead of print.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [CrossRef]
- Vincent, A.; Herman, J.; Schulik, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [CrossRef]
- Sohal, D.P.; Kennedy, E.B.; Cinar, P.; Conroy, T.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Lau, M.W.; Johnson, T.; Krishnamurthi, S.; et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J. Clin. Oncol. 2020, 27, 3217–3230. [CrossRef][PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; di Giacomo, A.M.; de Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J Clin. Oncol. 2020, 38, 1–10. [CrossRef] [PubMed]
- Henriksen, A.; Dyhl-Polk, A.; Chen, I.; Nielsen, D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat. Rev. 2019, 78, 17–30. [CrossRef] [PubMed]
- Ribas, A.;Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Zhang, B.; Liu, J.; Liang, C.; Meng, Q.; Hua, J.; Yu, X.; Shi, S. The reciprocal regulation between host tissue and immune cells in pancreatic ductal adenocarcinoma: New insights and therapeutic implications. Mol. Cancer. 2019, 18, 184. [CrossRef] [PubMed]
- Wellenstein, M.D.; de Visser, K.E. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity 2018, 48, 399–416. [CrossRef]
- Cox, A.D.; Fesik, S.W.; Kimmelman, A.C.; Luo, J.; Der, C.J. Drugging the undruggable RAS:Mission possible? Nat. Rev. Drug Discov. 2014, 13, 828–851. [CrossRef]
- [10] Bailey P., Chang D. K. Nones K., Johns A. L., Patch A. M. Gingras M. C., et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016, 531: 47-52.
- [11] Kamisawa T., Wood L. D. Itoi T., Takaori K. Pancreatic cancer. Lancet. 2016, 388:73-85.
- [12] Gruber R., Panayiotou R., Nye E., Spencer-Dene B., Stamp G., Behrens A. YAP1 and TAZ Control Pancreatic Cancer Initiation in Mice by Direct Up-regulation of JAK-STAT3 Signaling. Gastroenterology. 2016, 151:526-39.
- [13] Humpton T. J., Alagesan B., DeNicola G. M., Lu D., Yordanov G. N. Leonhardt C. S., et al. Oncogenic KRAS Induces NIX-Mediated Mitophagy to Promote Pancreatic Cancer. Cancer Discov. 2019, 9:1268-1287.
- [14] Yamamoto K., Venida A., Yano J., Biancur D. E., Kakiuchi M., Gupta S., et al. Autophagy promotes immune evasion of pancre-atic cancer by degrading MHC-I. Nature. 2020, 581:100-105.
- [15] Blagih J., Zani F., Chakravarty P., Hennequart M., Pilley S., Hobor S., et al. Cancer-Specific Loss of p53 Leads to a Modulation of Myeloid and T Cell Responses. Cell Rep. 2020, 30:481-496.
- [16] Dong Z.Y., Zhong W. Z., Zhang X. C., Su J., Xie Z., Liu S. Y., et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res. 2017, 23:3021-3024.
- [17] Skoulidis F., Goldberg M. E., Greenawalt D. M., Hellman M. D. Awad M. M. Gainor J. F. et al. STK11/LKB1 Mutations and PD-1 Inhibitor Resistance in KRAS-Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8:822-835.
- [18] Liang T., Tong W., Ma S., Chang P. Standard therapies: solutions for improving therapeutic effects of immune checkpoint in-hibitors on colorectal cancer. OncoImmunology. 2020, 9:1773205.
- [19] Waters A. M., Der C. J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb Perspect Med. 2018, 8: a0311435.
- [20] He P., Yang J. W., Yang V. W., Bialkowska. A. B. Krüppel-like Factor 5, Increased in Pancreatic Ductal Adenocarcinoma, Pro-motes Proliferation, Acinar-to-Ductal Metaplasia, Pancreatic Intraepithelial Neoplasia, and Tumor Growth in Mice. Gastroen-terology. 2018, 154: 1494-1508.
- [21] Zhang W., Nandakumar N., Shi Y., Manzano M., Smith A., Graham G., et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014, 7:ra42.
- [22] DeNicola G. M., Karreth F. A., Humpton T. J., Gopinathan A., Wei C., Frese K., Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011, 475:106-9.
- [23] Chang C., Qiu J., O’Sullivan D., Buck M. D. Noguchi T., Curtis J. D., et al. Metabolic Competition in the Tumor Microenviron-ment Is a Driver of Cancer Progression. Cell. 2015, 162:1229-41.
- [24] Kottakis F., Nicolay B. N., Roumane A., Karnik R., Gu H., Nagle J. M., et al. LKB loss links serine metabolism to DNA methyla-tion and tumorigenesis. Nature. 2016, 539: 390-395.
- [25] Jones S. A., Jenkins B. J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018, 18:773-789.
- [26] Corcoran R. B., Contino G., Deshpande V., Tzatsos A., Conrad C., Benes C. H. et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011, 71:5020-9.
- [27] Eibl G., Rozengurt E. KRAS, YAP, and obesity in pancreatic cancer: A signaling network with multiple loops. Semin Cancer Biol. 2019, 54: 50-62.
- [28] Zhao X., Wang X., Fang L., Lan C., Zheng X., Wang Y., et al. A combinatorial strategy using YAP and pan-RAF inhibitors for treating KRAS-mutant pancreatic cancer. Cancer Lett. 2017, 402: 61-70.
- [29] Allende M., Zeron-Medina J., Hernandez J., Macarulla T., Balsells J., Merino X., et al. Overexpression of Yes Associated Protein 1, an Independent Prognostic Marker in Patients With Pancreatic Ductal Adenocarcinoma, Correlated With Liver Metastasis and Poor Prognosis. Pancreas. 2017, 46:913-920.
- [30] Yang W., Yang S., Zhang F., Cheng F., Wang X., Rao J. Influence of the Hippo-YAP signaling pathway on tumor associated macrophages (TAMs) and its implications on cancer immunosuppressive microenvironment. Ann Transl Med. 2020, 8:399.
- [31] Hingorani S. R., Wang L., Multani A. S., Combs C., Deramaudt T. B. Hruban R. H., et al. Trp53R172H and KRASG12D cooper-ate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005, 7:469-83.
- [32] Murakami S., Shabhazian D., Surana R., Zhang W., Chen H., Graham G. T., Yes-associated protein mediates immune repro-gramming in pancreatic ductal adenocarcinoma. Oncogene. 2017, 36:1232-1244.
- [33] Gan L. L., Hii L.W., Wong S. F., Leong C. O., Mai C. W. Molecular Mechanisms and Potential Therapeutic Reversal of Pancreatic Cancer-Induced Immune Evasion. Cancers (Basel). 2020, 12:1872.
- [34] Sivaram N., McLaughlin P. A., Han H. V., Petrenko O., Jiang Y. P., Ballou L. M., et al. Tumor-intrinsic PIK3CA represses tumor immunogenecity in a model of pancreatic cancer. J Clin Invest. 2019, 129:3264-3276.
- [35] Fukumura D., Kloepper J., Amoozgar Z., Duda D. G., Jain R. K. Enhancing cancer immunotherapy using antiangiogenics: op-portunities and challenges. Nat Rev Clin Oncol. 2018, 15:325-340.
- [36] Li S., Xu H. X., Wu C. T., Wang W. Q., Jin W., Gao H. L., et al. Angiogenesis in pancreatic cancer: current research status and clinical implications. Angiogenesis. 2019, 22:15-36.
- [37] Sodir N.M., Kortlever R. M., Barthet V. J. A., Campos T., Pellegrinet L., Kupczak S., et al. MYC Instructs and Maintains Pancre-atic Adenocarcinoma Phenotype. Cancer Discov. 2020, 10:588-607.
- [38] Lu C., Paschall A. V., Shi H., Savage N., Waller J.L., Sabbatini M. E., et al. The MLL1-H3K4me3 Axis-Mediated PD-L1 Expres-sion and Pancreatic Cancer Immune Evasion. J. Natl. Cancer Inst. 2017, 109: djw283.
- [39] Johnson B. A. 3rd., Yarchoan M., Lee V., Laheru D. A., Jaffee E. M. Strategies for Increasing Pancreatic Tumor Immunogenicity. Clin Cancer Res. 2017, 23:1656-1669.
- [40] Joyce J. A., Fearon D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015, 348: 74-80.
- [41] Feig C., Jones J. O., Kraman M., Wells R. J. B., Deonarine A., Chan D. S., Targeting CXCL12 from FAP-expressing carcino-ma-associated fibroblasts synergized with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013, 110: 20212-7.
- [42] Beatty G. L., Winograd R., Evans R. A., Long K. B., Luque S. L., Lee J. W., et al. Exclusion of T cells From Pancreatic Carcinomas in Mice Is Regulated by Ly6C(low) F4/80(+) Extracuumoral Macrophages. Gastroenterology. 2015, 149:201-10.
- [43] O’Reilly E.M., Oh D.Y., Dhani N., Renouf D.J. Lee M. A., Sun W., et al. Durvalumab With or Without Tremelimumab for Pa-tients With Metastatic Pancreatic Ductal Adenocarcinoma: A phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5: 1431-8.
- [44] Luke J.J., Lemons J.M., Karrison T. G., Pitroda S.P., Melotek J. M., Zha Y. Y., et al. Safety and Clinical Activity of Pembrolizumab and Multisite Stereotactic Body Radiotherapy in Patients With Advanced Solid Tumors. J Clin Oncol. 2018, 36:1611-1618.
- [45] Xie C., Duffy A. G., Brar G., Fioravanti S., Mabry-Hrones D., Walker M., et al. Immune Checkpoint Blockade in Combination with Stereotactic Body Radiotherapy in Patients with Metastatic Pancreatic Ductal Adenocarcinoma. Clin Cancer Res. 2020, 26:2318-2326.
- [46] Wang Q., Ju X., Wang J., Fan Y., Ren M., Zhang H. Immunogenic cell death in anticancer chemotherapy and its impact on clini-cal studies. Cancer Lett. 2018, 438: 17-23.
- [47] Weiss G. J., Waypa J., Blaydorn L., Coats J., McGahey K., Sangal A., et al. A phase Ib study of pembrolizumab plus chemother-apy in patients with advanced cancer (PmebroPlus). Br J Cancer. 2017, 117:33-40.
- [48] Kamath S. D., Kalyan A., Kircher S., Nimeiri H., Fought A.J., Benson 3rd Al., et al. Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A phase Ib Study. Oncologist. 2020, 25: e808-e815.
- [49] Dawood S., Austin L., Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park). 2014, 28:1101-7.
- [50] Riaz N., Havel J. J., Makarov V., Desrichard A., Urba W. J., Sims J. S., et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017, 171: 934-949.
- [51] Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., et al. FOLFIRINOX versus gemcitabine for meta-static pancreatic cancer. N Engl J Med. 2011, 364:1817-25.
- [52] Von Hoff D. D., Ervin T., Arena F. P., Chiorean E. G., Infante J., Moore M., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013, 369:1691-703.
- [53] Schumacher T.N., Scheper W., Kvistborg P. Cancer Neoantigens. Annu Rev Immunol. 2019, 37:173-200.
- [54] Aglietta M., Barone C., Sawyer M. B., Moore M. J., Miller Jr W. H., Bagalà C., et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014, 25:1750-5.
- [55] Wainberg Z. A., Hochster H.S., Kim E., George B., Kalyan A., Chiorean E.G., et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) + gemcitabine (Gem) in advanced pancreatic cancer (APC). J Clin Oncol. 2019, 37:298.
- [56] Wainberg Z. A., Hochster H. S., George B., Gutierrez M., Johns M. E., Chiorean E. G., et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) ± gemcitabine (Gem) in solid tumors: interim results from the pancreatic cancer (PC) cohorts [abstracts]. J Clin Oncol. 2017, 35: 412.
- [57] Renouf D.J., Dhani N.C., Kavan P., Jonker D.J., Wei AC-C., Hsu T., et al. The Canadian Cancer Trials Group PA.7 trial: results from the safety run in of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) versus GEM, nab-P, durvalumab (D), and tremelimumab (T) as first-line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). J Clin Oncol. 2018, 36: 349.
- [58] Borazanci E.H., Jameson G.S., Borad M.J., Ramanathan R.K., Korn R.L., Caldwell L., et al. A phase II pilot trial of nivolumab (N) + albumin bound paclitaxel (AP) + paricalcitol (P) + cisplatin (C) + gemcitabine (G) (NAPPCG) in patients with previously un-treated metastatic pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol. 2018, 36:358.
- [59] Luchini C., Brosens L. A. A., Wood L. D. Chatterjee D., Shin J. II., Sciammarella C., et al. Comprehensive characterization of pancreatic ductal adenocarcinoma with microsatellite instability: histology, molecular pathology and clinical implications. Gut. 2020. Online ahead of print.
- [60] Allen E., Jabouille A., Rivera Lee B., Lodewijckx I., Missiaen R., Steri V., et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates immunity through HEV formation. Sci Transl Med. 2017, 9: eaak9679.
- [61] Mace T. A., Shakya R., Pitaarresi J. R., Swanson B., McQuinn C. W., Loftus S., et al. IL-6 and PD-L1 antibody blockade combina-tion therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018, 67:320-332.
- [62] Zhang Q., Green M. D., Lang X., Lazarus J., Parsels J. D., Wei S., Inhibition of ATM Increases Interferon Signaling and Sensitizes Pancreatic Cancer to Immune Checkpoint Blockade Therapy. Cancer Res. 2019, 79:3940-3951.
- [63] Zhu Y., Knolhoff B.L., Meyer M.A., Nywening T.M., West B.L., Luo J., et al. CSF1/CSF1R blockade reprograms tu-mor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014, 74:5057-69.
- [64] Stewart R. A., Pilié P.G., Yap T. A. Development of PARP and Immune-Checkpoint Inhibitor Combinations. Cancer Res. 2018, 78: 6717-6725.
- [65] Keenan B. P., Saenger Y., Kafrouni M. I., Leubner A., Lauer P., Maitra A., et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology. 2014, 146: 1784-94.
- [66] Jiang H., Hegde S., Knolhoff B. L., Zhu Y., Herndon J. M., Meyer M. A., et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016, 22:851-60.
- [67] Sanford D. E., Belt B. A., Panni R. Z., Mayer A., Deshpande A. D., Carpenter D., et al. Inflammatory monocyte mobilization de-creases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin Cancer Res. 2013, 19:3404-15.
- [68] Blair A.B., Kleponis J., Thomas D.L. 2nd., Muth S. T., Murphy A. G., Kim V., et al. IDO1 inhibition potentiates vaccine-induced immunity against pancreatic adenocarcinoma. J Clin Invest. 2019, 129:1742-1755.
- [69] Burrack A. L., Spartz E. J., Raynor J. F., Wang I., Olson M., Stromnes I.M. Combination PD-1 and PD-L1 Blockade Promotes Durable Neoantigen-Specific T Cell-Mediated Immunity in Pancreatic Ductal Adenocarcinoma. Cell Rep. 2019, 28:2140-2155.
- [70] Christenson E. S., Jaffee E., Azad N. S. Current and emerging therapies for patients with advanced pancreatic ductal adenocar-cinoma: a bright future. Lancet Oncol. 2020, 21: e135-e145.
- [71] Nollmann FI., Ruess DA. Targeting Mutant KRAS in Pancreatic Cancer: Futile or Promising? Biomedicines. 2020,8(8):281.
- [72] Canon J., Rex K., Saiki A.Y., Mohr C., Cooke K., Bagal D., et al. The clinical KRAS(G12C) inhibitor AMG510 drives anti-tumour immunity. Nature. 2019, 575(7781):217-223.
- [73] Hallin J., Engstrom L.D., Hargis L., Calinisan A., Aranda R., Briere D. M., et al. The KRASG12C Inhibitor MRTX849 Provides In-sight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10(1): 54-71.
- [74] Hong D. S., Fakih M. G., Strickler J. H., Desai J., Durm G. A., Shapiro G.I., et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020, 383(13):1207-1217.
- [75] Briere D., Calinisan A., Aranda R., Sudhakar N., Hargis L., Gatto S., The KRASG12C Inhibitor MRTX849 Reconditions the Tumor Immune Microenvironment and Leads to Durable Complete Responses in Combination with Anti-PD-1 Therapy in a Syngeneic Mouse Model. AACR 2019. [Abstract]
- [76] Tran E., Robbins P.F., Lu Y.C., Prickett T.D., Gartner J.J., Jia L., et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016, 375(23):2255-2262.
- [77] Vakiani E., Janakiraman M., Shen R., Sinha R., Zeng Z., Shia J., et al. Comparative genomic analysis of primary versus meta-static colorectal carcinomas. J Clin Oncol. 2012, 30: 2956-2962.
- [78] Yoshimura A., Muto G. TGF-β function in immune suppression. Curr Top Microbiol Immunol. 2011, 350:127-47.
- [79] Boutin A. T., Liao W. T., Wang M., Hwang S. S., Karpinets T. V., Cheung H., et al. Oncogenic KRAS drives invasion and main-tains metastases in colorectal cancer. Genes Dev. 2017, 31:370-382.
- [80] Smeby J., Sveen A., Merok M. A., Danielssen S. A., Eilertsen I. A., Guren M. G., et al. CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer. Ann Oncol. 2018, 29:1227-1234.
- [81] Guinney J., Dienstmannn R., Wang X., deReyniès A., Schlicker A., Soneson C., et al. The consensus molecular subtypes of colo-rectal cancer. Nat Med. 2015, 21:1350-6.
- [82] Le D. T., Kim T. W., Van Cutsem E., Geva R., Jäger D., Hara H., et al. Phase II Open-Label Study of Pembrolizumab in Treat-ment-Refractory, Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: KEYNOTE-164. J Clin Oncol. 2020, 38:11-19.
- [83] Overman M. J., Lonardi S., Wong K. Y. M., Lenz H. J., Gelsomino F., Aglietta M., et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018, 36:773-779.
- [84] Andre T., Shiu K. K., Kim T. W., Jensen B. V., Jensen L. H., Punt C., et al. KEYNOTE-177: Phase 3, Open-label, randomized study of first-line pembrolizumab (Pembro) versus investigator-choice chemotherapy for mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) metastatic colorectal carcinoma (mCRC)[Abstract]. J Clin Oncol. 2018, 4-suppl (Feb-ruary 26th).
- [85] Ganesh K., Stadler Z. K., Cercek A., Mendelsohn R. B., Shia J., Segal N. H., et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019, 16:361-375.
- [86] Lal N., White B. S., Goussous G., Pickles O., Mason M. J., Beggs A. D., et al. KRAS Mutation and Consensus Molecular Subtypes 2 and 3 Are Independently Associated with Reduced Immune Infiltration and Reactivity in Colorectal Cancer. Clin Cancer Res. 2018, 24:224-233.
- [87] Liao W., Overman M. J., Boutin A. T., Shang X., Zhao D., Dey P., et al. KRAS-IRF2 Axis Drives Immune Suppression and Im-mune Therapy Resistance in Colorectal Cancer. Cancer Cell. 2019, 35:559-572.
- [88] Tran E., Ahmadzadeh M., Lu Y. C., Gros A., Turcotte S., Robbins P. F., et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015, 350:1387-90.
- [89] Yuki S., et al. Short-term results of VOLTAGE-A: Nivolumab monotherapy and subsequent radical surgery following preoper-ative chemoradiotherapy in patients with microsatellite stable and microsatellite instability-high locally advanced rectal can-cer. 2020 ASCO annual meeting. [Abstract 4100]
- [90] Ghiringhelli F., et al. Durvalumab and tremelimumab in combination with FOLFOX in patients with RAS-mutated, microsatel-lite-stable, previously untreated metastatic colorectal cancer (mCRC): Results of the first intermediate analysis of the phase Ib/II MEDETREME trial. J Clin Oncol. 2020 38: 15-suppl, 3006.
- [91] Shota F., et al. Regorafenib plus nivolumab in patients with advanced gastric (GC) or colorectal cancer (CRC): An open-label, dose-finding, and dose-expansion phase 1b trial (REGONIVO, EPOC1603). 2019 ASCO annual meeting. [Abstract]
- [92] Wang G., Wang J. J., Yin P. H., Xu K., Wang Y. Z., Shi F., et al. Strategies to target energy metabolism in consensus molecular subtype 3 along with Kirsten rat sarcoma viral oncogene homolog mutations for colorectal cancer therapy. J Cell Physiol. 2019, 234:5601-5612.
- [93] Yun J., Rago C., Cheong I., Pagliarini R., Angenendt P., Rajagopalan H., et al. Glucose deprivation contributes to the develop-ment of KRAS pathway mutations in tumor cells. Science. 2009, 325: 1555-9.
- [94] Kerr E. M., Gaude E., Turrell F. K., Frezza C., Martins C. P. Mutant KRAS copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature. 2016, 531: 110-3.
- [95] Gu M., Xu T., Chang P. KRAS/LKB1 and KRAS/TP53 co-mutations create divergent immune signatures in lung adenocarcino-mas. Ther Adv Med Oncol. 2020, ahead of print.
