Aroma profile is one of the main features for the acceptance of wine. Yeasts and bacteria are the responsible organisms to carry out both, alcoholic and malolactic fermentation. Alcoholic fermentation is in turn, responsible for transforming grape juice into wine and providing secondary aromas. Secondary aroma can be influenced by different factors; however, the influence of the microorganisms is one of the main agents affecting final wine aroma profile. Saccharomyces cerevisiae has historically been the most used yeast for winemaking process for its specific characteristics: high fermentative metabolism and kinetics, low acetic acid production, resistance to high levels of sugar, ethanol, sulfur dioxide and also, the production of pleasant aromatic compounds. Nevertheless, in the last years, the use of non-saccharomyces yeasts has been progressively growing according to their capacity to enhance aroma complexity and interact with S. cerevisiae, especially in mixed cultures.
- wine secondary aroma
- fermentation
- non-saccharomyces yeasts
- lactic acid bacteria
- volatile compounds
- strain variability
1. Introduction
1.1. Secondary Wine Aroma
1.2. Fermentation Implication on Wine Secondary Aroma
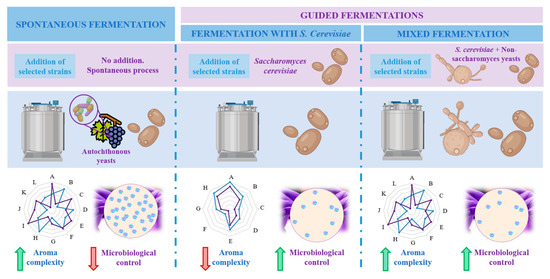
1.3. Microorganisms Implied in Wine Aroma
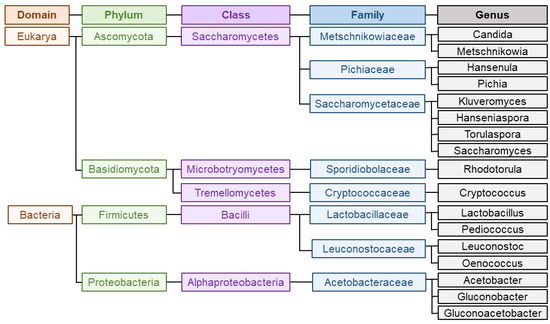
Wine is a complex matrix where the development of alcoholic fermentation, leaded by different yeasts coupled to the volatile compounds released during malolactic fermentation, leaded by LAB and acetic acid bacteria (AAB), defines wine secondary aroma [8]. Yeasts are responsible for alcoholic fermentation, and particularly, the unicellular fungi Saccharomyces cerevisiae governs the process, which can occur spontaneously or guided by the use of starter cultures [17]. Yeast domain counts up to more than 2000 species, among which Saccharomyces has traditionally been the most studied and important genus for industrial fermentation [8]. Within Saccharomyces species, S. cerevisiae is the most known since the first inoculation processes with a pure yeast culture were carried out with this species. This trend continued for many decades and resulted in the generalized use of S. cerevisiae as starter yeasts inmost wine fermentations [28]. However, as aforementioned, non-saccharomyces species also play an important role during fermentation. Among this group, the genera most commonly present and studied are Hanseniaspora, Hansenula, Metschnikowia, Candida, Pichia, Lachancea, Brettanomyces, Kluyveromyces, Schizosaccharomyces, Torulaspora, Zygosaccharomyces and Saccharomycodes [5,8][5][8]. In respect of bacteria, most abundant LAB belongs to genera Lactobacillus, Oenococcus, Pediococcus and Leuconostoc whereas most predominant AAB during winemaking are Acetobacter, Gluconobacter or Gluconacetobacter [29]. Figure 2 represents the main groups and taxonomy of the microorganisms implied in wine aroma. The challenge of winemakers and researches lies on the detection, characterization and quantification of all these microorganisms populations during fermentation to assess their participation on the development of wine secondary aroma [29].
Therefore, this review presents an overview of the main secondary aromas present in wine, the microorganisms involved in the spontaneous and guided (simultaneous or mixed) fermentations as well as an approach to the aroma variation that wine can suffer when different strains and genetic modifications have occurred.
2. Compounds Involved in Secondary or Fermentative Aroma
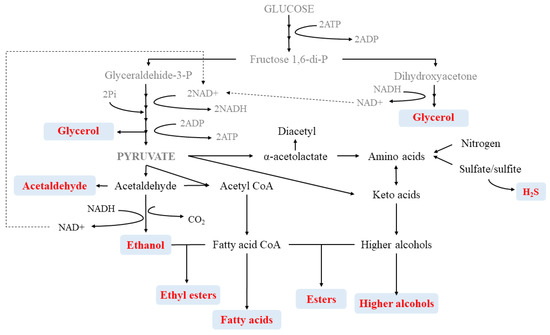
The quality of wine is derived from its aroma which is, in turn, characterized by its volatile composition, mainly created during the fermentation stages. Fermentation is highly dependent on the species and strains selected but also on the components of the wine matrix. Among the main volatiles that define wine, higher alcohols, esters and fatty acids play a key role in the creation of secondary aromas (Table 1 and Figure 3).
| Aromatic Class | Main Compounds | Desirable Concentration | Sensorial Properties | Producer Organism | Ref. |
|---|---|---|---|---|---|
| Fatty acids | Acetic acid, pentanoic acid, hexanoic acid, octanoic acid, decanoic acid, 9-Decenoic acid, 3-methylbutanoic acid, sobutyric acid | 200–700 mg/L | In excessive amount: rancid, greasy, and cheesy notes | S. cerevisiae, P. fermentas, C. zemplinina, H. guilliermondii, H. vineae, H. uvarum | [3,19,31][3][19][31] |
| Higher alcohols | 1-Propanol-isobutanol, isoamyl alcohol, 2-Phenylethanol, tyrosol, tryptophol, 2-methylbutanol-1, 3-methyl-1-butanol-1 | <300 mg/L | Floral, honey, and fruity notes (<300 mg/L). Pungent aroma (>400 mg/L) |
S. cerevisiae, C. zemplinin, H. uvarum, H. osmophila, H. guilliermondii, P. anomala, P. membranifaciens | [2,3,5,8,19][2][3][5][8][19] |
| Esters | Ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl acetate, isobutyl acetate, amyl acetate, hexyl acetate, 2PA, isoamyl acetate | 150–200 mg/L | Fruity aroma, including banana or apple, honey, and floral tones | S. cerevisiae, Candida, Hansenula, Pichia | [2,3,7,19][2][3][7][19] |
| Phenolics | 4-Vinyl guajacol, 4-Vinylphenol | - | Sweet vanillin aroma | LAB | [1,7,31][1][7][31] |
2.1. Volatile Fatty Acids
In the category of aliphatic fatty acids, apart from the most abundant volatile acid, i.e., the acetic acid, the major medium chain fatty acids are hexanoic, octanoic or decanoic. Besides, in the group of the unsaturated fatty acids is worthy to mention 9-decenoic acid which possesses preservative properties and is relevant from an aroma point of view when transformed into ethyl ester [31]. Yeasts are the primary producers of these fatty acids which are the initial substrate for the final formation of ethyl esters. Among yeasts, S. cerevisiae is capable of synthesizing mainly hexanoic and octanoic acids in high amounts, but also pentanoic, decanoic and 3-methylbutanoic acids. Other non-saccharomyces species such as the genus Hanseniaspora has been described to produce acetic acid (in very variable ranges, from 0.6 up to 3.4 g/L) and species such as Hanseniaspora vineae, H. uvarum, H. guilliermondii or Candida zemplinina displayed higher synthesis rates for isobutyric acid [3,19][3][19]. However, it has been stated that this group of yeasts does not present a distinct biosynthesis of fatty acids. In fact, the use of mixed fermentations including S. cerevisiae and non-saccharomyces can modify the chemical profile of the single S. cerevisiae model. In general terms, this combination shows a reduction in the amount of medium-chain fatty acids, as it happens when inoculating S. cerevisiae with H. osmophila. Even though, the utilization of a mixture of C. stellata and S. cerevisiae could increase the amount of hexanoic and octanoic acids, followed Pichia fermentas. Similarly, the application of sequential inoculations based on S. cerevisiae and non-saccharomyces usually provides wines with lower concentrations of fatty acids [3]. The use of mixed or sequential fermentations can have benefits to regulate the content of these medium chain fatty acids, since their excessive presence may provide negative aromas with greasy, rancid and cheesy notes [3,32][3][32].2.2. Higher Alcohols
The most abundant alcohols in wine, apart from ethanol and glycerol, are diols, higher alcohols and esters. Ethanol provides viscosity, balance taste and fix odors while higher alcohols and glycerol strongly contribute to the aroma complexity of wine and to the overall mouthfeel of wine. Higher alcohols are the result of the catabolism of amino acids by a process known as Ehrlich reaction, which affect directly or indirectly to the synthesis of aroma compounds. Higher alcohols are also involved as ester precursors which are important compounds in wine aroma [5,19][5][19]. Major higher alcohols are 1-propanol, isobutanol and isoamyl alcohol. Other important volatiles are the precursors of aromatic alcohols such as 2-phenylethanol, tyrosol or tryptophol and other higher alcohols but present in lower amounts, like 2-methylbutanol-1, 3-or methyl-1-butanol-1. Moderate concentrations of some of the volatiles considered to have high odor intensity, such as 3-methyl-1-butanol, 2-phenylethanol or isoamyl alcohol, can provide positive impact in the wine providing flower, honey and fruit aroma notes. However, the higher alcohol concentration plays a key role in the complexity of the aroma composition. Optimal alcohol values under 300 mg/L provide fruity and flowery notes, whereas alcohol values above 400 mg/L become negative by adding pungent and unpleasant aromas [2,3,8,19][2][3][8][19]. Among the different fermentation parameters that affect the final concentration of alcohol in wine, yeast strain is one of the key parameters followed by temperature, pH or oxygen, apart from grape ripeness and variety [33]. Higher alcohol synthesis has been widely studied and related to different species and/or inoculation protocols to obtain wines with an equilibrated higher alcohol composition. Different works have evaluated the efficiency of S. cerevisiae in terms of higher alcohol production [34,35][34][35]. Generally, no significant differences have been observed for 1-propanol while isobutanol, isoamyl alcohol, 3-methyl-1-butanol or 2-phenylethanol production seems to be strain-dependent and related to the presence of S. cerevisiae, both as pure or mixed cultures. In general terms, H. uvarum, C. zemplinina or P. anomala are considered as high alcohol producers, used both as single and mixed (with S. cerevisiae) fermentation agents [3,8,19][3][8][19]. Nevertheless, the single application of non-saccharomyces yeasts has been stated to produce lower amounts of total alcohols than S. cerevisiae and so, a reduction of the final amount of higher alcohols when using mixed cultures [36]. Indeed, H. osmophila, H. guilliermondii and P. membranifaciens were demonstrated to produce lower amounts of higher alcohols when tested against S. cerevisiae, even though H. osmophila provided high levels of 2-phenylethanol and isoamyl alcohol. Similarly, for the genus Candida, C. zemplinina has been described to synthesize 2-phenylethyl, glycerol and low amounts of ethanol and acetic acid. This combination has prompted its classification as fructophilic species, whereas C. stellata is classified as low producer. Another study with H. uvarum strains displayed variability in all produced higher alcohols except for isobutanol whose production seems to be boosted by Hanseniaspora. Indeed, another species, H. guilliermondii, also has a higher production rate of isobutanol than S. cerevisiae. Besides, same species synthesized very low amounts of 1-propanol [3,8,19][3][8][19].2.3. Esters
Esters are another relevant group, also responsible for the aroma complexity of wines with more than 160 representatives already identified. From a chemical point of view, they can be classified into ethyl fatty acid esters or acetate esters. In the first category, ethyl hexanoate, ethyl octanoate, and ethyl decanoate are the most abundant ones. In these molecules, ethanol represents an important contribution to their structure. In the second class, higher alcohols are essential for the formation of these esters. The major acetate esters are isobutyl acetate, amyl acetate, hexyl acetate, ethyl acetate (fruity aroma), isoamyl acetate (banana aroma) and 2-phenylethyl acetate (2PA), which has been described to provide honey, fruity and floral aromas to the wine [2,3,7][2][3][7]. In white wine, the main fatty acid ethyl esters include ethyl butanoate, caproate, caprylate, caprate and laurate. As other esters, they can also provide fruity tones that become softer with the increasing number of carbons in their chemical structure of the formation of these esters depends on the selection of yeast species and other fermentation parameters such as low temperatures, are [7]. Different yeasts have been used to give complexity to wines through ester production including S. cerevisiae but also non-saccharomyces species such as Candida, Hansenula and Pichia since their differential enzymatic mechanisms allow the introduction of novel aromas in wines [3]. In general terms, esters have positive effects on the aroma of young wines, especially in those with neutral flavors. Nevertheless, as it happens in the case of higher alcohols, excessive amounts of esters may induce negative effects on the quality of wine. A high concentration of esters can hidden varietal aromas and simplify the composition of aroma of the final product or spoil wine, for instance, if ethyl acetate exceeds 150–200 mg/L [2,19][2][19].2.4. Volatile Phenols
The positive aroma notes of this group of molecules have been mainly related to the aging process where the main volatile phenols are guaiacol, 4-methyIguaiacol, 4-ethylguaiacol, phenol, o-cresol or vanillin. The enzymes involved in these metabolic steps are mainly associated with LAB, such as β-glucosidases, proteases, esterases, citrate lyases and phenolic acid decarboxylases. In fact, many malolactic fermentations take place in oak barrels even though LAB can synthesize oak-like derived compounds from non-volatile phenols present in wine. Among the non-volatile phenols present in grapes it is common to find phenolic acids (caffeic, ferulic and p-coumaric) or their tartaric esters (caftaric acid, feruloyl tartaric acid, p-coumaroyl tartaric). LAB have the capacity to metabolize cinnamic acids, such as p-coumaric or ferulic, that through a decarboxylation step can be transformed into 4-vinyl guajacol and 4-vinylphenol. Thus, the use of LAB to obtain these compounds before the aging step has gained attention since it can help to modify the aroma complexity of wine. LAB can transform non-volatile phenols that contribute with unpleasant aromas such as pharmacy, smoke, forest, leather or pepper, into volatile pleasant ones, such as those related to vanillin, methyl vanilla or homovainyl alcohol. Apart from those that can be synthesized during fermentation stages due to their presence in grapes, another volatile phenols not present in grapes can be found in wines, i.e., acetovanillone [1,7,31][1][7][31].
References
- Pereira, A.G.; Fraga, M.; Garcia-Oliveira, P.; Carpena, M.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Barros, L.; Ferreira, I.C.F.R.; Prieto, M.A.; Simal-Gandara, J. Management of Wine Aroma Compounds: Principal Basis and Future Perspectives. In Winemaking-Stabilization, Aging Chemistry and Biochemistry; IntechOpen: London, UK, 2020.
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An Approach of the Madeira Wine Chemistry. Beverages 2020, 6, 12.
- Jeromel, A.; Korenika, A.-M.J.; Tomaz, I. 6—An Influence of Different Yeast Species on Wine Aroma Composition. In Fermented Beverages; Grumezescu, A.M., Holban, A.M.B.T.-F.B., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 171–285. ISBN 978-0-12-815271-3.
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on varietal aromas during wine making: A review of the impact of varietal aromas on the flavor of wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450.
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159.
- Rapp, A. Volatile flavour of wine: Correlation between instrumental analysis and sensory perception. Food Nahr. 1998, 42, 351–363.
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884.
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189.
- Ribâereau-Gayon, P.; Dubourdieu, D.; Donáeche, B. Handbook of Enology: Volume 1; John Wiley & Sons: Hoboken, NJ, USA, 2006.
- Marín-San Román, S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in analytical techniques for the extraction of grape and wine volatile compounds. Food Res. Int. 2020, 137, 109712.
- Blanco-Padilla, A.; Soto, K.M.; Hernández Iturriaga, M.; Mendoza, S. Food antimicrobials nanocarriers. Sci. World J. 2014, 2014, 1–11.
- Van Wyk, N.; Grossmann, M.; Wendland, J.; Von Wallbrunn, C.; Pretorius, I.S. The Whiff of Wine Yeast Innovation: Strategies for Enhancing Aroma Production by Yeast during Wine Fermentation. J. Agric. Food Chem. 2019, 67, 13496–13505.
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255.
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine aging technology: Fundamental role of wood barrels. Foods 2020, 9, 1160.
- Tao, Y.; García, J.F.; Sun, D.W. Advances in Wine Aging Technologies for Enhancing Wine Quality and Accelerating Wine Aging Process. Crit. Rev. Food Sci. Nutr. 2014, 54, 817–835.
- Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological application of tannin-based extracts. Molecules 2020, 25, 614.
- Zohre, D.E.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process. Biochem. 2002, 38, 319–324.
- Tenorio, C.; Gutie, A.R. Analysis of yeast population during spontaneous alcoholic fermentation: Effect of the age of the cellar and the practice of inoculation. Int. J. Food Microbiol. 2005, 103, 49–56.
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411.
- Ciani, M.; Comitini, F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015, 1, 1–6.
- Rossouw, D.; Bauer, F.F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46.
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii for secondary fermentation in sparkling wine production. Food Microbiol. 2018, 74, 100–106.
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process. Biochem. 2020, 90, 44–49.
- Shi, W.K.; Wang, J.; Chen, F.S.; Zhang, X.Y. Effect of Issatchenkia terricola and Pichia kudriavzevii on wine flavor and quality through simultaneous and sequential co-fermentation with Saccharomyces cerevisiae. LWT 2019, 116, 1–9.
- Maturano, Y.P.; Mestre, M.V.; Kuchen, B.; Toro, M.E.; Mercado, L.A.; Vazquez, F.; Combina, M. Optimization of fermentation-relevant factors: A strategy to reduce ethanol in red wine by sequential culture of native yeasts. Int. J. Food Microbiol. 2019, 289, 40–48.
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48.
- Mina, M.; Tsaltas, D. Contribution of Yeast in Wine Aroma and Flavour. In Yeast—Industrial Applications; IntechOpen: London, UK, 2017; pp. 117–134.
- Marsit, S.; Dequin, S. Diversity and adaptive evolution of Saccharomyces wine yeast: A review. FEMS Yeast Res. 2015, 15, 1–12.
- Longin, C.; Petitgonnet, C.; Guilloux-Benatier, M.; Rousseaux, S.; Alexandre, H. Application of flow cytometry to wine microorganisms. Food Microbiol. 2017, 62, 221–231.
- National Center for Biotechnology NCBI Taxonomy Browser. Available online: (accessed on 15 November 2020).
- Schreier, P.; Jennings, W.G. Flavor composition of wines: A review. Crit. Rev. Food Sci. Nutr. 1979, 12, 59–111.
- Louw, L.; Tredoux, A.G.J.; Van Rensburg, P.; Kidd, M.; Naes, T.; Nieuwoudt, H.H. Fermentation-derived aroma compounds in varietal young wines from South Africa. S. Afr. J. Enol. Vitic. 2010, 31, 213–225.
- Muñoz, D.; Peinado, R.A.; Medina, M.; Moreno, J. Higher alcohols concentration and its relation with the biological aging evolution. Eur. Food Res. Technol. 2006, 222, 629–635.
- Tokpohozin, S.E.; Fischer, S.; Becker, T. Selection of a new Saccharomyces yeast to enhance relevant sorghum beer aroma components, higher alcohols and esters. Food Microbiol. 2019, 83, 181–186.
- Stribny, J.; Gamero, A.; Pérez-Torrado, R.; Querol, A. Saccharomyces kudriavzevii and Saccharomyces uvarum differ from Saccharomyces cerevisiae during the production of aroma-active higher alcohols and acetate esters using their amino acidic precursors. Int. J. Food Microbiol. 2015, 205, 41–46.
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247.
