1. Introduction
Wound healing is a complex and well-regulated process controlled by extensive communication between cells, with a dynamic and complex crosstalk between different signaling pathways
[1].
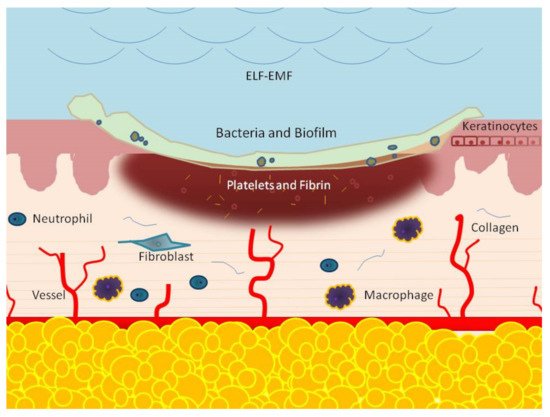
This process is composed of many phases, which include, consecutively, the hemostasis, inflammation response, new tissue formation, and tissue remodeling phases
[2][3][2,3]. The hemostatic phase results from the immediate activation of platelets. These cells release molecules, such as a growth factor and cytokines, that prevent bleeding and initiate wound repair. The second step is inflammation, which is characterized by the afflux 24 to 48 h after injury of different immune cells, such as neutrophils, monocytes, and lymphocytes. These cells work together and in close coordination to prevent infection and to remove dead tissue
[3][4][3,4]. Two to ten days following tissue injury, cellular proliferation, and migration of different cell types, such as fibroblasts, keratinocytes, and endothelial cells, new tissue formation occurs
[5][6][5,6]. Fibroblasts are known as the main actors regulating the wound repair process and, in the presence of the wound microenvironment, migration, proliferation
[6][7][8][6,7,8] and synthesis, and secretion of many factors, such as Matrix metalloproteinase-14 (MMP-14), basic fibroblast growth factor (bFGF), and fibroblast growth factor-9 (FGF-9), collagen homeostasis and angiogenesis increase
[9][10][9,10].
Finally, in the re-modelling phase, two to three weeks after injury, fibroblasts differentiate into myofibroblasts
[11], which produces an extracellular matrix leading to a mature scar
[12]. The tissue remodeling process may last for a year or more. At this stage, all the processes started by injury will turn off through apoptosis of involved cells, fibroblasts, macrophages, and endothelial cells
[10][11][12][13][14][10,11,12,13,14]. The wound will be repaired only if all these classic healing steps work correctly and in close coordination.
The process is highly efficient, but sometimes it can deviate from its physiological course, resulting in an ulcerative skin defect (chronic wound) or an excessive scar formation (hypertrophic scar or keloid). Chronic wound development may be common in various conditions including pressure, diabetes, venous pathology (venous, arterial, mixed, and vasculitis), trauma, and surgery, with significant morbidity and mortality risk
[15][16][15,16] as well as impact for a healthy economy
[17][18][17,18].
ELF-EMFs are non-ionizing, low-energy, electromagnetic fields capable of inducing several biological effects. Frequencies considered to be ELFs range from 3 Hz to 300 Hz. The study of the interaction between ELF-EMFs and the tissue is not always easy since different biological effects are related to EMFs’ time of exposure, waveform, frequency, amplitude, cell type, and cell status
[19][20][19,20].
The ELF-EMFs are commonly produced by electrical devices, high tension electrical distribution networks, from residential and occupational sources, and by power lines. Low-frequency electric fields influence all systems characterized by charged particles as the human body. In fact, tiny electrical currents exist in the human body due to the chemical reactions that occur as part of normal bodily functions, even in the absence of external electric fields.
The interest in the biological interaction of ELF-EMFs with tissues has, nevertheless, increased due to their possible effect on human health as well as their potential therapeutic use. ELF-EMFs with frequencies less than 300 Hz do not have enough energy to break molecular bonds, nor cause DNA damage, ionization, or even to have thermal effects on cells and tissues
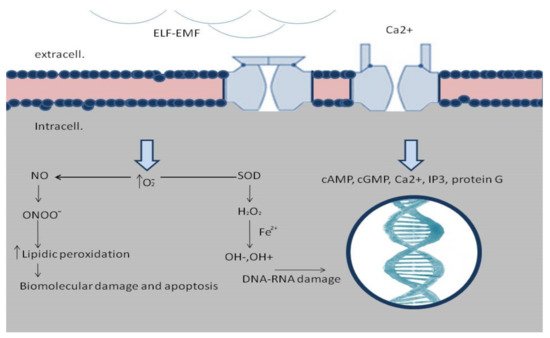
[21]. Biological effects modulated by EMFs are very wide and include cell migration, proliferation and differentiation, cytokine and growth factors expression, and nitric oxide signaling alteration
[20][21][22][23][24][25][20,21,22,23,24,25]. ELF-EMFs can interact with the chemical and biological processes modulating the physiological homeostasis, and, thus, can interact in wound healing.
Despite some studies reporting potential negative effects of ELF-EMFs, such as increased risk of childhood cancer, breast cancer, neoplastic development, neurodegenerative diseases, and in fertility, cardiovascular disorders, disease promotion, and progression
[26][27][28][29][30][31][32][33][26,27,28,29,30,31,32,33], no convincing evidence was ever provided for a direct relationship between ELF-EMFs and disease development. In the last few years, an increasing number of reports have evaluated the effects of ELF-EMFs on tissue repair.