Five-membered 1,2,4-oxadiazole heterocyclic ring has received considerable attention because of its unique bioisosteric properties and an unusually wide spectrum of biological activities. Thus, it is a perfect framework for the novel drug development. After a century since the 1,2,4-oxadiazole have been discovered, the uncommon potential attracted medicinal chemists’ attention, leading to the discovery of a few presently accessible drugs containing 1,2,4-oxadiazole unit. It is worth noting that the interest in a 1,2,4-oxadiazoles’ biological application has been doubled in the last fifteen years. Herein, after a concise historical introduction, we present a comprehensive overview of the recent achievements in the synthesis of 1,2,4-oxadiazole-based compounds and the major advances in their biological applications in the period of the last five years as well as brief remarks on prospects for further development.
- 1.2.4-oxadiazole
- synthetic methods
- drug design
- drug discovery
- structure-activity relationship
- medicinal application
1. Introduction
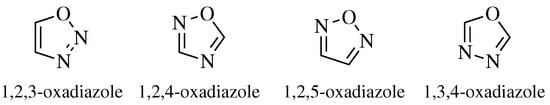
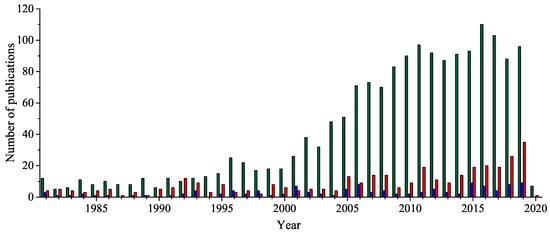
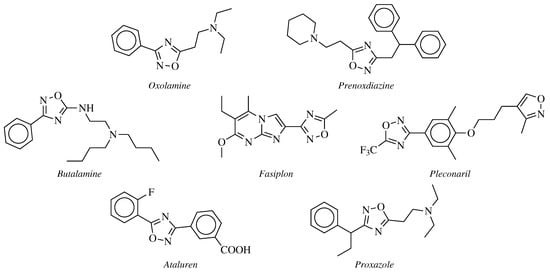
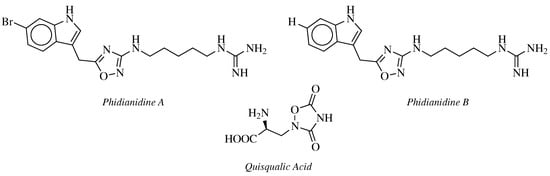
2. Historical Remarks—1,2,4-Oxadiazole
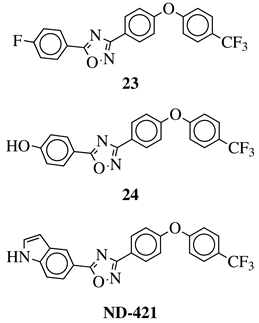
3. Anticancer Agents
Every year cancer impacts about 20 million people all over the world resulting in deaths counting in millions (Figure 5). Unfortunately, a number of new cancer cases is still rising and almost 30 million people will be diagnosed with carcinoma by 2040 in high-developed countries [30][50]. For that reason, finding new cancer treatments or effective drugs is one of the greatest needs of the current community and a challenge for modern medicine. Biological evaluation of 1,2,4-oxadiazoles revealed that some of their derivatives are potent anticancer agents. The greatest breakthrough came with the discovery of 3,5-diarylsubstituted derivatives of 1,2,4-oxadiazole as a new series of apoptosis inducers [31][51]. Since then, exploration of the anticancer activity of 1,2,4-oxadiazole derivatives has been started resulting in a creation of a wide library of compounds [32][33][52,53].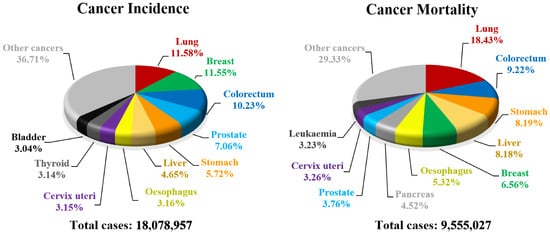
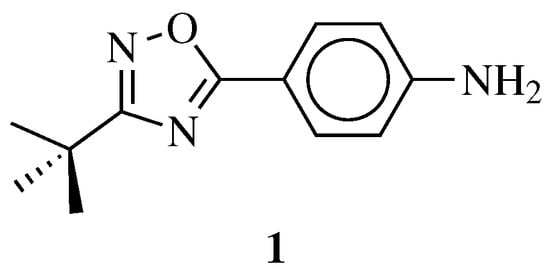
| General Structure | Substituents | The Most Active Derivatives | Activity | Ref. |
 |
R1 = H, NH2 and other (see Ref.); R2 = H or phenyl. |
 |
IC50 values of 2.76 and 9.27 μM against OVXF 899 and PXF 1752 cancer cell lines, respectively. |
| General Structure | Substituents | The Most Active Derivatives | Activity | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
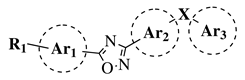 |
R1 = H, OH, OCH3, NH2, NHAc, NH3Cl, NHMs, NH-nBu, NH-tBu, NHCOPh, NH-iPr, PO3H2, PO(OEt)2, SO2NH2, CONH2, COOH, COOCH3 F, Cl, Br, I, NO2, ethynyl or CN; Ar1 = phenyl, benzyl, 2-pyrole, 3-pyridyl, 4-pyridyl, 5-indole, 3-pyrrazole, 2-imidazole and many others (see Ref.); Ar2, Ar3 = p-phenylene, 6-indole, 2-pyridyl, 6-chromene, carbazole, N-phenylpiperazine, N-phenylmorpholine and many others (see Ref.); X = NH, CH2, O, CO, NBn, SO or SO2. | [ | 35 | ] | ||||
 |
MIC | 50 | values <4 μg/mL against over 210 diverse, MRSA and VRE strains. | [65][67][68][72][96,98,99,103] |  |
X = Cl or Br; | ||
 |
XR1 = methyl, benzyl, 2-pyridinyl or anthracen-9-ylmethyl. | 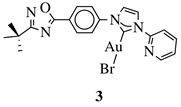 |
= NH or none; R1 = H, 3-chloro-4-fluorophenyl, 2-chlorophenyl, 2-ethyl, 4-ethyl, 5-bromo-2-fluorophenyl or 2-methylpyridin-5-yl. |
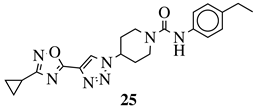 IC50 values of 3 nM against LXFA 629 and MAXF 401 cancer cell lines, respectively. IC50 values of 3 nM against LXFA 629 and MAXF 401 cancer cell lines, respectively. |
Grown inhibition zone within 20–25 mm against S. aureus, B. subtilis, E. coli, P. vulgaris, P. [36] | |||
| aeruginosa | , | C | . albicans. | [73][104] | 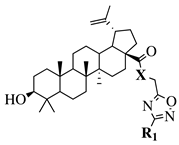 |
X = O or NH; R1 = phenyl, benzyl, 2-chlorophenyl, 4-fluorophenyl, 2-mehylphenyl, 4-bromophenyl, 4-methylphenyl, 4-methoxyphenyl, 4-pyridinyl, 2-methoxyphenyl, 2-benzyloxyphenyl or 3-pyridinyl. |
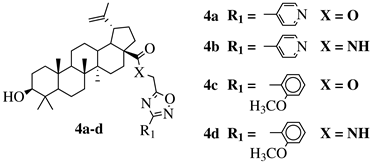 |
IC |
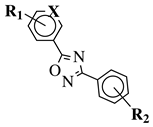 |
R1 = H, 2-chloro or 3-chloro; X = CH or N; R2 = H, 2-nitro, 2-chloro, 3-bromo, 2-chloro-5-nitro, 2-bromo, 3-nitro, 2-iodo, 3,5-dinitro, 4-nitro or 2-hydroxy. |
 | 50 values between 26.1–34.3 | MIC value of 60 μμM against Colo 205, Hep G2 and Hela cell lines. | M against E. coli.[37] | [74][38] | ||
| [ | 105 | ] |  |
R1 = methyl, chloromethyl or phenyl. | 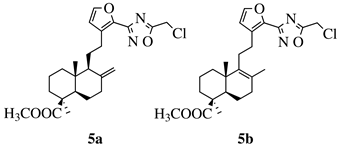 |
GI50 | ||
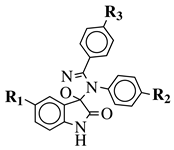 |
R1 = H, F, Cl, Br, I, methyl, ethyl, methoxy or iPr; R2 = H, methyl, methoxy, iPr, F, Cl, Br or I; R3 = H, F, Cl, Br, nitro, i | values of 0.08 ( | 5a) and 0.34 (5b) μM against CEM-13 cell line. | Pr, OBn, methoxy, ethoxy or CN. | 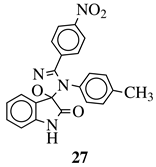 |
MIC value of 64 μg/mL against S. [39] | ||
| epidermidis | . | [ | 75 | ][106] |  |
R1 = H, Br, Cl, F, methoxy or NH | ||
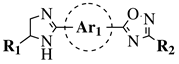 |
R2. | 1 = H or methyl; Ar 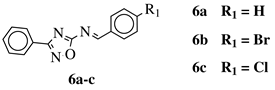 |
1 = p-phenylene or m-phenylene; R2 = methyl, cyclopropyl, 2-thienyl, 2-chlorophenyl, 3-chlorophenyl, 3,4-dichlorophenyl, 4-ethylphenyl, 4-t-butylphenyl, 4-methylphenyl, 3,4,-dimethylphenyl and many others (see Ref.).CC50 values of 137.3, 79.0 and 140.3 μM against Ca9-22 cell line, respectively. |
 |
MIC values in a range 8–16 μg/mL toward S. aureus, B. subtilis, E. coli[40] | |||
| , | P | . | fluorescent. | [76][107] |  |
R1 | ||
 | = H, 2-chloro, 3-chloro, 4-chloro, 4-nitro, 4-methyl, 4-methoxy, 4-trifluoromethyl, 2-bromo, 3-bromo, 4-bromo or 4-fluoro; R2 = N(CH |
R3)2, N(C2H5)2, pyrrolidine-1-yl, azepan-1-yl, morpholin-1-yl, thiomorpholine-1-yl, N-methylpiperazin-1-yl, N-phenylpiperazin-1-yl, 3-bromopropan-1-yl or 3-chloropropan-1-yl. | 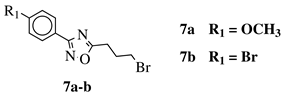 |
80% of death of NB4, K562 and MDA-MB-231 cancer cell lines at 25 (7a) and 10 (7b) μM. | [41] | |||
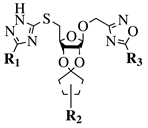 |
R1 = H or NH2; R2 = isopropylidene or cyclopentylidene; R3 = 4-nitrophenyl, 4-chlorophenyl or 3,4,5-trimethylphenyl. |
 |
GI50 of 4.5 μM against WiDr cancer cell line. | [42] | ||||
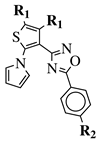 |
R1 = CH3 or —(CH2)4—; R2 = H, Cl, Br, methyl or methoxy. |
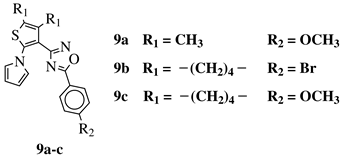 |
IC50 values of 0.48 (9a), 0.78 (9b), 0.19 (9c) μM against MCF-7 cancer cell line. | [43] | ||||
 |
R1 = H, 3-methyl, 4-methyl, 3-bromo, 4-methoxy, 4-trifluoromethyl, 4-chloro, 4-bromo or 4-fluoro. |  |
IC50 values between 13.6–48.37 μM against HCT-116, PC-3, SNB-19, B16F10, L929 cell lines. | [44] | ||||
 |
R1 = H, 3,4,5-trimethoxy, 4-methoxy, 4-chloro, 4-bromo, 4-fluoro, 4-trifluoromethyl, 4-nitro, 4-cyano or 4-methyl. | 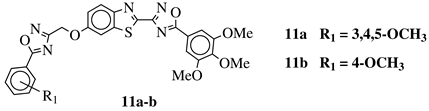 |
IC50 values in a range from 0.11 to 2.09 μM against MCF-7, A375 and HT-29 cancer cell lines. | [45] | ||||
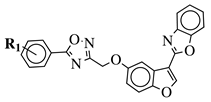 |
R1 = H, 3,4,5-trimethoxy, 4-methoxy, 4-chloro, 4-bromo, 4-fluoro, 4-trifluoromethyl, 4-nitro, 4-cyano or 4-methyl. | 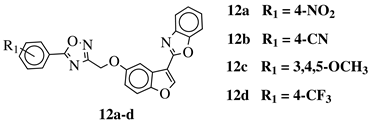 |
IC50 values between 0.011–1.89 μM against A549, MCF-7, A375 and HT-29 cancer cell lines. | [46] | ||||
 |
R1 = H, 3,4,5-trimethoxy, 4-methoxy, 4-chloro, 4-bromo, 4-fluoro, 4-trifluoromethyl, 4-nitro, 3-nitro or 4-methyl. | 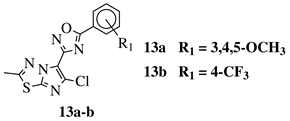 |
IC50 values in a range of 0.11–1.47 μM against A375, MCF-7 and ACHN cancer cell lines. | [47] | ||||
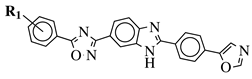 |
R1 = H, 3,4,5-trimethoxy, 4-methoxy, 4-chloro, 4-bromo, 4-fluoro, 4-trifluoromethyl, 4-nitro, 4-cyano or 4-methyl. | 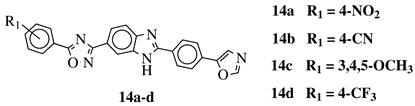 |
IC50 values between 0.12–2.78 μM against MCF-7, A549 and A375 cancer cell lines. | [48] | ||||
 |
R1 = methyl, phenyl, 4-fluorophenyl, benzyl or 4-methoxbenzyl; R2 = phenyl, 9-phenanthryl or 4-pyridinyl; R3 = 4-nitrophenyl, 4-chlorophenyl, 4-trifluoromethylphenyl or 4-fluorophenyl. |
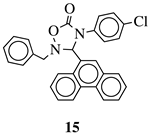 |
IC50 value of 10.38 μM toward MCF-7 cancer cell line. | [49] | ||||
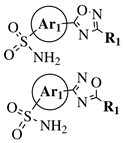 |
R1 = methyl, phenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-t-butylphenyl, 4-methylphenyl, 2-methoxyphenyl, 3-methoxyphenyl, cyclopropyl, 2-pyridinyl, 3-pyridinyl, 4-pyridinyl, 2-thienyl, 3-thienyl, 4-cyanophenyl, 2-fluorophenyl, 3-fluorophenyl, 4-fluorophenyl, 2-chlorophenyl or 3,4-dichlorophenyl; Ar1 = p-phenylene, m-phenylene, p-methoxyphenylene or 2,4-thienyl. |
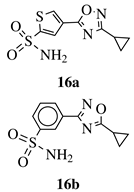 |
Ki value of 89 pm and 0.75 nm (hCA IX and hCA II, respectively) for 16a in CO2 hydration stopped-flow biochemical assay. 16b showed high selectivity toward PANC-1 cancer cell line. |
[50][51] | ||||
 |
R1 = H, F, Cl, Br or methoxy; R2 = H, F or Br. |
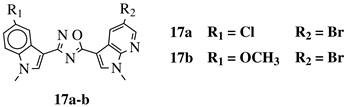 |
IC50 values of 0.65 (17a) and 2.41 μM (17b) against MCF-7 cancer cell line. | [52] | ||||
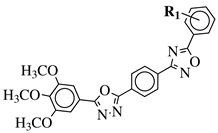 |
R1 = H, 3,4,5-trimethoxy, 4-methoxy, 4-chloro, 4-bromo, 4-fluoro, 4-nitro, 3-nitro, 4-cyano or 4-trifluoromethyl. | 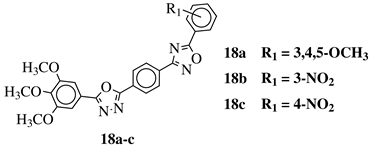 |
IC50 values in a range of 0.45–2.11 μM against MCF-7, A549, MDA-MB-231 cancer cell lines. | [53] | ||||
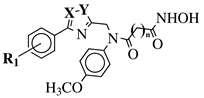 |
X, Y = N, O or O, N; n = 5 or 6; R1 = H, 2-methyl, 4-methyl, 4-methoxy, 2-fluoro, 3-fluoro, 4-fluoro, 4-bromo or 4-nitro. |
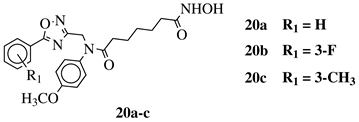 |
IC50 values of 8.2, 10.5, 12.1 nM (20a, 20b, 20c, respectively) toward HDAC-1. | [54][55] | ||||
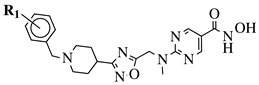 |
R1 = H, 4-methyl, 3-methyl, 2-fluoro, 4-fluoro, 2,4-difluoro, 2-chloro, 4-cyano, 4-trifluoromethyl or 2-chloro-4-fluoro. | 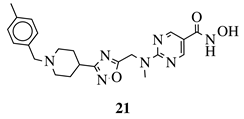 |
IC50 values of 1.8, 3.6 and 3.0 nM against HDAC-1, -2 and -3, respectively. | [56] | ||||
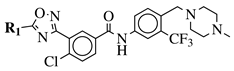 |
R1 = 3-pyridinyl, 4-pyridinyl, 4-methoxy-3-pyridinyl, 5-(2-methoxyethoxy)-3-pyridinyl, 5-morpholin-3-pyridinyl or 5-(1-methyl-1H-pyrazol-3-yl)-3-pyridinyl. | 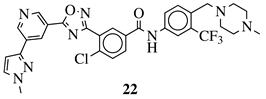 |
IC50 value of 7.3 nM against RET enzyme in ELISA assay. | [57] |
4. Antimicrobial Agents
| 1 |
| = phenyl, 4-mehtoxyphenyl, 4-chlorophenyl, 3-methylthienyl or 2-pyridinyl; | ||||
| R | ||||
| 2 | ||||
| , | ||||
| R | ||||
| 3 | = H, methyl, phenyl, 4-chlorophenyl, 4-methoxyphenyl, 3,4,-dimethoxyphenyl or 2,3-dimethoxyphenyl. |  |
MIC value of 0.68 mM against S. aureus. | [77][108] |
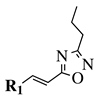 |
R1 = phenyl, 4-methylphenyl, 4-methoxyphenyl, 4-methylthiophenyl, 2-chlorophenyl, 4-chlorophenyl, 2-3-dichlorophenyl, 3,4-dichlorophenyl, 4-fluorophenyl, 4-bromophenyl, 4-hydroksyphenyl, 2-bromo-4-fluorophenyl, 4-cyanophenyl, 4-pyridinyl, 1-napthyl and others (see Ref.). | 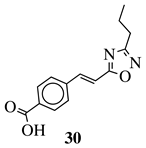 |
IC50 value of 0.045 μg/mL against M. tuberculosis (H37Ra). | [78][109] |
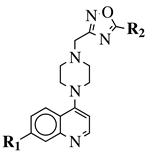 |
R1 = 4-pyridyl, 3-pyridinyl or 3,5-difluorophenyl; R2 = 3,5-dimethoxyphenyl, 3,5-difluorophenyl, 3-cyanophenyl, 2,3-dimethylphenyl, cyclopentyl or 4-izopropylphenyl. |
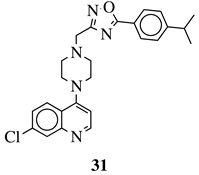 |
MIC value of 0.5 μg/mL against M. tuberculosis (H37Ra). | [79][110] |
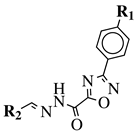 |
R1 = H, F, Cl, Br, methyl, nitro, methoxy or hydroxy; R2 = 4-hydroksy-3-methoxyphenyl, 2-styryl, ferrocene or 5-benzo[1,3]dioxole. |
 |
IC50 value of 0.02 μM against P. falciparum. In vivo studies failed—none in vivo activity. |
[80][111] |
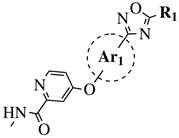 |
R1 = Me, Et, cyclopropyl, iPr, CF3, iBu or CH2OCH3; Ar1 = p-phenylene, p-2-methylphenylene, p-2,6-dimethylphenylene, 2,5-pyridinyl or 3-methylbenzothiophene |
 |
IC50 values of 66.0, 22.0 and 3.7 nM against hRV-B14, hRV-A21 and hRV-A71, respectively. | [81][112] |
