The surface-enhanced Raman scattering (SERS) technique, that uses magnetic plasmonic particles (MPPs), is an advanced SERS detection platform owing to the synergetic effects of the particles’ magnetic and plasmonic properties. As well as being an ultrasensitive and reliable SERS material, MPPs perform various functions, such as aiding in separation, drug delivery, and acting as a therapeutic material.
- Surface-Enhanced Raman Scattering (SERS)
- magnetic nanoparticles
- plasmonic nanoparticles
- detection
- drug delivery
- cancer therapy
- biological application
1. Introduction
2. Types of MPPs
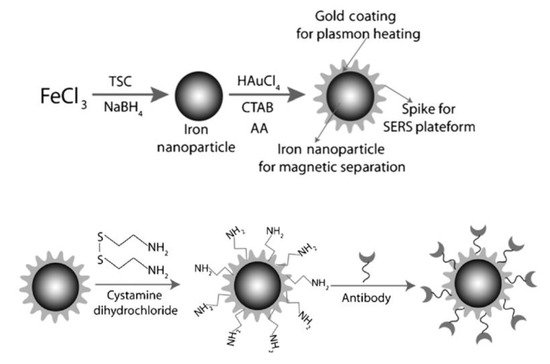
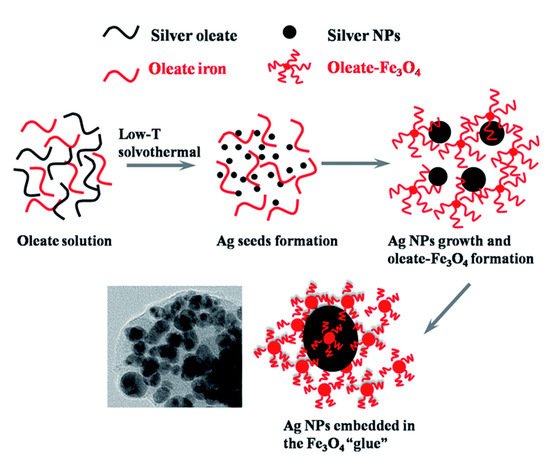
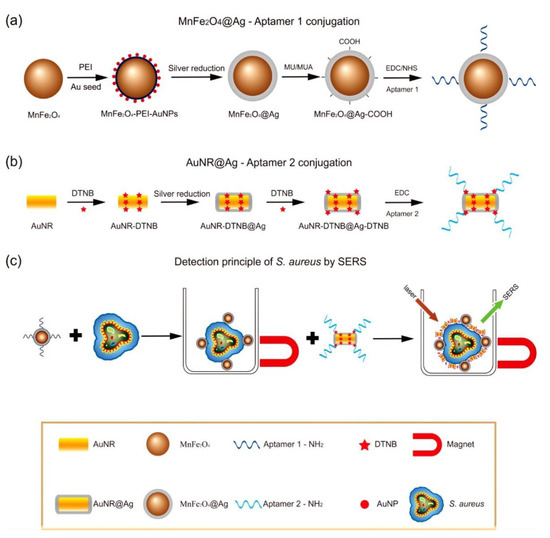
3. Bioapplication of MPPs
3.1. Detection and Separation
As SERS substrates, MPPs can detect targets with high sensitivity[22][76][77][78][79]. Additionally, as magnetic NPs, MPPs can be manipulated by an external magnetic field so that MPPs can be used to separate and enrich the target before SERS signal detection. Therefore, MPPs are often used to enhance the separation and detection of low concentration targets. These MPPs can also help isolate nucleic acids, proteins, and other small molecules in a complex biological matrix [59][80][81].
3.1.1. Nucleic Acids
Nucleic acid diagnostics, with its high sensitivity and specificity, plays a vital role in many fields, such as biology, medicine, and environmental science. Nucleic acid fragments and nucleotides can be targeted by magnetic SERS based detection. SERS enables high sensitivity and specificity, demonstrated by its ability to separate and collect targets with concentrations as low as fM[81][82][83], such as: typical Fe3O4 and Au based MPPs for adenine detection with detection limit as 0.7 µM[84]; dual MPPs including ultrabright Au- Ag core- gap-shell NPs with Raman reporter in the gap and magnetic NPs that were utilized for DNA detection with a detection limit as low as 100 aM[85]; and Fe3O4@Ag MPPs and duplex-specific nuclease signal amplification in microRNA detection with detection limit Nucleic acid diagnostics, with its high sensitivity and specificity.
Alula et al. developed MPPs containing iron and gold NPs for adenine detection [84]. A magnetic core is produced by coprecipitation and then coated by a polymer. Au NPs are then produced on the polymer-coated surface by a photochemical reduction method. The MPPs are stable in aqueous solution, disperse well in solution, and easily attach to the target adenine. Owing to their magnetic properties, the MPPs can concentrate the target to facilitate SERS imaging. Thereby, adenine can be detected at concentrations as low as 0.7 µM [84].
Ngo et al. reported an MPPs method to discriminate wild and mutant type malaria, via DNA detection and single nucleotide polymorphism[85]. Dual MPPs with magnetic NPs and Au/Ag NPs were labeled by DNA probes matching the target sequence. A hybridized sandwich of magnetic NPs-target sequence-Au/Ag NPs is formed via specific recognition of complementary DNAs, which are concentrated at the SERS detector. The limit of detection of the detection platform was approximately 100 aM.
MicroRNAs are short single stranded RNA molecules that are the primary regulatory factor in various biological pathways, such as mRNA degradation, translational inhibition, cell proliferation, differentiation, and apoptosis. They can also cause cancer[81][86][87][88][89][90][91]. Therefore, microRNAs are used as biomarkers for several diseases, including cancer. Pang et al. fabricated a Fe3O4@Ag MPPs designed for microRNA detection[81]. The Fe3O4@Ag MPPs are modified by Raman tags-DNA probes. Target microRNA are captured on the surface of Fe3O4@Ag MPPs through DNA/RNA hybridization. The SERS signal is significantly amplified by duplex specific nuclease, yielding a detection limit of 0.3 fM. The Fe3O4@Ag MPPs have been successfully applied in microRNAs’ capture, concentration, and direct quantification, overcoming disadvantages in previous microRNAs’ diagnostic tools, such as single sequence and low abundance[81]
In addition, MPPs have been employed in determining anti-cancer drug interaction with DNA [32], and for DNA methyltransferase activity[92]measurements, which uses SERS to detect DNA fragments via interaction between the fragments and plasmonic metals. These studies showed the MPPs also supported functionalization and recovery for recycling [32], and had excellent separation ability[92].
3.1.2. Protein
Like nucleic acids, proteins are key biological molecules that have a multitude of roles in organisms. There are numerous examples of proteins dispersed through an organism, including hormones, antibodies, cell-surfaced molecules, enzymes, and structural proteins. As abnormal protein quantities can lead to disease, sensitive and specific diagnosis techniques are necessary. Therefore, magnetic SERS has been applied in disease-based protein detection. Target proteins include human immunoglobulin G[20][36], prostate specific antigen[26][35][93], urinary erythropoietin[94], sepsis-specific biomarkers[95] carcinoembryonic antigen[96], thrombin, platelet derived growth factor BB, immunoglobulin E[62], and glycated hemoglobin[97]. These MPPs are designed with very specific components, such as antibodies, aptamers or chemical molecules[62][95][97]. The MPPs enable easy extraction, isolation, collection, and purification of the specific target from biological samples[62][93][94][95][96][97] and facilitate measurement at low concentrations.
3.1.3. Small Molecules
As an ultrasensitive sensor, SERS not only detects macromolecules like nucleic acids and proteins, but also single, small molecules[22]. Therefore, magnetic SERS have been applied in single molecule level diagnosis. The surfaces of the MPPs are labeled with a specific target antibody[30][98][99], aptamer[27], or chemical molecule [63]. The limit of target detection concentration can be as low as fg. mL−1 [30]. The magnetic parts act as a high-speed extractor[98] and simple isolator[99]. Additionally, the magnetic parts concentrate[63] and aggregate particles to generate hot spots, which increase the SERS intensity and thus aid sensitivity[100]. The process is used in a wide selection of applications, such as: determination of microcystin-LR in blood plasma[98]; anthrax biomarker poly-γ-D-glutamic acid in serum[99]; cotinine and benzoylecg onine in saliva[100]; thiocyanate and tetracycline in food products[27][41]; nitrite ions in environmental, biological, and food samples[63]; and N-terminal pro-brain natriuretic peptide as a biomarker for heart failure[30].
3.1.4. Cancer Diagnostic
Cancer is a group of diseases caused by abnormal growth of cells[101]. It can occur anywhere in the human body and often spreads. From the early stages of cancer, biomarkers are released[102]. If these biomarkers can be detected, then early diagnosis is possible, which aids in effective treatment. Magnetic SERS can accurately detect very low concentrations of biomarkers. In these MPPs, the plasmonic property targets the biomarkers and the magnetic property contributes to the agglomeration and the amplification of the signal. Many studies have demonstrated magnetic SERS’ usefulness in cancer marker detection with high sensitivity. MPPs are synthesized with specific biological components, including antibodies or aptamers, in order to target cancer markers or cancer cells, such as carcinoembryonic antigens[101], circulating tumor cells[102], RNA extract from cancer cells[81], breast-cancer cells, floating leukemia cells[57], and Bronchioalveolar stem cells[103]. Even at extremely low concentrations (~fM), the MPPs can separate and collect the target using an external magnetic field. The SERS signal is also amplified by the magnetic gathering[81]. Figure 4 shows an example of SERS detection of a cancer biomarker. In this case, the MPPs are formed from magnetic NiFe@Au and plasmonic Au NPs, with Raman labeling and suitable antibodies. The biomarker/MPPs combinations are separated, gathered by an external magnet, and detected by SERS amplification[101].
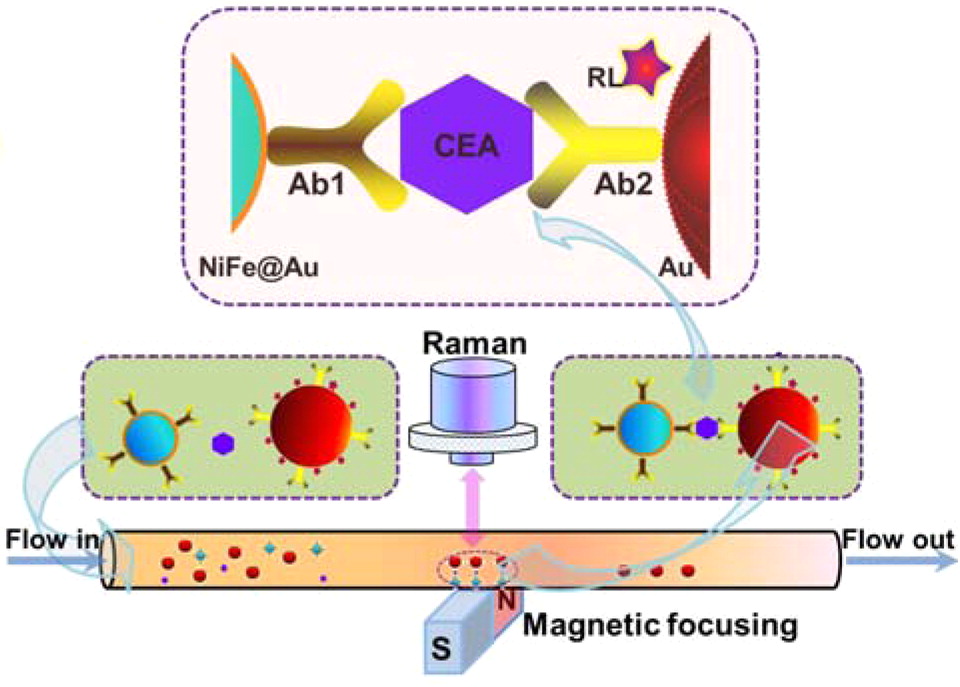
Figure 4. Illustration of SERS detection of cancer biomarker CEA (carcinoembryonic antigen). Using functional nanoprobes consisting of Au coated NiFe magnetic nanoparticles (NiFe@Au), capture antibodies (Ab1), detection antibodies (Ab2), and Raman labels (RL). Reprinted with permission from ref[101]. Copyright © 2015 American Chemical Society.
3.1.5. Detection of Pathogens
The magnetic SERS technique is one method for the diagnosis of infectious diseases caused by pathogens, such as bacteria or viruses[25][34][104][105]. Compared with other methods—including polymerase chain reaction, enzyme-linked immunosorbent assay, and culture and colony counting of bacteria—magnetic SERS has advantages, such as simpler sample preparation requirements, improved sensitivity, and rapid and multiplexed detection[104]. Additionally, magnetic properties assist in the concentration of the target to improve separation[15], accuracy, and sensitivity[106]. Typically, pathogens can be determined directly[34][106], or indirectly via specific capture with antibodies[24][104][107][108][109] or aptamers[25][110]. The MPPs used can be magnetic plasmonic core-shell mono particles[24] or dual particles including magnetic and plasmonic particles[25]. Additionally, the MPPs can be immobilized on a surface by specific capture[107], or without labeling[34]. Once these MPPs interact with targets, the targets are gathered by the magnetic force. The pathogens have a distinct, identifiable SER signal. Magnetic SERS techniques have ultrasensitive capabilities, down to 10 pathogen cells in 1 mL [25].
3.2. Drug Delivery and Therapy
The needs of drug delivery and therapy often coexist, in order to carry a drug to the affected location and provide medical treatment (Figure 5). Magnetic NPs have the potential to be next-generation drug carriers because of their unique physical-chemical properties[53][56]. Magnetic NPs can move easily and rapidly to the target position under a magnetic field. The toxicity and biocompatibility of magnetic NPs can be adjusted by surface modifications, such as coatings of polyethyleneimine (PEI)-g-polyethylene glycol (PEG), chitosan, dextran, N-(2-hydroxypropyl)methacrylamide (HPMA), polymeric micelles, starches, proteins, and polyvinyl alcohol (PVA)[52][53][55]. After being immobilized with the targeting ligands (enzymes, peptides, antibodies, aptamers etc.), the magnetic NPs accurately reach the target position and release drug molecules[54][55]. For cancer and tumor treatment, magnetic NPs have been utilized as a beneficial mechanism for thermotherapy[111][112][113][114][115]. In thermotherapy (high temperature treatment), magnetic particles act as heat transferrers, causing apoptosis of the tumor cells. In a study on mice by Xie et al., effective thermotherapy was achieved with four injections, each of dose 28 mg Fe. kg−1 body weight, using an alternating current magnetic field of 390 kHz and 12 A, with 30 min exposure at 43 °C [114]. Additionally, plasmonic NPs, such as Au and Ag, have been investigated for application in smart drug delivery and cancer therapy[54][55][111][116][117][118][119]. Several studies have also demonstrated that Au and Ag NPs possess antitumor properties and have the potential to enhance cancer therapy[120][121][122][123][124][125][126][127][128]. Briefly, endocytosis particles are carried by vesicles to a cell’s cytoplasm and nucleus; there they produce a toxic effect and cause apoptosis or programmed cell death through reactive oxygen species, tumor necrosis factor, or interleukin-6[117]. Meyers et al.[129] reported that peptide-targeted Au NPs successfully carried and delivered Phthalocyanine 4, an photodynamic agent, to cancer cells and then killed the cancer cells (concentration 1 µM). Ag NPs and their composite with other metals have also been investigated for antibacterial characteristics[127][130][131][132][133][134][135]. Ag NPs have been shown to effectively inhibit various pathogenic bacteria, fungi and viruses, including Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, dermatophyte, and HIV-1[127]. The antibacterial characteristics depend on the size, shape, concentration, and charge of the particles[111][127]. The optimum parameters for antibacterial Ag NPs were found to be spherical and less than 10 nm in size, rather than triangular, linear, or cubic in shape, and larger in size[111][127]. Additionally, accumulation over time and positive surface charge can help the Ag NPs increase their antibacterial effect[127]. An Ag-Co-Cr alloy has been investigated as an antibacterial medical implant[135].
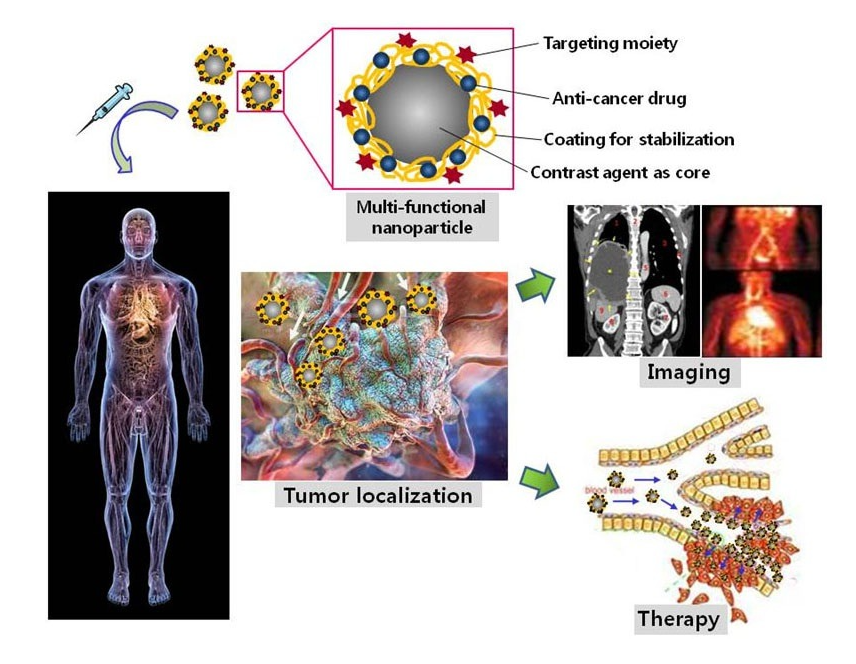
MPPs that combine magnetic and plasmonic nanoparticles have shown the same properties as the constituent magnetic NPs and plasmonic NPs. In a study by Tomitaka, magnetic plasmonic core-shell nanostar NPs demonstrated image-guided drug delivery and NIR-triggered drug release applications[51]. Under an alternating magnetic field, MPPs act as imaging contrast agents, assisting in the production of clear magnetic particle images. The Au parts of the MPPs bind the drug, which is then released at the desired location under NIR stimulation. Another study by Lal et al. demonstrated that iron oxide gold nanobowls can be guided magnetically, and that they possess distinct SERS capabilities. These characteristics show MPPs’ potential as a therapeutic drug storing and imaging contrast material[136]. Moreover, iron gold alloy NPs, in a study by Li et al., demonstrated their potential in thermotherapy-mediated controlled drug release for cancer therapy. According to this report, the thermotherapeutic properties of MPPs trigger drug release from the drug-MPPs conjugate. Furthermore, the magnetic properties in MPPs also aid in the drug unbinding under an external magnetic field[137].
In addition, the MPPs have exhibited photothermal ability that enables thermotherapeutic destruction of bacteria[24][108]. Popcorn shaped Fe core-Au shell MPPs have shown their antibacterial properties via a study on the multidrug resistant Salmonella DT104 bacterial strain[24]. The experiment achieved approximately 100% bacteria cell death using 2 mL of bacteria cells, at 1.2 × 105 CFU mL−1, incubated with 100 µL of particles and exposed to light (670 nm at 1.5 W cm−2) for 10 min. In another study, a mixture containing 150 µL of 1.3 × 104 CFU mL−1 E. coli cells, with 50 µL of MPPs solution, was exposed to light (670 nm, 2.5 W cm−2) for 12 min, and showed approximately 90% cell death[108]. In addition, the magnetic properties of MPPs aid the rapid separation of target pathogens in solution.
3.3. Imaging
Iron or gold-based NPs have been utilized as imaging contrast agents under alternating magnetic fields, such as in MRI and computed tomography; specifically, aimed at bio-imaging tumors and enabling their associated cancer diagnosis[111][138][139][140][141][142][143][144]. Iron and gold-based MPPs’ properties are used to upgrade the quality of cancer therapy by facilitating real-time diagnosis or image-guided drug delivery and therapy, and provides clear magnetic particle imaging. The high-quality images enable the control of the MPPs’ position under a magnetic environment. Therefore, once the MPPs reach the target position (tumor or cancer cell), the local environment’s structure is imaged and can help in diagnosis. Moreover, in drug delivery, observable drug release improves control of the drug dose and thereby ensures more effective treatment. Therefore, by imaging, a drug can be monitored at the tumor’s location and the drug’s concentration controlled[21][28][51][56][104] (Figure 5). For example, in Tomitaka et al.‘s research, MPPs demonstrated image-guided, near-infrared (NIR) responsive, and triggered drug release capabilities[51]. The MPPs produced clear magnetic particle imaging that enabled the MPPs to be guided to the correct position, and thereby deliver the drug to the required location. The Au parts in MPPs act as drug binders that release the drug under NIR stimulation. Thus, MPPs are candidates for applications in image-guided drug delivery therapy. These novel imaging properties could improve human health care in the future.
4. Conclusion and Future Perspectives
The above discussions demonstrate that MPPs are multifunctional nanomaterials that combine the synergistic effects of their magnetic and plasmonic parts. MPPs have improved the quality of existing imaging techniques and have the potential to monitor exceptionally low concentration targets and provide real-time delivery and release of therapeutic drugs. The latter has already been successfully applied at the in vitro and in vivo levels in rats. MPPs can be efficiently used in various systems to provide improved disease diagnosis, monitoring, and treatment. With real-time control, drug dose can be easily monitored, and release rates appropriately adjusted for each individual situation.
Application MPPs in thermotherapy is further widely performed due to not only high effectiveness but also less side effects. For example, in the case of intracranial thermotherapy of glioblastoma multiforme, magnetic NPs combined with external beam radiotherapy did not produce the side effects commonly seen with traditional treatments, such as headaches, nausea, vomitus, and allergic reactions. Additionally, no neurological deficits or infections were evident in the treated regions[112]. This study has shown the potential of next-generation MPPs for cancer treatments that decrease side effects and aid in healing.
MPPs also exhibit potential antibacterial properties. Bacterial inhibition without the use of antibiotics will help prevent the evolution of bacteria capable of defeating antibiotics. Overall, the potential and prospective applications of MPPs could bring significant benefits in the future.
Beyond this, there are many reports that have discussed the application of machine and artificial intelligence in the SERS technique[145][146][147][148]. It has provided a novel idea that it is possible for artificial intelligence which learns using MPPs in bioapplication to raise a new approach in future chemistry.
